Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation
Abstract
:1. Introduction
2. Results
2.1. Papain Decreased Fat Accumulation, Body Weight and Serum Lipid Content in HFD-Induced Obese Mice
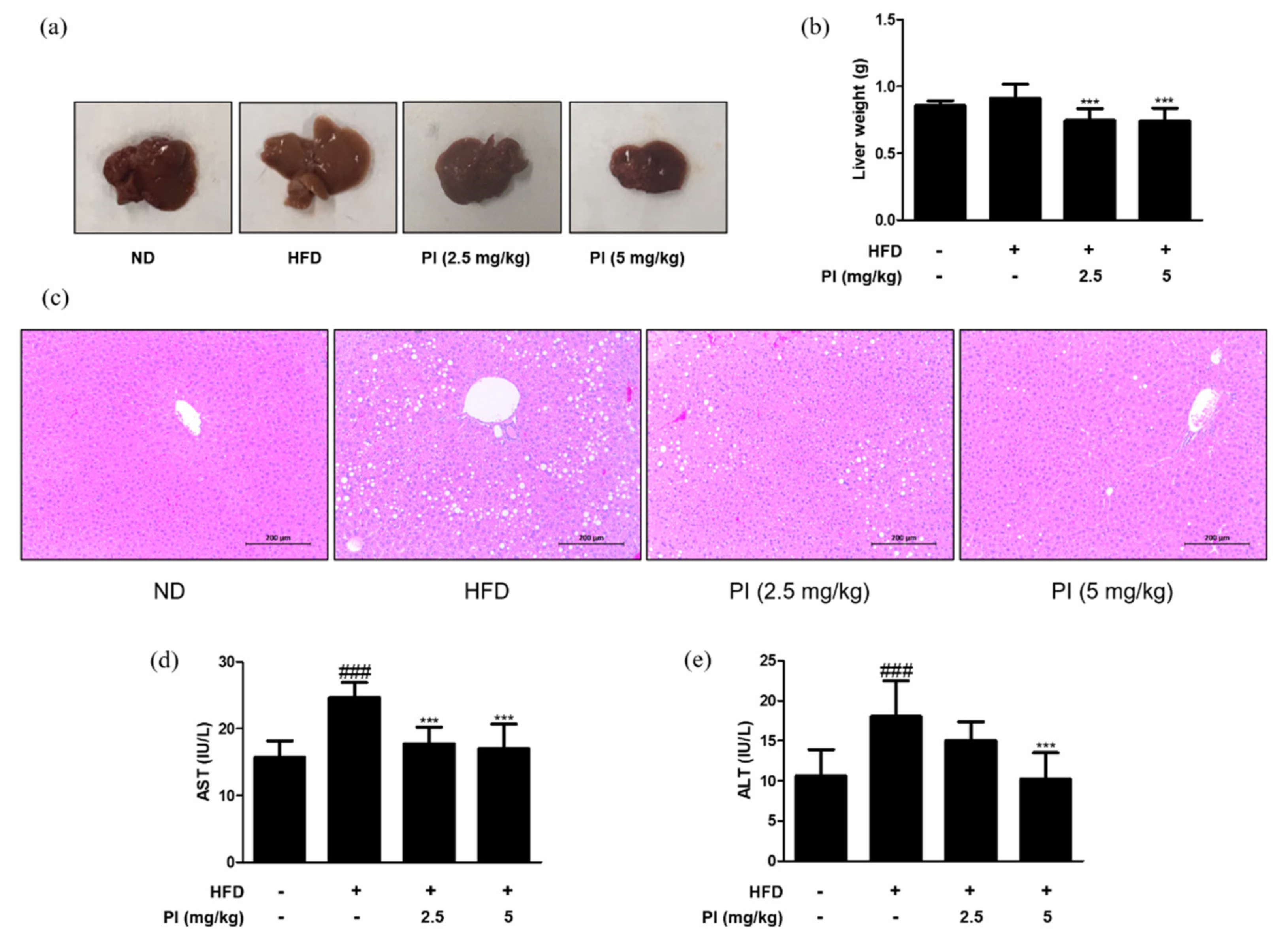
2.2. Papain Decreased the Liver Weight and Liver Lipid Accumulation in HFD-Induced Obese Mice
2.3. Papain Regulated the Expression of Adipogenic and Inflammatory Markers in Liver of HFD-Induced Obese Mice
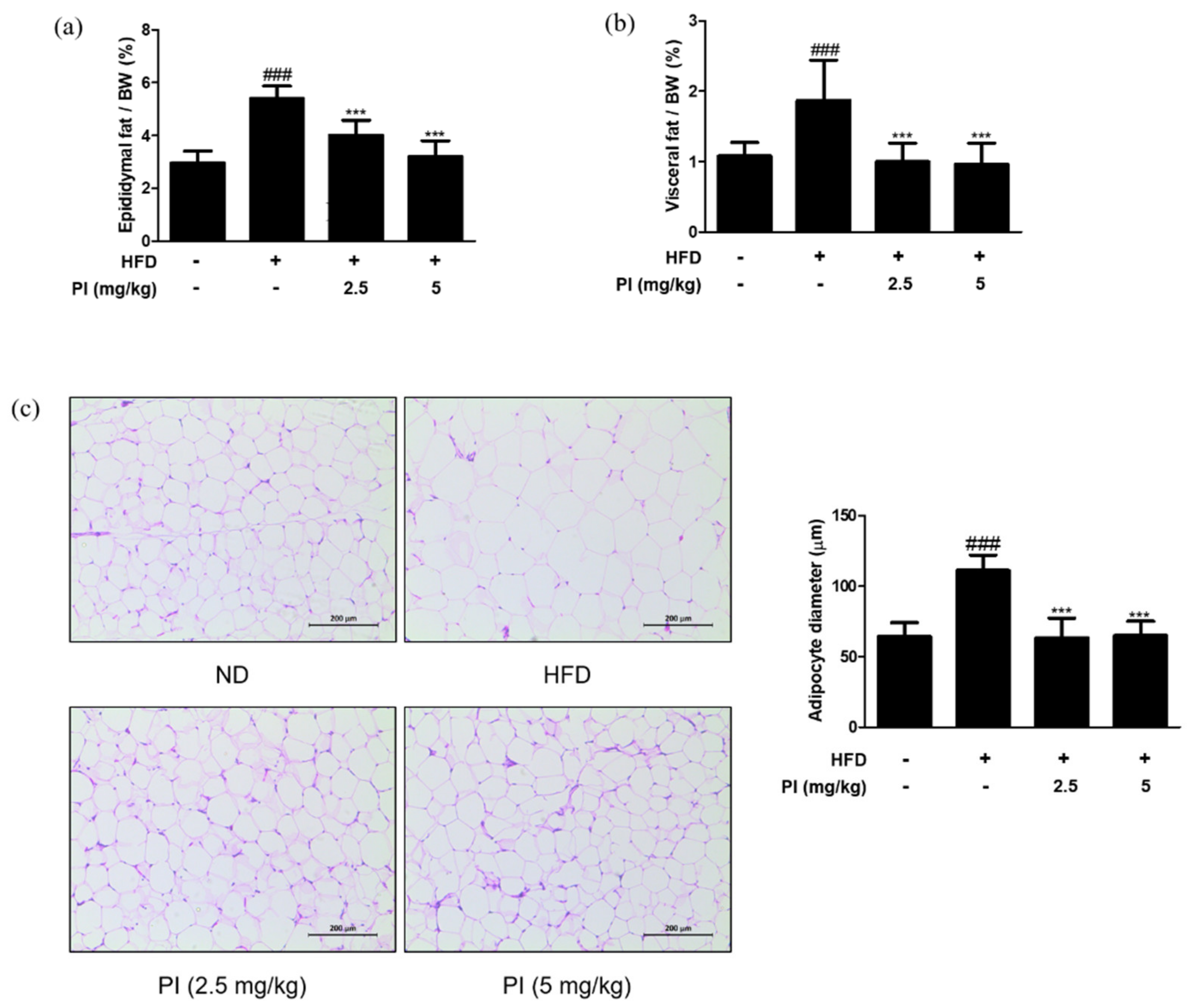
2.4. Papain Reduced the Adipose Fat Weight and Inhibited the Adipocyte Hypertrophy in HFD-Induced Obese Mice
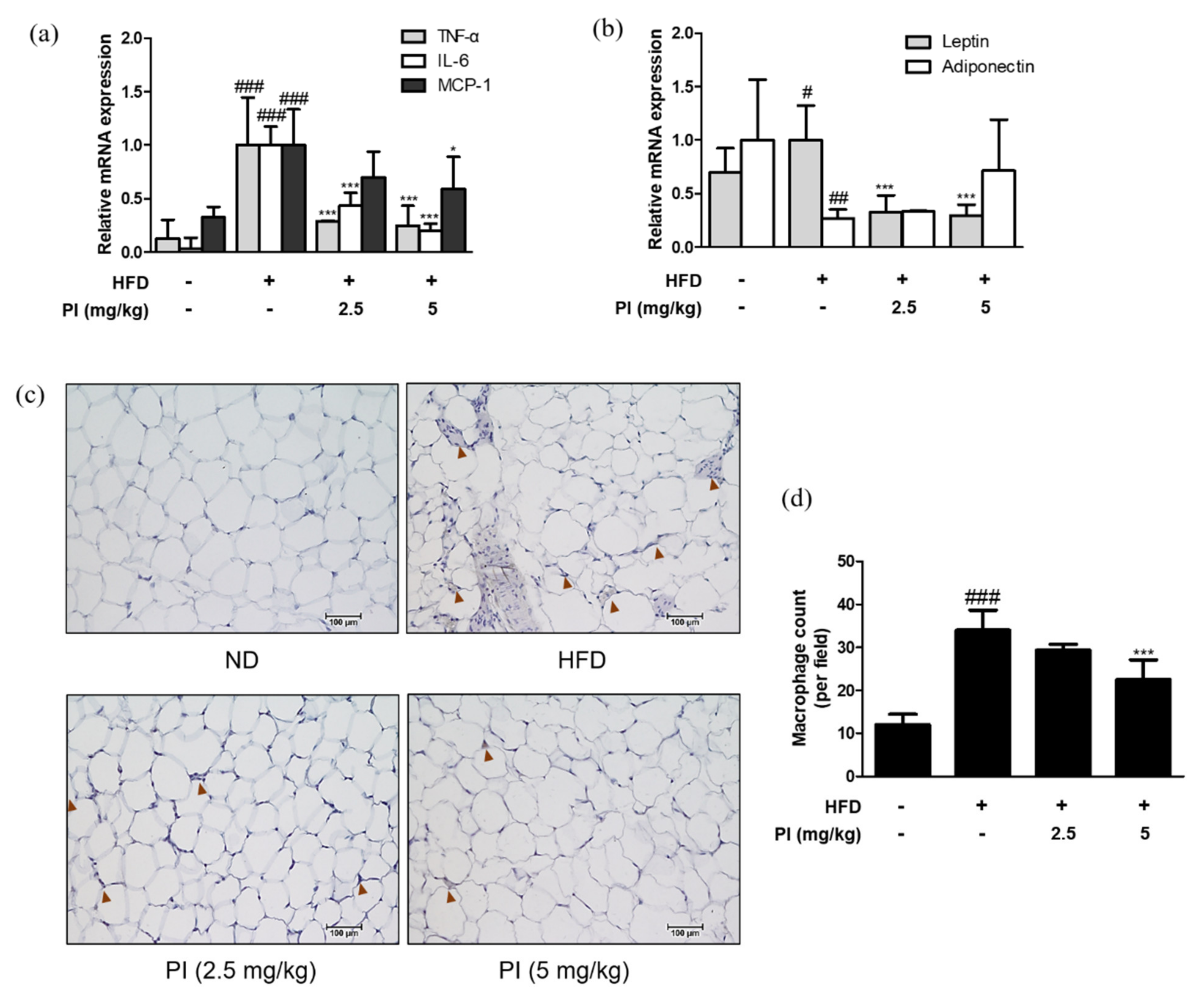
2.5. Papain Regulated Adipokines and Macrophage Infiltrations in Adipose Tissue of HFD-Induced Obese Mice
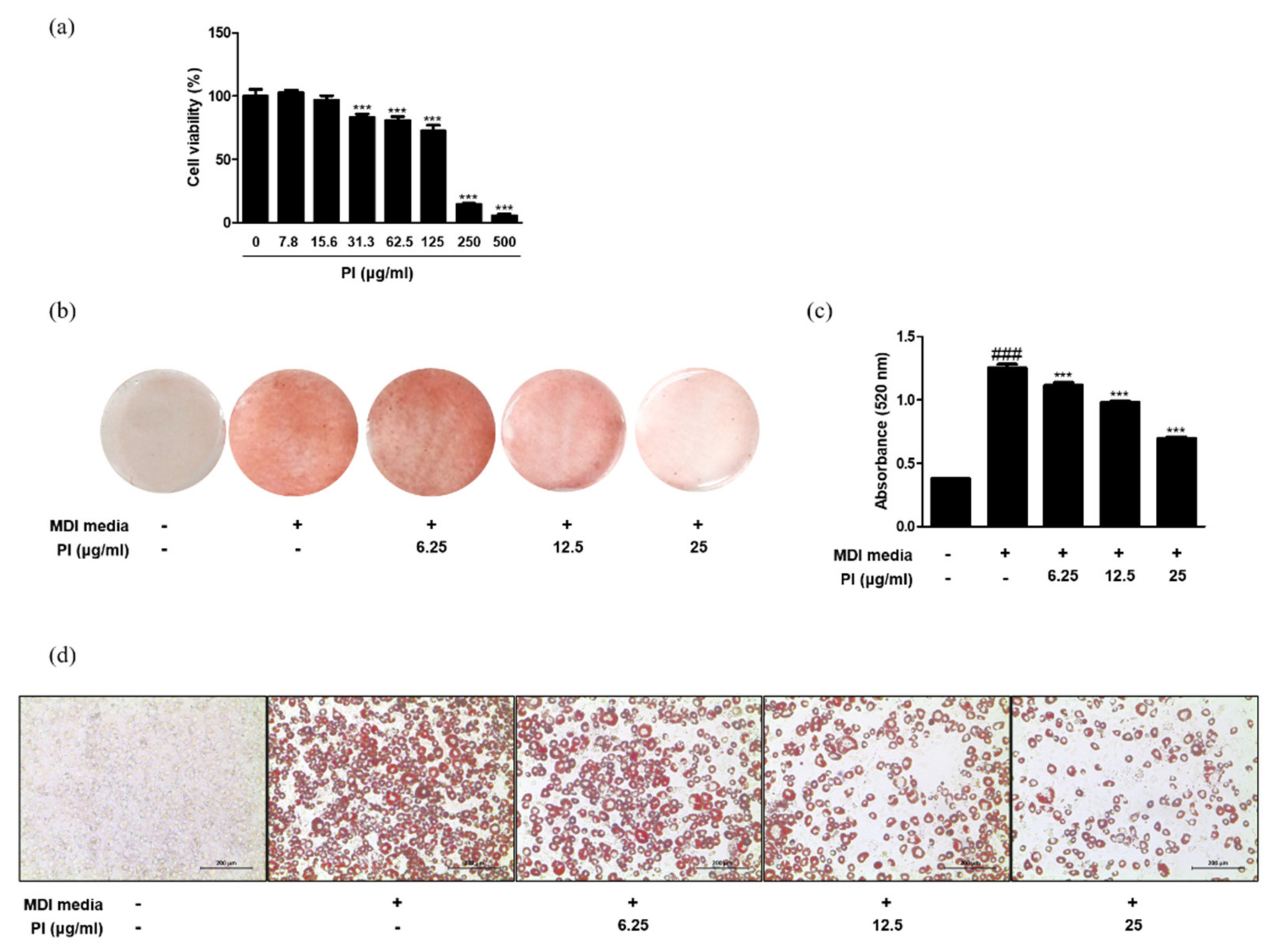
2.6. Papain Decreased Adipocyte Differentiation in 3T3-L1 Preadipocytes and Primary Preadipocytes
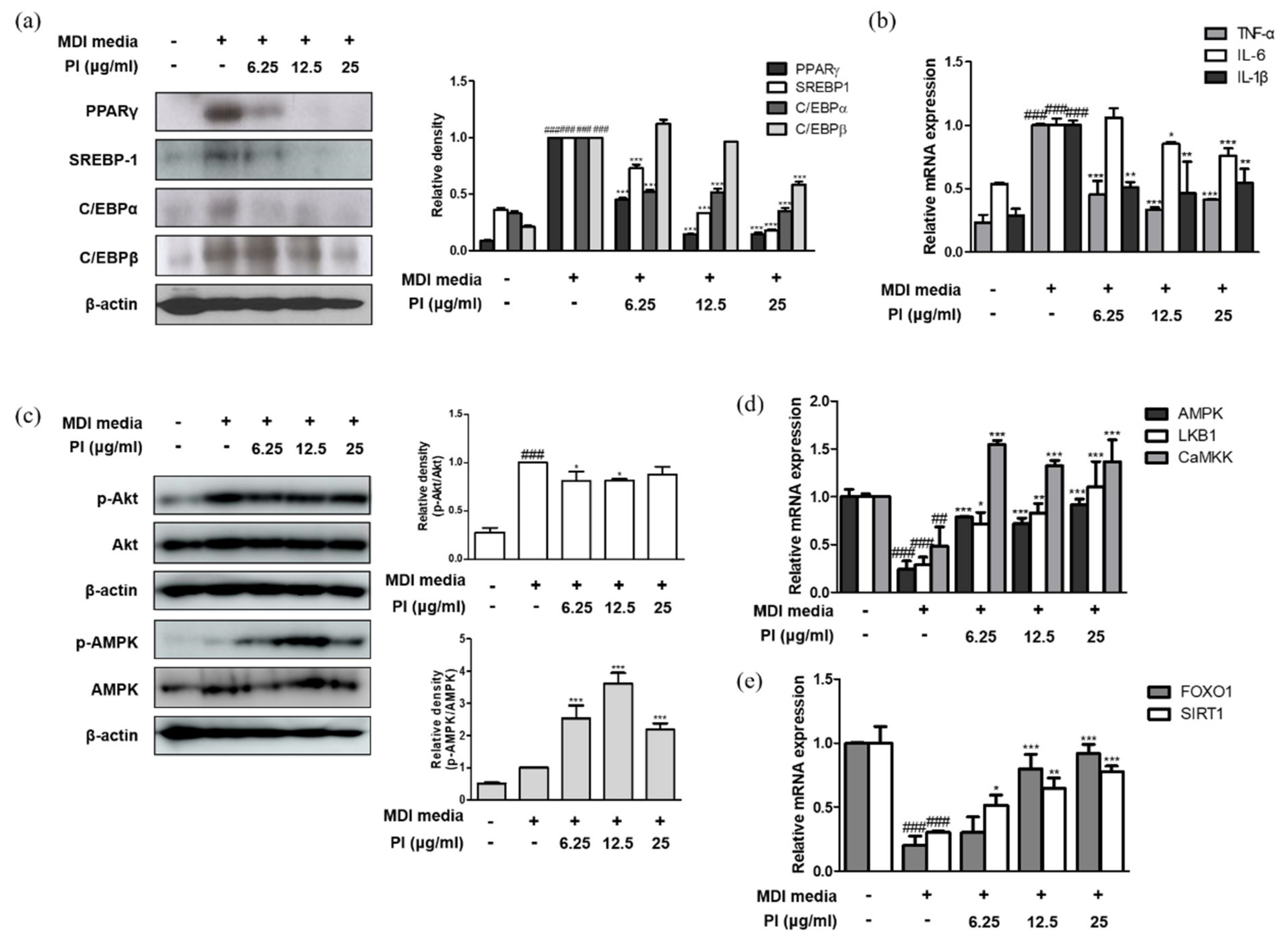
2.7. Papain Inhibited the Expression of Adipogenic Markers in MDI-Treated 3T3-L1 Preadipocytes
2.8. Papain Regulated the Expression of AMPK/SIRT1-FoxO1 in MDI-Treated 3T3-L1 Preadipocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Experiments
4.3. Histological Analysis
4.4. Analysis of Serum Lipid Profile
4.5. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
4.6. Western Blot Analysis
4.7. Immunohistochemical (IHC) Analysis
4.8. 3T3-L1 Preadipocytes Culture and Cell Viability
4.9. Rat Primary Preadipocytes Isolation
4.10. Adipocyte Differentiation and Oil Red O Staining
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef] [PubMed]
- De Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef] [Green Version]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Hwang, K.A.; Hwang, Y.J.; Im, P.R.; Hwang, H.J.; Song, J.; Kim, Y.J. Platycodon grandiflorum Extract Reduces High-Fat Diet-Induced Obesity Through Regulation of Adipogenesis and Lipogenesis Pathways in Mice. J. Med. Food 2019, 22, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.L.; Wang, T.Y.; Li, M.; Shang, Y.X. Alleviating airway inflammation by inhibiting ERK-NF-kappaB signaling pathway by blocking Kv1.3 channels. Int. Immunopharmacol. 2018, 63, 110–118. [Google Scholar] [CrossRef]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Ezekiel Amri, F.M. Papain, a Plant enzyme of biological importance: A review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar]
- Mota Vde, S.; Turrini, R.N.; Poveda, V.d.B. Antimicrobial activity of Eucalyptus globulus oil, xylitol and papain: A pilot study. Rev. Esc. Enferm. USP 2015, 49, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Dos Anjos, M.M.; da Silva, A.A.; de Pascoli, I.C.; Mikcha, J.M.; Machinski, M., Jr.; Peralta, R.M.; de Abreu Filho, B.A. Antibacterial activity of papain and bromelain on Alicyclobacillus spp. Int. J. Food Microbiol. 2016, 216, 121–126. [Google Scholar] [CrossRef]
- Fei, X.M.; Zhou, Y.L.; Qi, J.W.; Wu, J.G.; Qiu, L.N.; Wang, Z.N. In vitro inhibitory effects of papain on platelet aggregation. Chin. J. Clin. Pharmacol. Ther. 2009, 14, 906–911. [Google Scholar]
- Jiang, L.; Fei, X.M.; Miao, C.L.; Yuan, W.F.; Qiu, L.N. Effects of papain on monocyte-platelet aggregates formation and monocyte activation induced by monocyte-platelet aggregates. Chin. J. Clin. Pharmacol. Ther. 2015, 20, 289–292. [Google Scholar]
- Moraes, D.; Levenhagen, M.A.; Costa-Cruz, J.M.; Costa, A.P.N.; Rodrigues, R.M. In vitro efficacy of latex and purified papain from Carica papaya against Strongyloides venezuelensis eggs and larvae. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, X.; Yuan, W.; Zhao, Y.; Wang, H.; Bai, S.; Huang, Q. Papain Ameliorates the MPAs Formation-Mediated Activation of Monocytes by Inhibiting Cox-2 Expression via Regulating the MAPKs and PI3K/Akt Signal Pathway. BioMed Res. Int. 2018, 2018, 3632084. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, A.; Storck, A. The Prevention of Peritoneal Adhesions by Papain: A Clinical Study. Ann. Surg. 1936, 104, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Athesh, K.; Karthiga, D.; Brindha, P. Anti-obesity effect of aqueous fruit extract of Carica papaya L. in rats fed on high fat cafeteria diet. Int. J. Pharm. Pharm. Sci. 2012, 4, 327–330. [Google Scholar]
- Maniyar, Y.; Bhixavatimath, P. Antihyperglycemic and hypolipidemic activities of aqueous extract of Carica papaya Linn. leaves in alloxan-induced diabetic rats. J. Ayurveda Integr. Med. 2012, 3, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Santana, L.F.; Inada, A.C.; Espirito Santo, B.; Filiu, W.F.O.; Pott, A.; Alves, F.M.; Guimaraes, R.C.A.; Freitas, K.C.; Hiane, P.A. Nutraceutical Potential of Carica papaya in Metabolic Syndrome. Nutrients 2019, 11, 1608. [Google Scholar] [CrossRef] [Green Version]
- Adedapo, A.; Orherhe, V. Anti-nociceptive and anti-inflammatory studies of the aqueous leaf extract of Carica papaya in laboratory animals. Asian J. Exp. Biol. Sci. 2013, 4, 89–96. [Google Scholar]
- Amazu, L.U.; Azikiwe, C.C.A.; Njoku, C.J.; Osuala, F.N.; Nwosu, P.J.; Ajugwo, A.O.; Enye, J.C. Anti-inflammatory activity of the methanolic extract of the seeds of Carica papaya in experimental animals. Asian Pac. J. Trop. Med. 2010, 3, 884–886. [Google Scholar] [CrossRef] [Green Version]
- Stevens, L.E. A reassessment of papain in preventing peritoneal adhesions. Am. J. Surg. 1968, 115, 535–539. [Google Scholar] [CrossRef]
- Kellerman Slotemaker, J.P. Preventive with papain of formation of peritoneal adhesions. Ned. Tijdschr. Voor Verloskd. Gynaecol. 1956, 56, 462–469. [Google Scholar]
- Parra, O.M.; Saad, W.A.; Ferri, S.; Peduto, L.; Ferraz-Neto, J.B.; Dal Colletto, G.M. Dose-dependence of the carboxymethylcellulose-papain combination in the prevention of peritoneal adhesions. Study in rats. Arq. Gastroenterol. 1991, 28, 99–102. [Google Scholar]
- Seo, M.; Goo, T.W.; Chung, M.Y.; Baek, M.; Hwang, J.S.; Kim, M.A.; Yun, E.Y. Tenebrio molitor Larvae Inhibit Adipogenesis through AMPK and MAPKs Signaling in 3T3-L1 Adipocytes and Obesity in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2017, 18, 518. [Google Scholar] [CrossRef] [Green Version]
- Chusyd, D.E.; Wang, D.; Huffman, D.M.; Nagy, T.R. Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front. Nutr. 2016, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zheng, L.D.; Zou, P.; Brooke, J.; Smith, C.; Long, Y.C.; Almeida, F.A.; Liu, D.; Cheng, Z. FoxO1 antagonist suppresses autophagy and lipid droplet growth in adipocytes. Cell Cycle 2016, 15, 2033–2041. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Han, J.; Lee, H.J. Anti-Adipogenic Effect of Neferine in 3T3-L1 Cells and Primary White Adipocytes. Nutrients 2020, 12, 1858. [Google Scholar] [CrossRef]
- Canbay, A.; Bechmann, L.; Gerken, G. Lipid metabolism in the liver. Z. Gastroenterol. 2007, 45, 35–41. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.F.; Jiang, S.; Wu, J.; Liu, J.; Yuan, X.W.; Shen, D.; Zhang, J.Z.; Zhou, N.; He, J.; et al. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat. Commun. 2020, 11, 719. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, S.; Manabe, I.; Nagai, R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov. Med. 2009, 8, 55–60. [Google Scholar]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.E.; Kim, J.M.; Joung, K.H.; Lee, J.H.; You, B.R.; Choi, M.J.; Ryu, M.J.; Ko, Y.B.; Lee, M.A.; Lee, J.; et al. The Roles of Adipokines, Proinflammatory Cytokines, and Adipose Tissue Macrophages in Obesity-Associated Insulin Resistance in Modest Obesity and Early Metabolic Dysfunction. PLoS ONE 2016, 11, e0154003. [Google Scholar] [CrossRef] [Green Version]
- Bruun, J.M.; Lihn, A.S.; Verdich, C.; Pedersen, S.B.; Toubro, S.; Astrup, A.; Richelsen, B. Regulation of adiponectin by adipose tissue-derived cytokines: In vivo and in vitro investigations in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E527–E533. [Google Scholar] [CrossRef] [Green Version]
- Gormand, A.; Henriksson, E.; Strom, K.; Jensen, T.E.; Sakamoto, K.; Goransson, O. Regulation of AMP-activated protein kinase by LKB1 and CaMKK in adipocytes. J. Cell. Biochem. 2011, 112, 1364–1375. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Zachariah Tom, R.; Garcia-Roves, P.M.; Sjogren, R.J.; Jiang, L.Q.; Holmstrom, M.H.; Deshmukh, A.S.; Vieira, E.; Chibalin, A.V.; Bjornholm, M.; Zierath, J.R. Effects of AMPK activation on insulin sensitivity and metabolism in leptin-deficient ob/ob mice. Diabetes 2014, 63, 1560–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habegger, K.M.; Hoffman, N.J.; Ridenour, C.M.; Brozinick, J.T.; Elmendorf, J.S. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology 2012, 153, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, F.; Nanetti, L.; Montecchiani, G.; Borroni, F.; Salvolini, E.; Faloia, E.; Ferretti, G.; Mazzanti, L.; Vignini, A. In vitro effects of fermented papaya (Carica papaya, L.) on platelets obtained from patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. NMCD 2015, 25, 224–229. [Google Scholar] [CrossRef]
- Yang, Z.; Kahn, B.B.; Shi, H.; Xue, B.Z. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010, 285, 19051–19059. [Google Scholar] [CrossRef] [Green Version]
- Velagapudi, R.; El-Bakoush, A.; Lepiarz, I.; Ogunrinade, F.; Olajide, O.A. AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol. Cell. Biochem. 2017, 435, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Stepek, G.; Behnke, J.M.; Buttle, D.J.; Duce, I.R. Natural plant cysteine proteinases as anthelmintics? Trends Parasitol. 2004, 20, 322–327. [Google Scholar] [CrossRef]
- Huet, J.; Looze, Y.; Bartik, K.; Raussens, V.; Wintjens, R.; Boussard, P. Structural characterization of the papaya cysteine proteinases at low pH. Biochem. Biophys. Res. Commun. 2006, 341, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Rotondo, F.; Romero, M.D.; Ho-Palma, A.C.; Remesar, X.; Fernandez-Lopez, J.A.; Alemany, M. Quantitative analysis of rat adipose tissue cell recovery, and non-fat cell volume, in primary cell cultures. PeerJ 2016, 4, e2725. [Google Scholar] [CrossRef] [Green Version]


| Gene | Sequence (Forward) | Sequence (Reverse) |
|---|---|---|
| SREBP-1 | CATCGCAAACAAGCTGACCT | AGATCCAGGTTTGAGGTGGG |
| PPARγ | ATCGAGTGCCGAGTCTGTGG | GCAAGGCACTTCTGAAACCG |
| C/EBPα | TCGGTGCGTCTAAGATGAGG | TCAAGGCACATTTTTGCTCC |
| TNF-α | ATGAGCACAGAAAGCATGAT | TACAGGCTTGTCACTCGAAT |
| IL-1β | CCCCAAAAGATGAAGGGCTG | CTGGAAGGTCCACGGGAAAG |
| IL-6 | TTCCATCCAGTTGCCTTCTTG | GGGAGTGGTATCCTCTGTGAAGTC |
| MCP-1 | AGGTCCCTGTCATGCTTCTG | TCTGGACCCATTCCTTCTTG |
| leptin | CTCCAAGGTTGTCCAGGGTT | AAAACTCCCCACAGAATGGG |
| adiponectin | CTGGAGGTGGGAGACCAAGT | TGGGCTATGGGTAGTTGCAG |
| FOXO1 | CCGGAGTTTAACCAGTCCAA | TGCTCATAAAGTCGGTGCTG |
| SIRT1 | TGCCATCATGAAGCCAGAGA | AACATCGCAGTCTCCAAGGA |
| AMPK | GGTGGATTCCCAAAAGTGCT | AAGCAGTGCTGGGTCACAAG |
| LKB1 | AAGGGGACGAGGACAAAGAG | GTACTTGCCGATGAGCTTGG |
| CaMKK | CCCAGTTGGTCCTCTCTGTT | AGAGATCTGAGTACGCCAGC |
| GAPDH | CCCACTCTTCCACCTTCGAT | CCACCACCCTGTTGCTGTAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.-M.; Kang, H.-A.; Cominguez, D.C.; Kim, S.-H.; An, H.-J. Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation. Int. J. Mol. Sci. 2021, 22, 9885. https://doi.org/10.3390/ijms22189885
Kang Y-M, Kang H-A, Cominguez DC, Kim S-H, An H-J. Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation. International Journal of Molecular Sciences. 2021; 22(18):9885. https://doi.org/10.3390/ijms22189885
Chicago/Turabian StyleKang, Yun-Mi, Hyun-Ae Kang, Divina C. Cominguez, Su-Hyun Kim, and Hyo-Jin An. 2021. "Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation" International Journal of Molecular Sciences 22, no. 18: 9885. https://doi.org/10.3390/ijms22189885







