1. Introduction
Perinatal insults, such as inflammation and birth asphyxia, often contribute to both preterm and term brain injury [
1]. Hypoxic-ischemic encephalopathy (HIE) occurs in 1–4 per 1000 live births in high-income countries, and around 12 per 1000 live births in low- and middle-income countries [
2]. The incidence of HIE in preterm populations is likely underestimated because of its lack of a clear clinical definition, but a retrospective report of infants born at less than 37 weeks’ gestation in the United States between 2008 and 2011 showed an incidence of moderate to severe HIE of 37.3 per 1000 live births [
3]. TH has been shown to significantly improve the outcomes of term and near-term infants with HIE in high resource settings, with a number needed to treat of 7 [
4]. TH has not been proven to be beneficial in term infants in lower resource settings [
5], or in preterm infants, so new approaches to neuroprotection are needed for these populations.
Animal models that accurately and reproducibly reflect human postnatal brain development as well as the pathophysiology and sequelae of HIE are also needed to test potential neurotherapeutics. For instance, few gyrencephalic or large animal models exist that allow for long-term assessment of injury to the developing cortex and white matter, which are particularly vulnerable to inflammation and HI in both preterm and term infants [
6]. The ferret is an ideal animal model to examine the long-term effects of injury on brain development and response to treatment in prematurity and asphyxia-related disease states [
7,
8]. Postnatal ferret brain development is histologically similar to the rapid brain maturation that occurs in the second half of human gestation [
9,
10]. Our group recently published a protocol utilizing lipopolysaccharide (LPS) inflammation presensitized hypoxia-ischemia plus hyperoxia (HIH) to model hypoxic-ischemic (HI) brain injury in the near-term (P17) ferret [
11]. The P17 ferret brain developmentally correlates to an infant at 32–36 week’s gestation [
12].
The present study presents two cohorts: an initial cohort testing a putative neuroprotectant, uridine monophosphate, with a later cohort testing two established neurotherapeutics-erythropoietin (Epo) and TH. We hypothesized that Ur and Epo would provide significant neuroprotection against inflammation-sensitized, hypoxic-ischemic brain injury, but that LPS presensitization would negate the protective effect of TH in this model [
13].
3. Discussion
Neonatal encephalopathy in both preterm and term infants is often associated with hypoxia-ischemia, and is often initiated or exacerbated by exposure to infection or other sources of inflammation [
15]. The ferret provides an exciting, highly translatable animal model to study neonatal encephalopathies. As the smallest gyrencephalic mammal, the ferret is born altricial and begins to develop cortical folds in the first 6–8 days of postnatal life [
16]. This feature allows for the study of injury patterns that impede normal cortical development. We have previously described a reproducible injury protocol to model neonatal encephalopathies in the P17 ferret, when the brain is approximately equivalent to human brain at 32–36 weeks of gestation. Utilizing this model, we sought to test both known and potential neurotherapeutics. After LPS-sensitized HIH in the P17 ferret, neither Ur nor TH were robustly beneficial, though TH was associated with preservation of certain white matter areas on DTI compared to Veh-treated injured controls. By comparison, Ur may have contributed to an increase in mortality and worse performance during reflex development. In contrast, Epo was partially neuroprotective, particularly with respect to white matter and cortical structure, which is consistent with multiple other preclinical studies [
17,
18,
19,
20,
21,
22]. This suggests that the ferret HIH model responds as expected to established neuroprotective agents in the setting of LPS-sensitized HI brain injury, providing a robust gyrencephalic, large animal model of neonatal encephalopathies amenable to multiple long-term behavioral and neuropathological assessments.
Uridine represents a potential therapy that would be particularly advantageous in low resource countries, as it is inexpensive and shelf-stable. When administered at a dose of 500 mg/kg for three consecutive days, Ur provided modest neuroprotection in a rat model of HIE [
14,
23]. The neuroprotective effects of Ur administration in rodents seems to be mediated through its antiapoptotic [
14] and antioxidant actions [
24]. However, despite being administered at a similar dose, Ur was not neuroprotective in this model and may have contributed to the increase in mortality. Even with the increased mortality in Ur treated animals, which likely removed the most severely injured animals, Ur treatment still delayed the acquisition of basic reflexes, decreased exploratory behavior in the open field and MWM arena, and increased brain pathology beyond that incurred from the HIH insult alone. Initially, there was a concern that exogenous Ur administration could result in a higher death rate due to altered thermoregulation, as high doses of Ur administered in human and rabbit experiments have been shown to induce hypothermia [
25]; however, rectal temperatures in our ferret model were not affected by Ur treatment (data not shown). Additionally, metabolism of Ur by ferrets differs from humans, in which Ur is the primary circulating pyrimidine nucleoside. By comparison, the ferret appears to convert the majority of circulating Ur to uracil, unless given in high doses. Therefore, the lack of neuroprotection conferred by administering Ur in this model does not preclude its potential success in other HI models or modalities of brain injury.
Epo is an erythropoietic hormone produced endogenously in response to chronic hypoxic events, including hypoxia and fetal stress in utero [
26,
27,
28,
29,
30,
31]. After acute neonatal brain injury, treatment with high doses of Epo confers neuroprotection acutely through antiapoptotic and anti-inflammatory actions in multiple animal models [
17,
18], as well as through longer acting protective mechanisms including angiogenesis, neurogenesis, and oligodendrogenesis [
19,
20,
21,
22]. Treatment with Epo in this model resulted in quicker weight recovery after HIH, with Epo-treated animals showing a growth rate more similar to controls than to the other HIH-exposed animals. There was also a trend across the late behavioral tests of the Epo animals behaving more similarly to controls than Veh, Ur, or TH treated animals, though this was rarely significantly different. However, interestingly, a separate swim pattern analysis found that compared to Veh and TH animals, Epo animals were less likely to have swim patterns similar to that of control animals. Understanding the variable effects of Epo on behavior is outside the scope of this paper but will be an important avenue for further investigation. Although Epo treatment was unable to significantly recover locomotor abilities after HIH, partial neuroprotection was observed in a number of neuropathological and morphological assessments. For example, animals in the Epo group had significantly improved cortical pathology scores and normalization of total gyral widths. Epo-treated animals also had significantly thicker white matter tracts in their SWM when compared to the Veh and Ur brains, but they still displayed significantly thinner white matter tracts in the SWM and CC regions when compared to the control brains. Similarly, although animals in the Epo treatment group had evidence of greater peri-hippocampal white matter injury than control animals by DTI, Epo-treated animals had DTI markers consistent with improved white matter preservation relative to the Veh group, particularly in the internal capsule. This suggests that the Epo treatment had localized effects on white matter protection and maturation following HIH, with region-specific responses to injury and therapy. It also aligns with some preclinical Epo studies in which Epo has not consistently been neuroprotective depending on model, dosing strategy, and outcome measures [
32,
33,
34,
35]. An important component of our future work in this model will be determining whether combinations of therapies are necessary to provide global neuroprotection after neonatal HI brain injury.
The benefit of administering TH within 6 h of an HI event has been demonstrated through meta-reviews and large clinical trials [
36], and we aimed to replicate its therapeutic effect in this model. In the setting of term HIE, however, TH does not provide full neuroprotection when it is deployed on its own, and infants can still suffer from debilitating long-term outcomes after an HI insult [
37]. Part of the lack of complete neuroprotection by TH may be due to heterogeneous etiologies of injury in the clinical setting, including HIE complicated by infection or chronic placental insufficiency [
38,
39]. In particular, TH has not been neuroprotective in preclinical models of HI sensitized with LPS [
13,
40,
41]. The lack of significant neuroprotection conferred by TH in our ferret model is therefore not unexpected due to the use of LPS for inflammatory presensitization, though TH animals did demonstrate improved behavioral outcomes in some tests compared to other HIH-exposed animals. For example, the TH group’s time to complete the reflex testing and activity level in the open field and MWM testing arenas were more consistent with the control group than the other HIH groups. However, gait analysis on the CatWalk showed that TH failed to provide behavioral recovery after HIH. Similar to the other HIH-exposed animals, the TH brains had shorter sulci, thinner gyri, and greater cerebellar exposure than the control animals, suggesting significant cortical dysgenesis after injury that was not ameliorated by TH. Additionally, and interestingly, despite evidence of diffuse white matter preservation on DTI after TH treatment, the TH group had the thinnest corpus callosum measurements out of all treatment groups. Though modeling complete chronic intra-uterine exposures such as chorioamnionitis remains a preclinical challenge, a significant number of infants still experience incomplete neuroprotection from TH treatment, and therefore models that respond similarly therefore remain important to the development of novel neurotherapeutics for scenarios where TH is not fully neuroprotective.
Overall, exposure to HIH at P17 disrupted ferret locomotor function at P42, as seen through CatWalk, open field, and testing in a water maze arena. Ur failed to repair and appeared to significantly worsen these deficits. This aligns with our current findings for Ur across other behavioral and morphological parameters and further highlights Ur’s neuroprotective inadequacy in this model. Compared to animals in the Veh and Ur groups, Epo and TH animals tended to behave most like controls during late behavioral tests. Despite this apparent improvement, it is important to note that Epo and TH medians differed in some way from controls for most late motor parameters, indicating the persistence of neurobehavioral deficits in those animals despite any therapeutic signals. Considering the practical reliance on TH in the clinical setting, we find it necessary to highlight that, although TH animals showed potential improvement in some behavioral parameters, they often fell short on several behavioral parameters compared to Epo animals.
This study has some limitations. Firstly, due to the lack of any therapeutic signal in the Ur group, these animals were not included in all of the final outcome analyses (particularly MRI and more extensive IHC measures). Regardless of the between-group differences observed across the behavioral parameters mentioned here, our findings are also more variable and nuanced compared to published studies using similar behavioral tests on rodents. Some of this may be due to the addition of confounding factors that arise when adapting rodent testing arenas to fit the larger body size of the ferret. The CatWalk system, in particular, seemed to confine and obstruct some ferrets from full ranges of motion. Few of the previous studies have examined motor function in juvenile ferrets beyond the development of reflexes [
42]. Consequently, we have attempted to develop novel paradigms that seek to identify and understand the ferret-specific behavior we observed during testing at P42, with further development of testing paradigms including more complex cognitive function ongoing. A disadvantage of using the P17 ferret to model neonatal encephalopathies is that its human developmental correlate falls somewhere between late preterm and term gestational age, similar to the P7 rat [
43]. This may help to explain why the partial neuroprotection from Epo seen in the model does not align with the lack of benefit from Epo treatment recently described in extremely preterm infants in the PENUT trial [
44]. In its current state, the P17 model appears to more closely correlate with near-term HIE rather than preterm brain injury, though our future work in the model is planned to be either earlier (P12-~28 weeks’ gestation) or later (P21–full-term equivalent) to provide clear separation in developmental stage. It is worth mentioning that no sex differences in treatment response were observed in this study. However, there is a significant body of literature of both human and animal studies to support the hypothesis that there would be a difference in response to treatment observable in this model [
45,
46,
47], and it is likely that sex-based differences would become apparent once larger group sizes are included. Another limitation is the relatively complex nature of the model, which was developed over several years of iteration [
7]. As such, we are unable to know which of the individual components of the experimental protocol—LPS presensitization, hypoxia, hyperoxia, and reperfusion—drive a certain therapeutic response. Examining the mechanisms of Epo neuroprotection in this model are beyond the scope of this manuscript but will form the basis of future work. By comparison, although we have not studied response to these therapies without the presensitizing effect of LPS in the model, it is likely that the inclusion of LPS is the primary reason for a lack of neuroprotective effect of TH [
13,
40,
41]. However, as more definitive evidence is accumulating regarding the lack of TH neuroprotection in lower income settings [
5], perhaps due to more protracted or chronic perinatal insults, the model as used here may be particularly useful for examining therapies for use in scenarios where TH is not beneficial.
In summary, this model responds as expected to a known neurotherapeutic and provides a platform to model complex long-term cortical development and white matter pathology assessments not feasible in most rodent or other large animal models. The ability of translational research to bring new therapeutics to the bedside relies upon animal models that are able to faithfully replicate several aspects of the human disease state. We believe this model will help the field to achieve that goal.
4. Materials and Methods
4.1. Animals and Housing
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Washington. Procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and reported in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. Ferret jills with cross-fostered and sex-balanced litters of 8 kits each were purchased from Marshall Bioresources (North Rose, NY, USA) and delivered to a centralized animal facility on postnatal (P) day 8. Ferrets were housed in a dedicated animal vivarium with ad libitum access to food and water before and after experimental procedures under standard conditions. Standard conditions included a 16:8 h light–dark cycle, room temperature range of 61–72 °F (16–22 °C), humidity of 30–70%, and 10–15 fresh air changes per hour.
4.2. Circulating Uridine and Uracil Levels
At P17, a single dose of uridine 5′ monophosphate (Sigma cat. U1752, St. Louis, MO, USA) was administered subcutaneously (s.c.) at a concentration of either 330 mg/kg (
n = 5, low-dose Ur group) or 1000 mg/kg (
n = 5, high-dose Ur group). Blood samples (150–200 μL; anticoagulated with EDTA) were obtained from the saphenous vein at 0 time point (baseline,
n = 8) prior to the administration of Ur or saline vehicle. Subsequent blood samples were collected at 30 min, 1 h, 2 h, and 4 h. Circulating levels of uridine and uracil were measured using high pressure liquid chromatography-mass spectrometry (
Supplementary Methods). Our goal was to determine a Ur dose that resulted in circulating concentrations similar to those conferring neuroprotection in a rodent model of neonatal brain injury: 10,000 ng/μL [
14].
4.3. Optimized P17 Injury Model
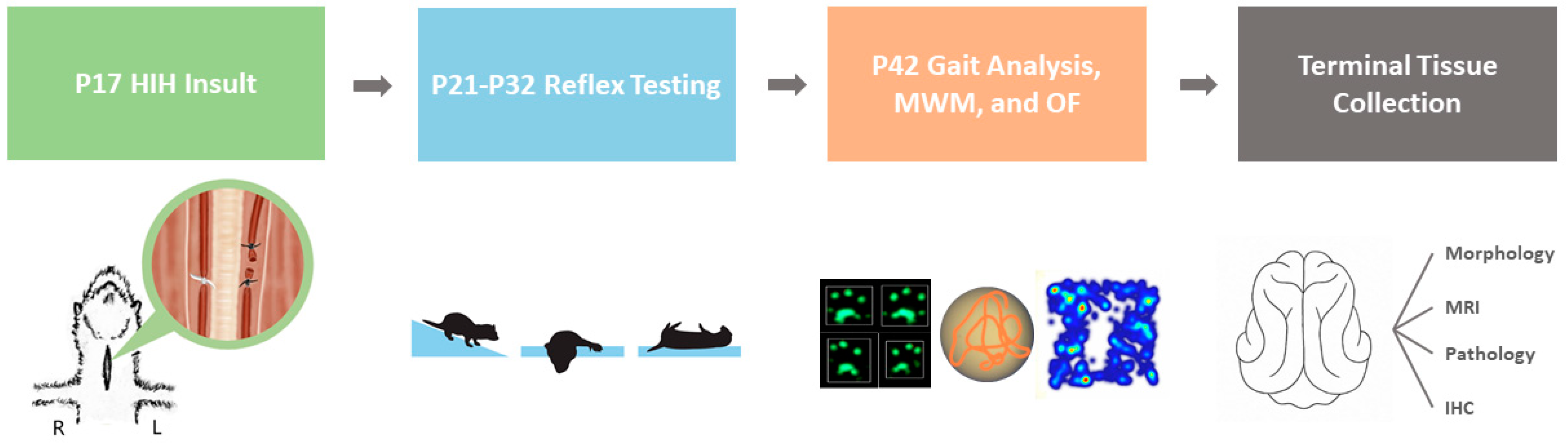
An overview of the experimental procedures is shown in
Figure 6. At P17, ferret kits were removed from the nest, weighed, and randomized to either control or HIH conditions. Control animals and HIH ferrets received 3 mL/kg saline vehicle (Veh) or 3 mg/kg LPS (freshly prepared ULTRA PURE LPS from
E. coli 055:B5, Lot #4231A, List Biological, Campbell, CA, USA) intraperitoneally, respectively. Inflammatory presensitizing injections were given approximately 4 h before the start of hypoxia to coincide with the peak cytokine response to LPS [
40,
48]. Animals who received LPS underwent bilateral carotid ligation under isoflurane anesthesia (3–5%) plus buprenorphine (0.05 mg/kg) analgesia. A 2 cm midline incision was made along the neck and the underlying tissue layers were dissected. Special care was taken to isolate the right and left carotid arteries from surrounding connective tissue and their accompanying nerves. The left carotid artery (LCA) was ligated twice with silk suture (5-0, Fine Science Tools, Foster City, CA, USA) and then transected between the two ligations. The right carotid artery (RCA) was temporarily occluded with umbilical tape (1/8 in, GF Health Products, Atlanta, GA, USA). The incision site was closed with wound clips, and the animals were taken to a separate room to recover. After a 30 min rest period, kits were placed in a prewarmed chamber and exposed to hypoxia (9% oxygen for 30 min) followed by hyperoxia (80% oxygen for 30 min), then hypoxia again (9% oxygen for 30 min). During the gas exposures, the internal body temperatures of two sentinel ferrets were closely monitored (Precision 4000A thermometer, YSI, Yellow Springs, OH, USA), to maintain normothermia (target rectal temperature 37 °C). After the second period of hypoxia, kits were returned to the surgical suite and the RCA umbilical tape was removed under anesthesia. In most cases, reperfusion of the RCA was observed. Incision sites were closed with wound clips and a 1 mL bolus of s.c. normal saline was given to maintain hydration. The kits were placed back with their jills to nurse and recover for 1 h.
4.4. Treatment
Initiation of treatment began 90–120 min after the end of the HIH insult across all cohorts. In the first cohort, HIH-exposed animals were randomized to receive either s.c. Veh or Ur (500 mg/kg every 12 h for 5 total doses). Ur dosing was determined based on pharmacokinetic data provided in
Supplemental Figure S1. In the second cohort, HIH-exposed animals were randomized to either Veh, Epo (2000 IU/kg s.c. at 0 h, 24 h, 48 h, and day 7 for 4 total doses), or TH (target rectal temperature 33.5 °C for 6 h). Epo dosing was based on previous rodent work in a similar model that conferred neuroprotection [
49] and converted to an equivalent dose using the ferret’s body surface area [
50]. Ferret kits not assigned to the TH group received their first dose of treatment (Veh, Ur, or Epo) and were placed in a plastic chamber in a heated water bath to maintain a normothermic rectal temperature of 36–37 °C.
Supplemental Figure S6 shows normal nesting temperatures of a P17 ferret. Animals randomized to TH were placed in a separate water bath and water temperature was adjusted to maintain a rectal temperature of 33–34 °C. During the temperature management period, each animal had its rectal temperature measured every 15–30 min for 6 h.
4.5. Behavioral Testing
4.5.1. Early Reflex Testing
Behavioral tests were performed daily from P21 to P27 and then 3×/week from P28 to P39. Kits were removed from the nest and placed in a heated chamber for 1 h prior to all testing. The tests were modified from rodent reflex testing and performed as previously published [
7,
11]. The tests included negative geotaxis (NG), cliff aversion (CA), and righting reflex (RR). NG was performed at both 25° and 45° and the time to rotate 90° and 180° (with a full step towards the top) was recorded. For CA, kits were positioned with their forepaws at the edge of the testing benchtop, and the times to back away from the edge and take a full step at least 90° away from the starting position were recorded. For RR, kits were positioned with their backs touching the testing benchtop. The experimenter would release the kit and the time it took to ground all 4 paws and the time to take a full step in any direction were recorded. All tests were performed by experimenters blinded to treatment groups. Kits underwent 3 trials each on NG and CA tests, and five trials each for the RR test. The animal was given a failing score if they were unable to complete the full test within 60 s or if they fell during NG or CA testing. A total time (sum of the mean time for each of the three tasks) score on each day, and the area under the curve (AUC) across the testing period (P21–P39) was calculated for each test to measure skill acquisition.
4.5.2. Gait Analysis
Gait characteristics were assessed using the CatWalkXT system (Noldus Information Technology, Wageningen, Netherlands). The protocol for kit handling, experiment setup, and testing process has been previously published [
11]. On P42, three compliant runs were collected from each animal. A compliant run was characterized by the ferrets’ ability to travel through the entire length of the testing platform without pausing. Paw print size, paw print intensity, base of support, phase dispersion, and lateral support were analyzed as measures of gait development. The software-detected paw print classifications were manually adjusted where needed to capture the ferret paw print more accurately, as the software was developed for use in rodents. In addition to the automated outputs, all runs were visually analyzed to determine the number of attempted runs. An attempted run was defined as the ferrets’ forward progression at least halfway through the CatWalk runway, regardless of full completion. A ratio of completed runs to attempted runs was calculated for each animal to measure animal compliance and ability to complete the trial.
4.5.3. Open Field
After the CatWalk test on P42, the ferret’s behavior was observed in an open field. Animals were tested in an acrylic box measuring 55 × 55 cm with opaque walls measuring 63.5 cm in height. Using EthoVisionXT software (Stoelting Co., Wood Dale, IL, USA) and a video camera, the behavior and movement of the ferrets were recorded for 5 min. The outputs generated included total distance moved, velocity, time spent in the center, and time spent in the corners.
4.5.4. Morris Water Maze (MWM)
A ferret-adapted version of the MWM test was performed on the final day of testing (P42) in a subset of the second cohort of animals (
n = 76). Each animal was first given a 60 s acclimation period prior to the testing trials. The behavior and movement of each animal was filmed and tracked using EthoVision software. Each trial lasted up to 60 s or until the animal either mounted the escape platform or required rescue. Data collected during MWM were analyzed for frequency and velocity of movement and distance traveled. Aside from the computerized outputs, a secondary analysis was conducted by generating an image file of each animal’s complete swim path for every trial. The images were evaluated on a dichotomous scale for the following parameters: greater than 50% arena coverage and circuitous exploration in open water. A trial was deemed “circuitous exploration in open water” if it was a mainly non-linear swim path and if the ferret avoided the perimeter of the pool arena. An additional analysis calculated exploratory behavior as a function of distance traveled and time spent moving (see
Supplementary Methods).
4.6. Ex Vivo MRI
At P42, kits were euthanized and perfusion fixed with phosphate-buffered saline (PBS) followed by 10% neutral buffered formalin (NBF). Subsequently, the brains were removed and immersion-fixed in NBF for at least 72 h before being rinsed and submerged in PBS at 4 °C to rehydrate for a further 72 h. Brains were then mounted on agarose gel sleds inside 50 mL Falcon tubes and immersed in Fomblin (Perfluoropolyether, PFPE; Solvay Specialty Polymers, GA). Nuclear magnetic resonance (NMR) data were collected on the Bruker Avance III, 4.7 Tesla (200 MHz, 1H), 20 cm, horizontal bore magnet with ParaVision version 6.0.1 software. All settings were applied as described previously and provided in
Supplemental Methods [
6]. Diffusion tensor images (DTIs) were obtained by processing diffusion-weighted images using the FMRIB Software Library (FSL v6.0, Oxford, UK) eddy software (
https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/eddy, accessed 4 January 2021) and FSL’s dtifit and fslmaths-fmedian options as previously detailed in Wood et al. [
7]. The resultant FA maps were co-registered onto a generated template for tract-based spatial statistics (TBSS). Fractional anisotropy (FA) maps were presented using Threshold-Free Cluster Enhancement (TFCE) adjusted for multiple comparisons, with significance levels set at 0.05 and 0.15.
4.7. Brain Measurements
After MRI were complete, brains were rinsed with PBS to remove remaining Fomblin and placed back into NBF for a minimum of 48 h. The brain was then removed from NBF and placed onto a paper towel to absorb excess liquid. External cortical features were measured using digital calipers with fine pointed jaws (SRA Measurements Products, Walpole, MA, USA). Sulci were measured from the beginning and end of the most distinct portion of the corresponding sulcus. Gyri were measured from the widest aspect of each corresponding gyrus. A map of all brain measurements is shown in
Supplemental Figure S7. As measures of overall cortical development, all gyral and sulcal measurements for each animal were summed for comparison.
4.8. Immunohistochemistry (IHC)
After brain measurement, coronal slices at the level of the caudate nucleus were taken from each brain, embedded in paraffin, and 4 μm sections were prepared for hematoxylin and eosin (H&E) staining and IHC. Glial fibrillary acidic protein (GFAP, 1:300 dilution; Agilent (Dako)) and Iba-1 (1:1500 dilution, WAKO Chemicals USA, 019-19741) IHC was performed at the University of Washington Harborview Medical Center Histology IHC lab. Myelin basic protein (MBP, 1:500 dilution, Abcam, AB7349) and Oligodendrocyte transcription factor 2 (Olig2, 1:500 dilution, Millipore, AB9610) IHC was performed at the University of Washington Histology and Imaging Core. For GFAP, staining was performed using rabbit polyclonal anti-GFAP using the Leica Bond III IHC stainer, the polymer refine detection kit, and the Bond Epitope retrieval 1 solution for 20 min. Iba-1 slides were run on the same Bond autostainer platform using the Leica Bond refine detection kit. Heat epitope retrieval was used to unmask the antigen for 20 min in Leica Epitope Retrieval 1 solution. MBP slides were baked for 30 min at 60 °C and deparaffinized on the Leica Bond Automated Immunostainer (Leica Microsystems, Buffalo Grove, IL, USA). Antigen retrieval was performed by placing slides in EDTA for 20 min at 100 °C. The primary antibody in Leica Primary Antibody Diluent was applied for 30 min. A secondary antibody, unconjugated rabbit anti-rat IgG (1:300 + 5% NGS in TBS, Vector, AI-4001), was then applied for 8 min. Goat anti rabbit horseradish peroxidase Leica Bond Polymer was added for 8 min. Antibody complexes were visualized using Leica Bond Mixed Refine (DAB, 3,3′-diaminobenzidine) detection 2× for 10 min at room temperature (RT). For Olig2, staining was performed using rabbit polyclonal anti-Olig2 (Olig2; Millipore, Cat No. AB9610) on formalin-fixed paraffin-embedded sections. Antigen retrieval was performed by placing slides in Citrate for 20 min at 100 °C. The primary antibody, Olig2 (1:500) or Rabbit IgG (1:1000) in Leica Primary antibody diluent, was applied for 30 min at room temperature.
4.9. Quantitative IHC
Image analysis was performed using whole slide digital images and automated image analysis. Slides were scanned in bright field with a 20× objective using a Nanozoomer Digital Pathology slide scanner (Hamamatsu; Bridgewater, NJ, USA) and the digital images were imported into Visiopharm software (Hoersholm, Denmark) for quantitative analysis. Prior to analysis, regions of interest (ROIs) were manually traced on each image for each stain. The ROIs were traced following a standardized protocol on both the left and right hemisphere and included the thalamus, subcortical white matter (SWM), dorsal cortex, hippocampus, and the corpus callosum (CC). The software then converted the initial digital imaging into gray scale values using two features, RGB-R with a mean filter of 5 pixels by 5 pixels and an RGB-B feature. Visiopharm was then trained to label positive staining and the background tissue counter stain using a stain-specific configuration based on threshold pixel values. Images were processed in batch mode using this configuration to generate positively stained to unstained tissue ratios. The research staff were blinded to the treatment groups during all assessments.
Using GFAP-stained slides, the thickness of white matter in the sub-sulcal regions of the SWM was measured. The images were imported into NDP.view2 (Hamamatsu Photonics, Bridgewater, NJ, USA) and measured using the annotation tool. White matter thickness was assessed in 4 ROIs: the CC and three white matter regions inferomedial to the lateral sulci, suprasylvian sulci, and pseudosylvian sulci.
MBP-stained slices were also used to measure the area of SWM in the control, Veh, and TH groups. The images were exported from NDP.view2 at 0.63× after adjusting the image settings to sharpen and lower gamma correction (0.5), which darkened and clarified the white matter tracts. The images were then imported into ImageJ, where the freehand selection tool was used to trace three subcortical ROIs-lateral, suprasylvian, and pseudosylvian-named for the corresponding sulcus perpendicular to each band of white matter (
Supplemental Figure S8).
4.10. Cortical Neuropathology Scoring
The H&E-stained slides were evaluated by a board-certified veterinary pathologist (JMS), who was blinded to treatment group and scored for cortical lesion and mineralization on a scale of 0 to 4. Cortical lesions were scored based on modifications of previous scoring systems used in rat models of hypoxic-ischemic brain injury [
51,
52] as follows: 0 = no detectable lesion; 1 = small focal or multifocal (<3) area of neuronal necrosis/loss and/or rarefaction, comprising <10% of the cortex; 2 = regionally extensive or multifocal lesion with neuronal necrosis/loss and/or rarefaction/cavitation, affecting 10–33% of the cortex unilaterally or 5–25% bilaterally; 3 = multifocal to coalescing lesion affecting 33–66% of the cortex unilaterally or 26–50% bilaterally; and 4 = severe lesion with cavitation affecting >66% of the cortex unilaterally or >50% bilaterally. Mineralization, which has been previously described following neuronal degeneration in animal models of transient forebrain ischemia [
53], was scored as follows: 0 = no detectable lesion; 1 = up to two small regions of mineralization; 2 = multifocal mineralization, unilateral or bilateral (3–10 foci); 3 = regionally extensive and multifocal to coalescing mineralization, bilateral, >10 foci; and 4 = severe bilateral multifocal to coalescing mineralization with thalamic involvement to any extent. Mineralization was characterized histologically by basophilic punctate, granular material on H&E and confirmed in an affected animal with von Kossa staining.
4.11. Statistical Analysis
In order to directly compare the Ur and Epo/TH cohorts but account for inter-cohort variability, data from behavioral testing and brain measurements were adjusted relative to the median control animal value of that sex for that cohort. For cortical neuropathology scores, animals that died were included and given a score of one increment greater than the worst possible score, to allow for assessment of combined outcomes. Data across multiple groups were compared using a Kruskal–Wallace test with Dunn’s post hoc test for pairwise tests adjusted for multiple comparisons. Data are presented as median with interquartile range (IQR). Adjusted p-values < 0.05 were considered statistically significant. Statistical analyses were performed in Prism (GraphPad Software, San Diego, CA, USA) and R Version 3.6.3 (Vienna, Austria).