9-O-Terpenyl-Substituted Berberrubine Derivatives Suppress Tumor Migration and Increase Anti-Human Non-Small-Cell Lung Cancer Activity
Abstract
1. Introduction
2. Results
2.1. NSCLC Cell Growth Inhibition Induced by Berberrubine and Its Derivatives
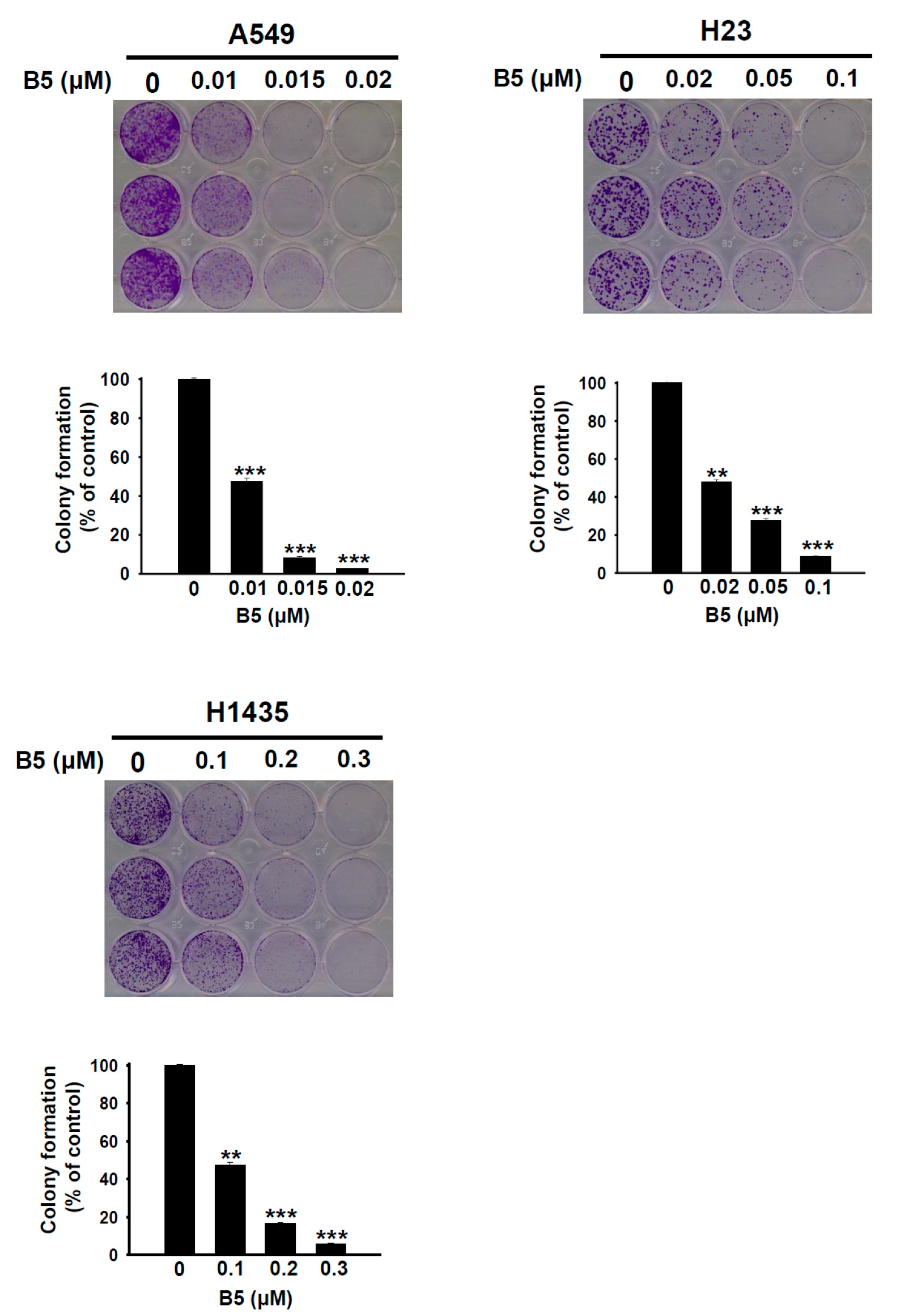
2.2. B5-Induced In Vitro Tumorigenesis Suppression in Human NSCLC Cells
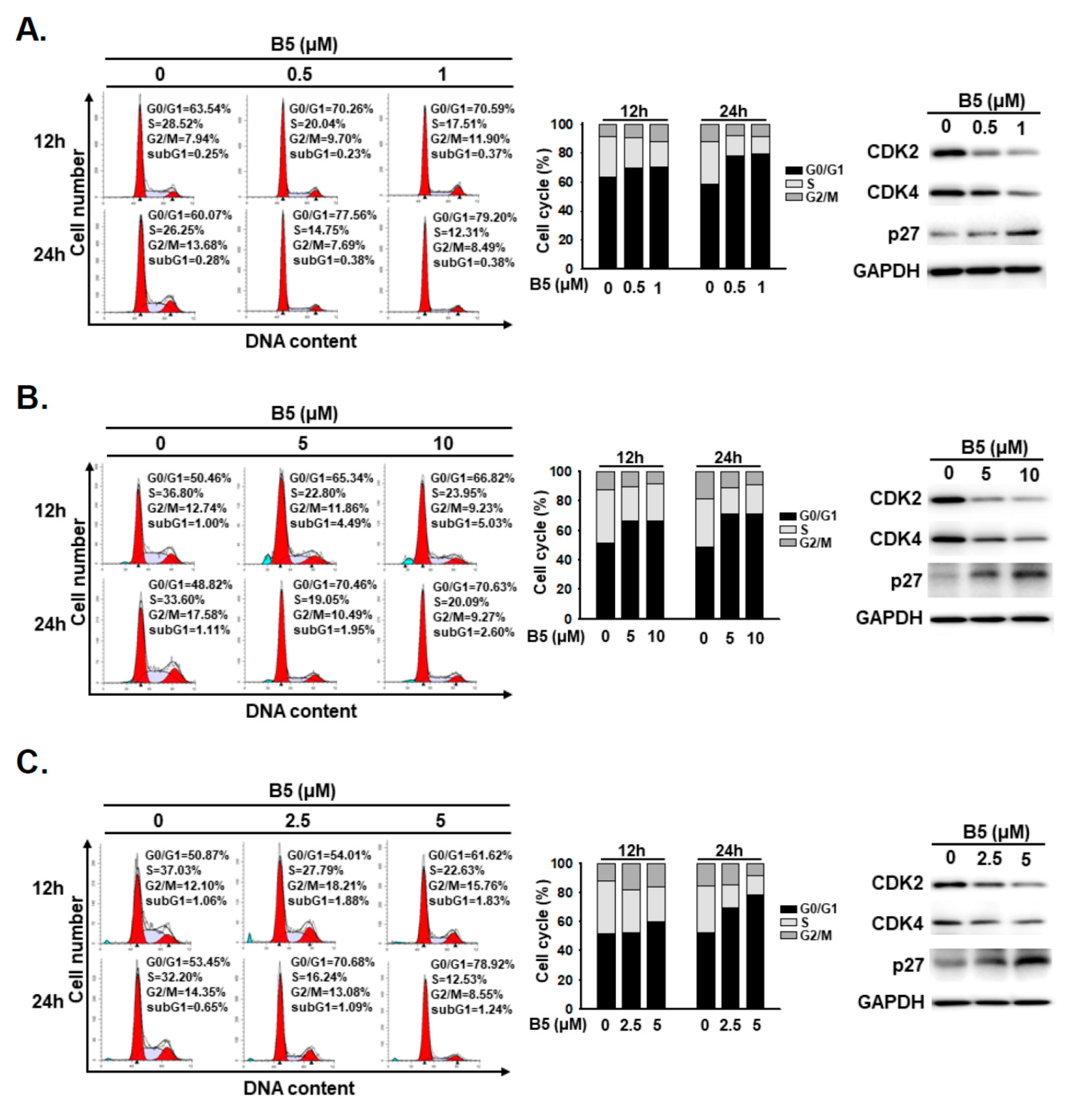
2.3. B5-Induced Cell-Cycle Regulation in Human NSCLC Cells
2.4. Partial Apoptosis Induction in B5-Treated Human NSCLC Cells
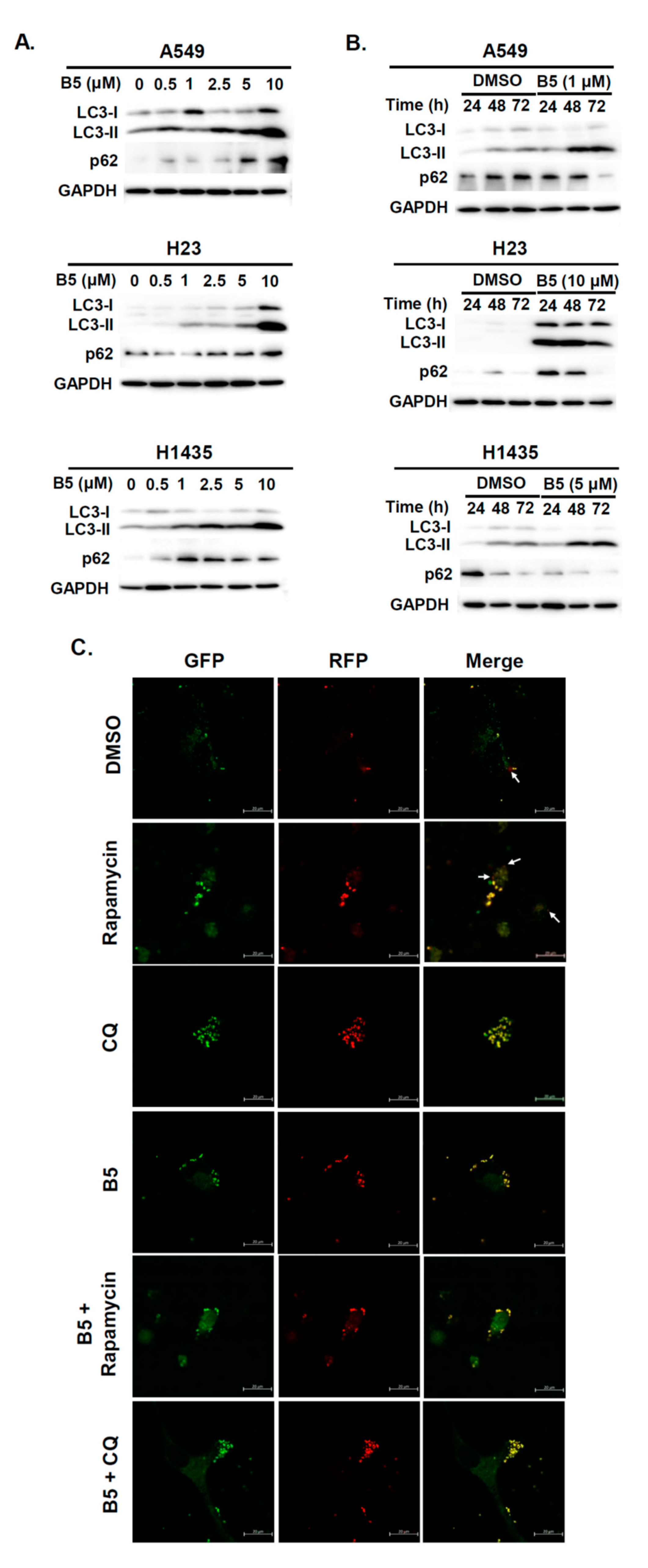
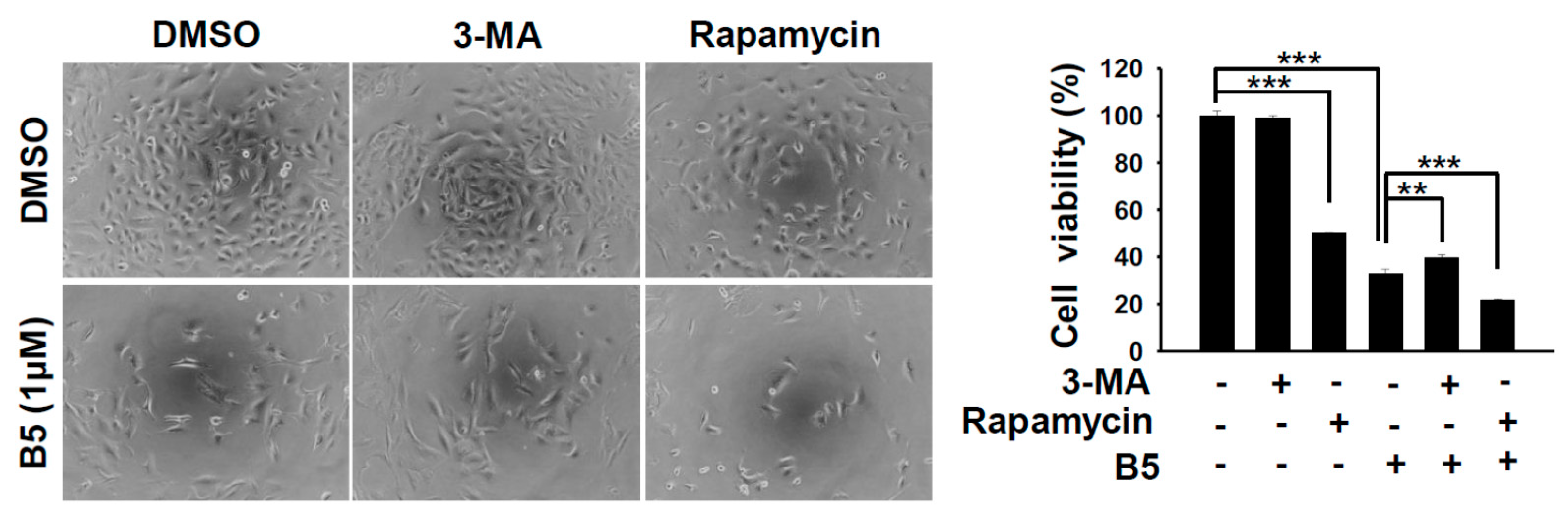
2.5. Autophagy Modulation in B5-Treated NSCLC Cells
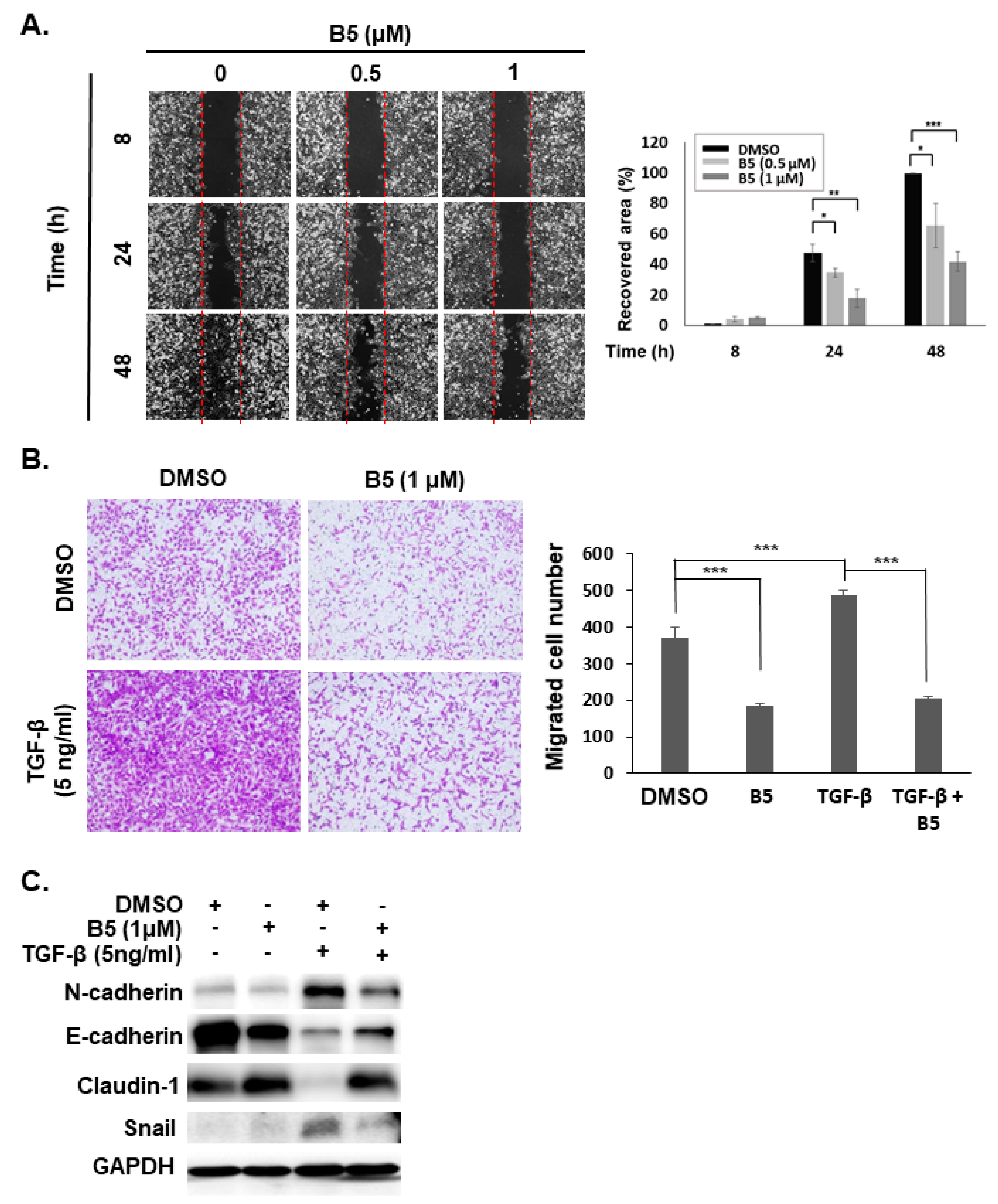
2.6. B5-Induced Tumor Migration Suppression in Human NSCLC Cells
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Cell Lines and Cell Cultures
4.3. Cellular Viability Assay
4.4. Western Blot Analysis
4.5. Cell Cycle Analysis
4.6. Colony Formation Assay
4.7. Autophagic Flux Observation
4.8. Migration Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Pourbagheri-Sigaroodi, A.; Kaveh, V.; Abolghasemi, H.; Ghaffari, S.H.; Momeny, M.; Bashash, D. Recent advances in immune checkpoint therapy in non-small cell lung cancer and opportunities for nanoparticle-based therapy. Eur. J. Pharmacol. 2021, 909, 174404. [Google Scholar] [CrossRef]
- Russo, A.; Cardona, A.F.; Caglevic, C.; Manca, P.; Ruiz-Patino, A.; Arrieta, O.; Rolfo, C. Overcoming TKI resistance in fusion-driven NSCLC: New generation inhibitors and rationale for combination strategies. Transl. Lung Cancer Res. 2020, 9, 2581–2598. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kwon, Y.; Kim, J.H.; Muller, M.T.; Chung, I.K. Induction of topoisomerase II-mediated DNA cleavage by a protoberberine alkaloid, berberrubine. Biochemistry 1998, 37, 16316–16324. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, F.; Song, Y.; Liu, H. The status of and trends in the pharmacology of berberine: A bibliometric review [1985–2018]. Chin. Med. 2020, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.Y.; Li, B.; Zhu, W.L.; Shi, J.Y.; Jia, Q.; Li, Y.M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017, 38, 157–167. [Google Scholar] [CrossRef]
- Lin, H.J.; Ho, J.H.; Tsai, L.C.; Yang, F.Y.; Yang, L.L.; Kuo, C.D.; Chen, L.G.; Liu, Y.W.; Wu, J.Y. Synthesis and In Vitro Photocytotoxicity of 9-/13-Lipophilic Substituted Berberine Derivatives as Potential Anticancer Agents. Molecules 2020, 25, 677. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Kam, K.H.; Chao, W.Y.; Zhao, P.W.; Chen, S.H.; Chung, H.C.; Li, Y.Z.; Wu, J.Y.; Lee, Y.R. Berberine Derivatives Suppress Cellular Proliferation and Tumorigenesis In Vitro in Human Non-Small-Cell Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4218. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. p53 Cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol. Carcinog. 2009, 48, 24–37. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Jiang, S.; Liu, J.; Chen, X.; Zhang, S.; Zhao, H. Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non-small cell lung cancer via the suppression of the MMP2 and Bcl-2/Bax signaling pathways. Oncol. Lett. 2018, 15, 7409–7414. [Google Scholar] [CrossRef]
- Liu, D.; Meng, X.; Wu, D.; Qiu, Z.; Luo, H. A Natural Isoquinoline Alkaloid With Antitumor Activity: Studies of the Biological Activities of Berberine. Front. Pharmacol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Buchanan, B.; Meng, Q.; Poulin, M.M.; Zuccolo, J.; Azike, C.G.; Gabriele, J.; Baranowski, D.C. Comparative pharmacokinetics and safety assessment of transdermal berberine and dihydroberberine. PLoS ONE 2018, 13, e0194979. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Chang, H.C. Determination of berberine in plasma, urine and bile by high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 1995, 665, 117–123. [Google Scholar] [CrossRef]
- Lo, C.Y.; Hsu, L.C.; Chen, M.S.; Lin, Y.J.; Chen, L.G.; Kuo, C.D.; Wu, J.Y. Synthesis and anticancer activity of a novel series of 9-O-substituted berberine derivatives: A lipophilic substitute role. Bioorg. Med. Chem. Lett. 2013, 23, 305–309. [Google Scholar] [CrossRef]
- Huang, K.J.; Kuo, C.H.; Chen, S.H.; Lin, C.Y.; Lee, Y.R. Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J. Cell Mol. Med. 2018, 22, 1894–1908. [Google Scholar] [CrossRef]
- Peng, P.L.; Hsieh, Y.S.; Wang, C.J.; Hsu, J.L.; Chou, F.P. Inhibitory effect of berberine on the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Toxicol. Appl. Pharmacol. 2006, 214, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nau, M.M.; Chiba, I.; Birrer, M.J.; Rosenberg, R.K.; Vinocour, M.; Levitt, M.; Pass, H.; Gazdar, A.F.; Minna, J.D. p53: A frequent target for genetic abnormalities in lung cancer. Science 1989, 246, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Horio, Y.; Takahashi, T.; Kuroishi, T.; Hibi, K.; Suyama, M.; Niimi, T.; Shimokata, K.; Yamakawa, K.; Nakamura, Y.; Ueda, R.; et al. Prognostic significance of p53 mutations and 3p deletions in primary resected non-small cell lung cancer. Cancer Res. 1993, 53, 1–4. [Google Scholar]
- Chou, C.W.; Lin, C.H.; Hsiao, T.H.; Lo, C.C.; Hsieh, C.Y.; Huang, C.C.; Sher, Y.P. Therapeutic effects of statins against lung adenocarcinoma via p53 mutant-mediated apoptosis. Sci. Rep. 2019, 9, 20403. [Google Scholar] [CrossRef]
- Horio, Y.; Hasegawa, Y.; Sekido, Y.; Takahashi, M.; Roth, J.A.; Shimokata, K. Synergistic effects of adenovirus expressing wild-type p53 on chemosensitivity of non-small cell lung cancer cells. Cancer Gene Ther. 2000, 7, 537–544. [Google Scholar] [CrossRef][Green Version]
- Leung, E.L.H.; Luo, L.X.; Liu, Z.Q.; Wong, V.K.W.; Lu, L.L.; Xie, Y.; Zhang, N.; Qu, Y.Q.; Fan, X.X.; Li, Y.; et al. Inhibition of KRAS-dependent lung cancer cell growth by deltarasin: Blockage of autophagy increases its cytotoxicity. Cell Death Dis. 2018, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Lin, C.F.; Lin, Y.S.; Su, W.C.; Chiu, W.H. Autophagy regulates vinorelbine sensitivity due to continued Keap1-mediated ROS generation in lung adenocarcinoma cells. Cell Death Discov. 2018, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Abukhdeir, A.M.; Vitolo, M.I.; Argani, P.; De Marzo, A.M.; Karakas, B.; Konishi, H.; Gustin, J.P.; Lauring, J.; Garay, J.P.; Pendleton, C.; et al. Tamoxifen-stimulated growth of breast cancer due to p21 loss. Proc. Natl. Acad. Sci. USA 2008, 105, 288–293. [Google Scholar] [CrossRef]
- Piyanuch, R.; Sukhthankar, M.; Wandee, G.; Baek, S.J. Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett. 2007, 258, 230–240. [Google Scholar] [CrossRef]
- Devarajan, N.; Jayaraman, S.; Mahendra, J.; Venkatratnam, P.; Rajagopal, P.; Palaniappan, H.; Ganesan, S.K. Berberine-A potent chemosensitizer and chemoprotector to conventional cancer therapies. Phytother. Res. 2021, 35, 3059–3077. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Zhang, N.; Yin, M.; Dong, J.; Zeng, Q.; Mao, G.; Song, D.; Liu, L.; Deng, H. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm. Sin. B 2020, 10, 2299–2312. [Google Scholar] [CrossRef]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef]
- Kimura, T.; Takabatake, Y.; Takahashi, A.; Isaka, Y. Chloroquine in cancer therapy: A double-edged sword of autophagy. Cancer Res. 2013, 73, 3–7. [Google Scholar] [CrossRef]
- Chen, Z.F.; Li, Y.B.; Han, J.Y.; Wang, J.; Yin, J.J.; Li, J.B.; Tian, H. The double-edged effect of autophagy in pancreatic beta cells and diabetes. Autophagy 2011, 7, 12–16. [Google Scholar] [CrossRef]
- Yu, H.I.; Shen, H.C.; Chen, S.H.; Lim, Y.P.; Chuang, H.H.; Tai, T.S.; Kung, F.P.; Lu, C.H.; Hou, C.Y.; Lee, Y.R. Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment. Int. J. Mol. Sci. 2019, 20, 5315. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Li, Y.; Yang, L.; Ma, Y.; Peng, X.; Yang, S.; Liu, J.; Li, H. Autophagy: A novel mechanism of chemoresistance in cancers. Biomed. Pharmacother. 2019, 119, 109415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. Berberine Reverses Doxorubicin Resistance by Inhibiting Autophagy Through the PTEN/Akt/mTOR Signaling Pathway in Breast Cancer. Onco Targets Ther. 2020, 13, 1909–1919. [Google Scholar] [CrossRef]
- Lin, Y.; Sheng, M.; Ding, Y.; Zhang, N.; Song, Y.; Du, H.; Lu, N.; Yu, W. Berberine protects renal tubular cells against hypoxia/reoxygenation injury via the Sirt1/p53 pathway. J. Nat. Med. 2018, 72, 715–723. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, J.F.; Song, J.; Bai, Y.; Deng, L.; Feng, C.P.; Xu, X.Y.; Guo, H.X.; Wang, Y.; Gao, X.; et al. Effects of Berberine on Diabetes and Cognitive Impairment in an Animal Model: The Mechanisms of Action. Am. J. Chin. Med. 2021, 49, 1399–1415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, H.; Gao, Q.; Xu, J.; Zhu, Y.; Zhai, M.; Zhang, P.; Shen, N.; Di, Y.; Wang, J.; et al. Berberine Attenuates Cerebral Ischemia-Reperfusion Injury Induced Neuronal Apoptosis by Down-Regulating the CNPY2 Signaling Pathway. Front. Pharmacol. 2021, 12, 609693. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, L.; Chen, Q.; Zhou, L. Exploring the Mechanism of Berberine Intervention in Ulcerative Colitis from the Perspective of Inflammation and Immunity Based on Systemic Pharmacology. Evid. Based Complement. Alternat. Med. 2021, 2021, 9970240. [Google Scholar] [CrossRef] [PubMed]
- Warowicka, A.; Nawrot, R.; Gozdzicka-Jozefiak, A. Antiviral activity of berberine. Arch. Virol. 2020, 165, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ye, C.; Chen, Y.; Chen, Y.; Diao, S.; Huang, M. Berberine Improves Behavioral and Cognitive Deficits in a Mouse Model of Alzheimer’s Disease via Regulation of beta-Amyloid Production and Endoplasmic Reticulum Stress. ACS Chem. Neurosci. 2021, 12, 1894–1904. [Google Scholar] [CrossRef]
- Lee, M.Y.; Li, Y.Z.; Huang, K.J.; Huang, H.C.; Lin, C.Y.; Lee, Y.R. Indirubin-3′-oxime suppresses human cholangiocarcinoma through cell-cycle arrest and apoptosis. Eur. J. Pharmacol. 2018, 839, 57–65. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chou, H.C.; Law, C.H.; Chang, W.T.; Wen, T.N.; Liao, E.C.; Lin, M.W.; Lin, L.H.; Wei, Y.S.; Tsai, Y.T.; et al. Progesterone receptor membrane component 1 is involved in oral cancer cell metastasis. J. Cell Mol. Med. 2020, 24, 9737–9751. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Lin, M.W.; Hsu, Y.W.; Chou, H.C.; Lin, L.H.; Law, C.H.; Lin, Y.C.; Hu, R.Y.; Kuo, W.H.; Ko, M.L.; et al. Biomarker discovery in highly invasive lung cancer cell through proteomics approaches. Cell Biochem. Funct. 2021, 39, 367–379. [Google Scholar] [CrossRef] [PubMed]

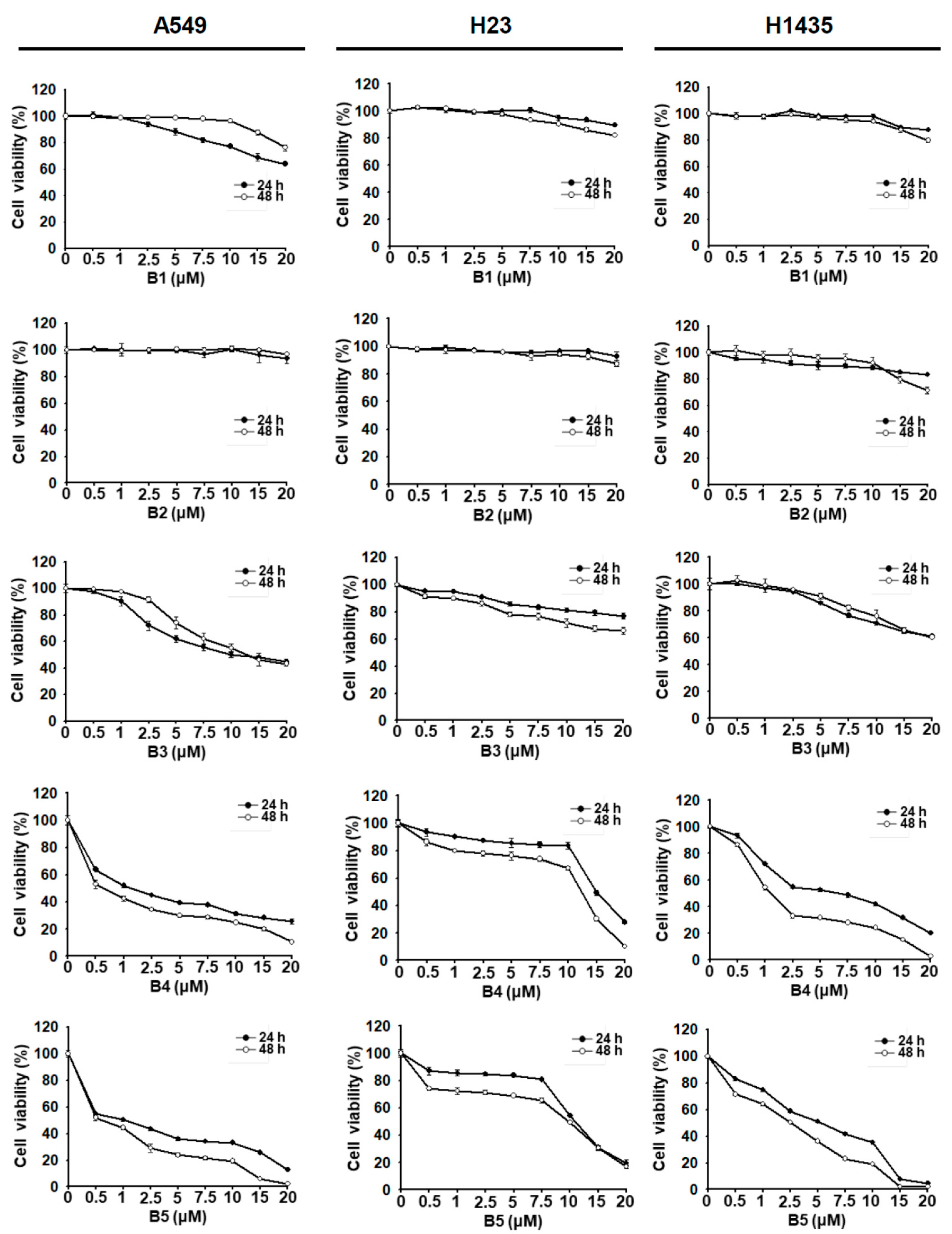

| Cell | Time (h) | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | B5 | ||
| A549 | 24 h | ND | ND | 10.0 ± 2.1 | 1.4 ± 0.7 | 1.0 ± 0.4 |
| 48 h | ND | ND | 12.9 ± 2.8 | 0.6 ± 1.0 | 0.6 ± 0.2 | |
| H23 | 24 h | ND | ND | ND | 14.9 ± 1.1 | 10.9 ± 1.7 |
| 48 h | ND | ND | ND | 12.4 ± 0.8 | 9.9 ± 0.1 | |
| H1435 | 24 h | ND | ND | ND | 6.7 ± 0.6 | 5.1 ± 1.0 |
| 48 h | ND | ND | ND | 1.4 ± 0.5 | 2.6 ± 0.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.-M.; Wu, J.-Y.; Chen, S.-H.; Chao, W.-Y.; Chuang, H.-H.; Kam, K.-H.; Zhao, P.-W.; Li, Y.-Z.; Yen, Y.-P.; Lee, Y.-R. 9-O-Terpenyl-Substituted Berberrubine Derivatives Suppress Tumor Migration and Increase Anti-Human Non-Small-Cell Lung Cancer Activity. Int. J. Mol. Sci. 2021, 22, 9864. https://doi.org/10.3390/ijms22189864
Chang J-M, Wu J-Y, Chen S-H, Chao W-Y, Chuang H-H, Kam K-H, Zhao P-W, Li Y-Z, Yen Y-P, Lee Y-R. 9-O-Terpenyl-Substituted Berberrubine Derivatives Suppress Tumor Migration and Increase Anti-Human Non-Small-Cell Lung Cancer Activity. International Journal of Molecular Sciences. 2021; 22(18):9864. https://doi.org/10.3390/ijms22189864
Chicago/Turabian StyleChang, Jia-Ming, Jin-Yi Wu, Shu-Hsin Chen, Wen-Ying Chao, Hsiang-Hao Chuang, Kam-Hong Kam, Pei-Wen Zhao, Yi-Zhen Li, Yu-Pei Yen, and Ying-Ray Lee. 2021. "9-O-Terpenyl-Substituted Berberrubine Derivatives Suppress Tumor Migration and Increase Anti-Human Non-Small-Cell Lung Cancer Activity" International Journal of Molecular Sciences 22, no. 18: 9864. https://doi.org/10.3390/ijms22189864
APA StyleChang, J.-M., Wu, J.-Y., Chen, S.-H., Chao, W.-Y., Chuang, H.-H., Kam, K.-H., Zhao, P.-W., Li, Y.-Z., Yen, Y.-P., & Lee, Y.-R. (2021). 9-O-Terpenyl-Substituted Berberrubine Derivatives Suppress Tumor Migration and Increase Anti-Human Non-Small-Cell Lung Cancer Activity. International Journal of Molecular Sciences, 22(18), 9864. https://doi.org/10.3390/ijms22189864






