Deletion of Col15a1 Modulates the Tumour Extracellular Matrix and Leads to Increased Tumour Growth in the MMTV-PyMT Mouse Mammary Carcinoma Model
Abstract
:1. Introduction
2. Results
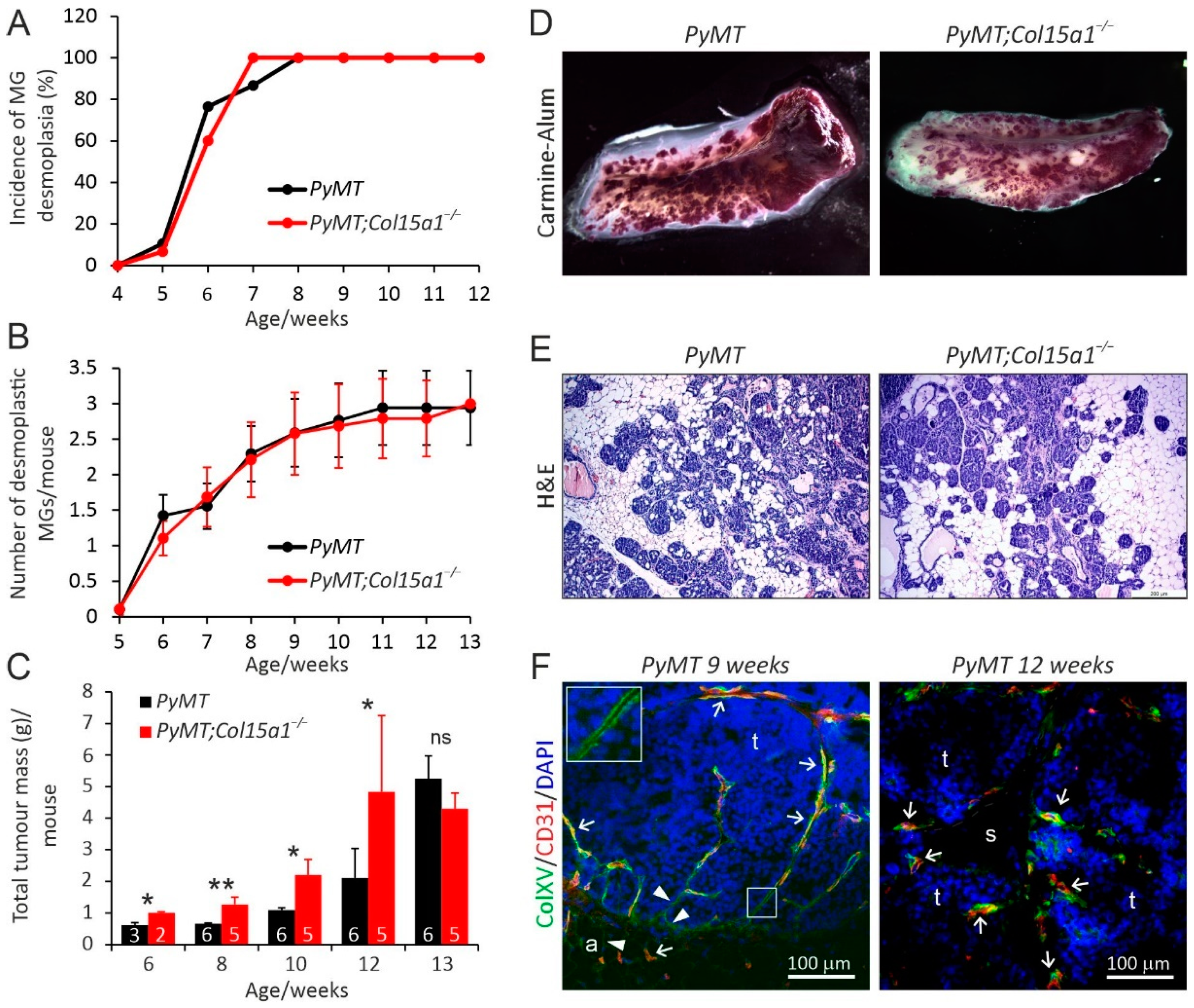
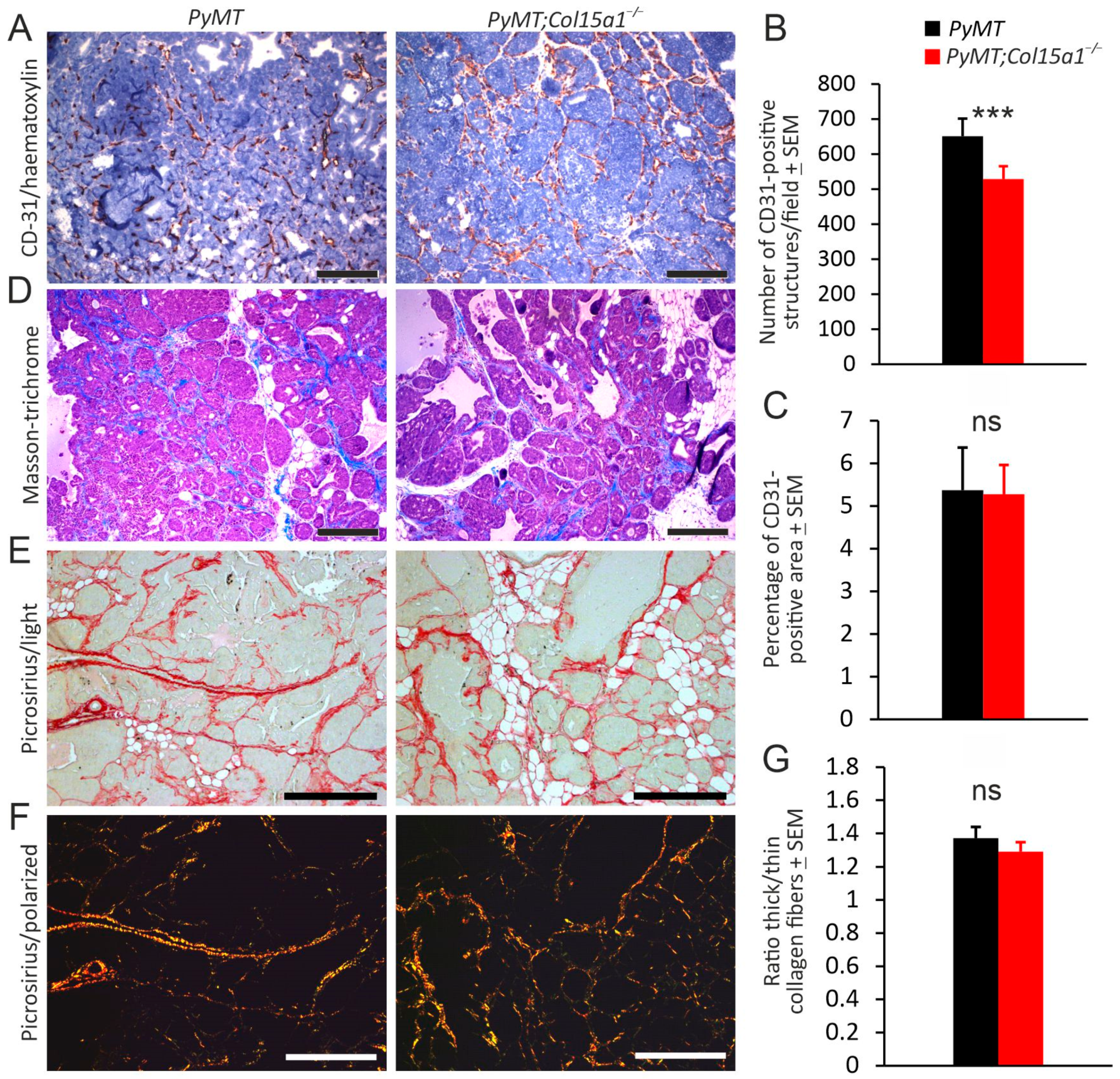
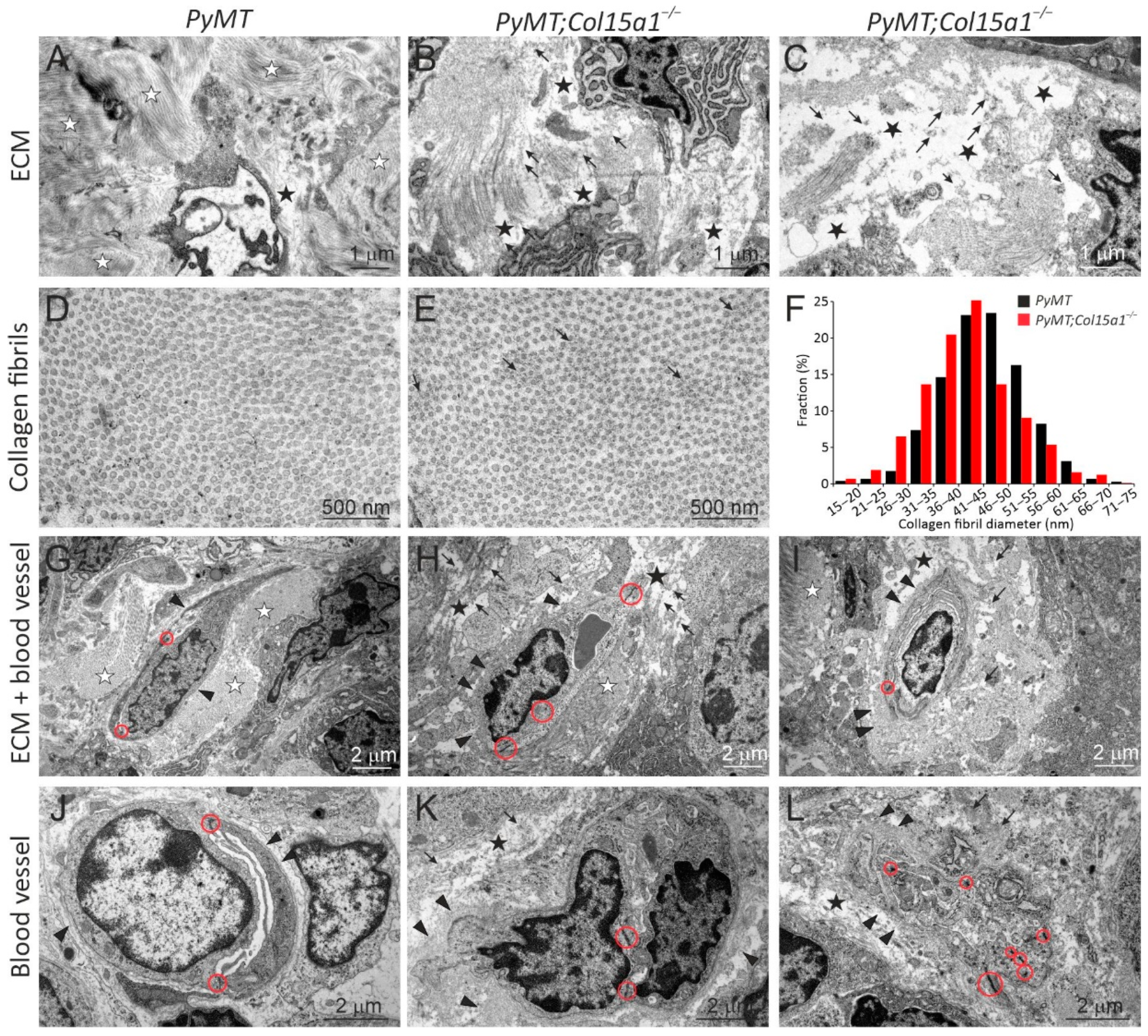
2.1. ColXV in Mouse Mammary Carcinogenesis
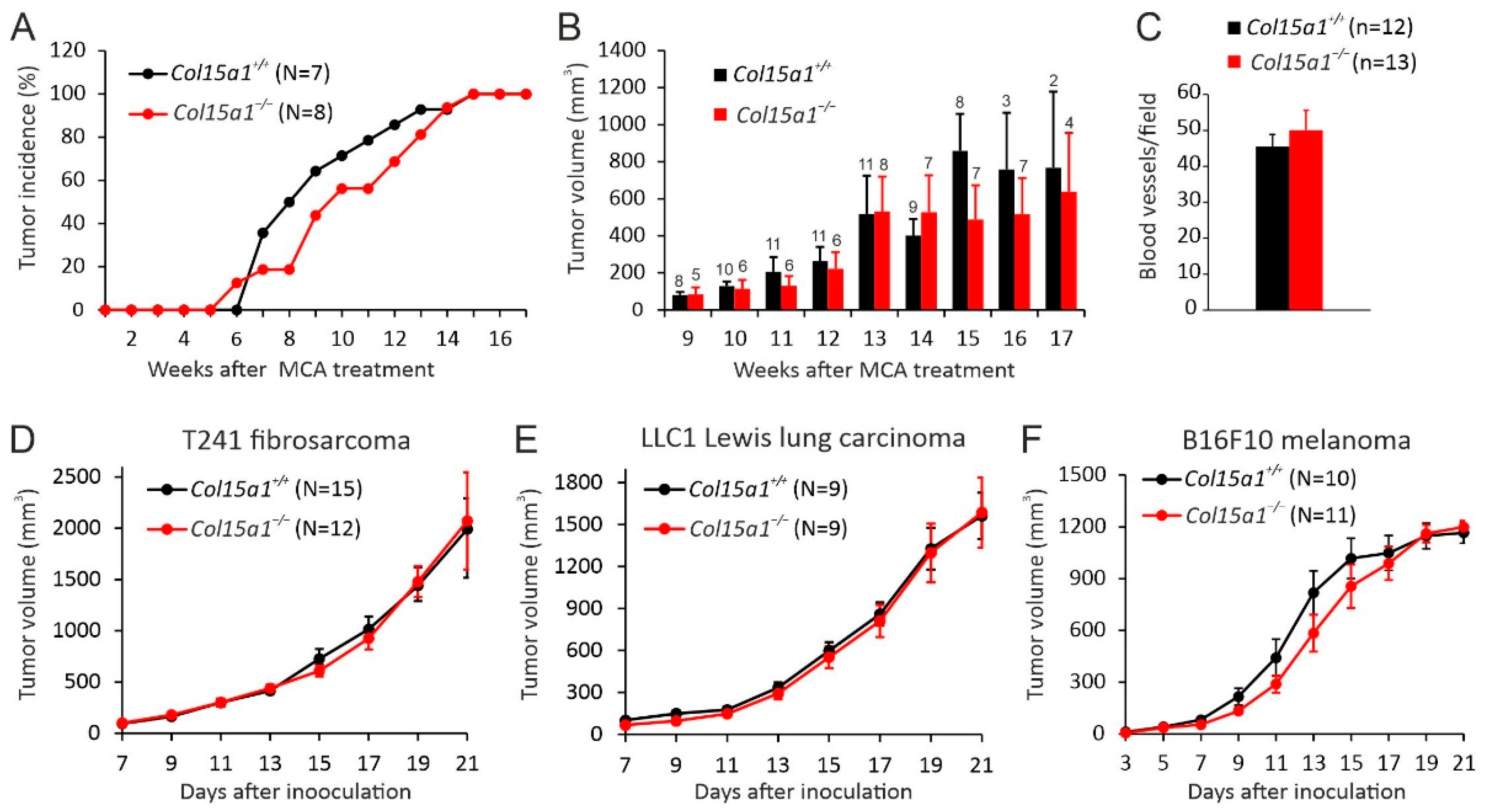
2.2. Other In Vivo Tumour Models
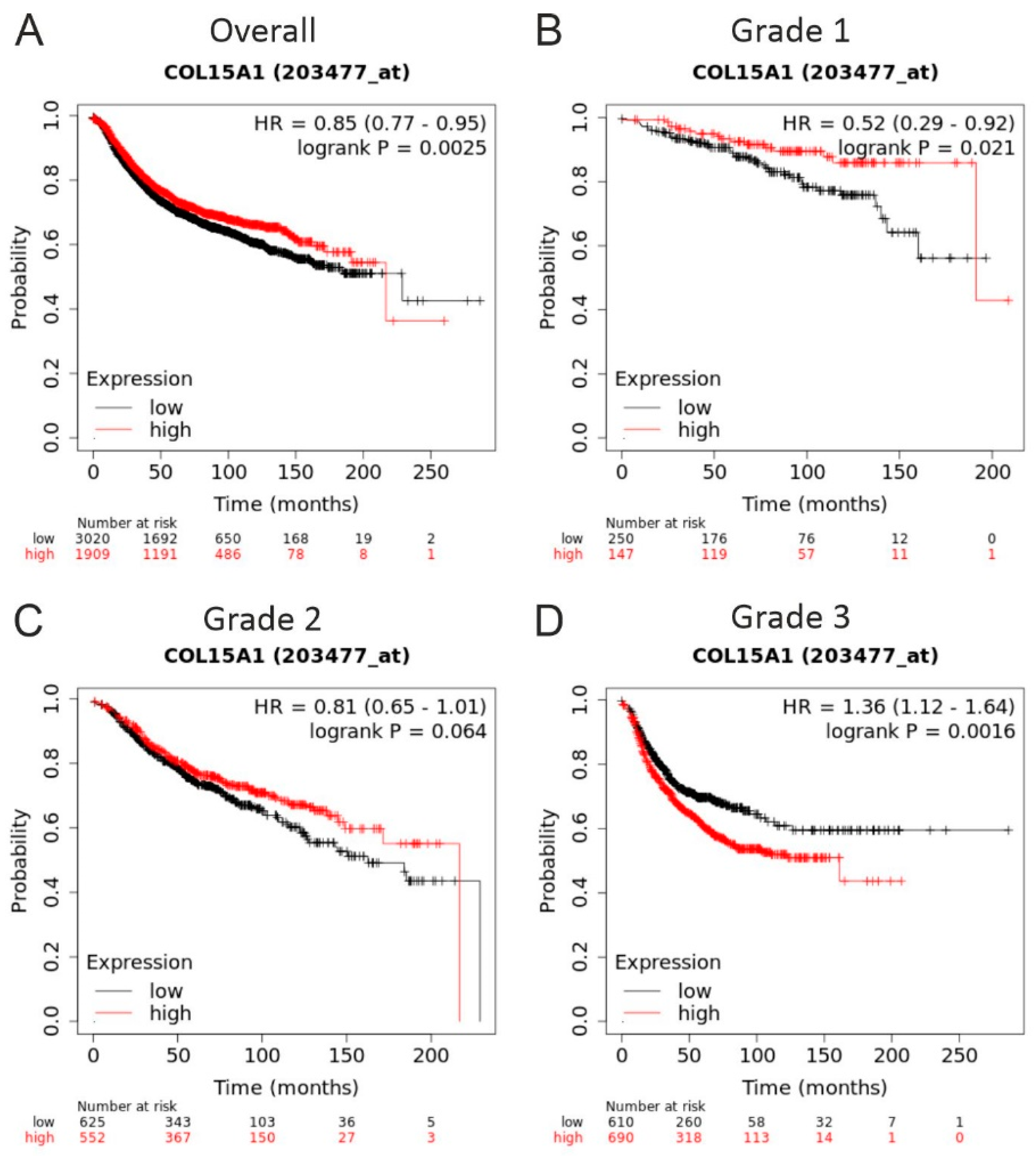
2.3. COL15A1 Expression-Based Survival Analysis for Breast Cancer Patients
3. Discussion
4. Materials and Methods
4.1. Mice and Licences
4.2. C57BL/6 Mouse Tumour Cell Lines
4.3. MMTV-PyMT Mammary Carcinogenesis Model
4.4. MCA-Induced Fibrosarcoma
4.5. Syngeneic Models
4.6. Tissue Analyses
4.6.1. Carmine Alum Staining on Whole Mounts
4.6.2. Haematoxylin-Eosin Staining
4.6.3. CD-31 Immunohistochemistry
4.6.4. Analysis of Fibrillar Collagen Content
4.6.5. Immunofluorescence Staining
4.6.6. Morphometric Analyses
4.6.7. Transmission Electron Microscopy
4.7. Kaplan-Meier Survival Analysis
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Myllyharju, J. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Ruggiero, F. The collagen superfamily: From the extracellular matrix to the cell membrane. Pathol. Biol. 2005, 53, 430–442. [Google Scholar] [CrossRef]
- Bretaud, S.; Guillon, E.; Karppinen, S.-M.; Pihlajaniemi, T.; Ruggiero, F. Collagen XV, a multifaceted multiplexin present across tissues and species. Matrix Biol. Plus 2020, 6–7, 100023. [Google Scholar] [CrossRef] [PubMed]
- Clementz, A.G.; Harris, A. Collagen XV: Exploring its structure and role within the tumor microenvironment. Mol. Cancer Res. 2013, 11, 1481–1486. [Google Scholar] [CrossRef] [Green Version]
- Heljasvaara, R.; Aikio, M.; Ruotsalainen, H.; Pihlajaniemi, T. Collagen XVIII in tissue homeostasis and dysregulation—Lessons learned from model organisms and human patients. Matrix Biol. 2017, 57–58, 55–75. [Google Scholar] [CrossRef] [Green Version]
- Izzi, V.; Heljasvaara, R.; Heikkinen, A.; Karppinen, S.-M.; Koivunen, J.; Pihlajaniemi, T. Exploring the roles of MACIT and multiplexin collagens in stem cells and cancer. Semin. Cancer Biol. 2019, 62, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Amenta, P.S.; Scivoletti, N.A.; Newman, M.D.; Sciancalepore, J.P.; Li, D.; Myers, J.C. Proteoglycan-collagen XV in human tissues is seen linking banded collagen fibers subjacent to the basement membrane. J. Histochem. Cytochem. 2005, 53, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Rasi, K.; Piuhola, J.; Czabanka, M.; Sormunen, R.; Ilves, M.; Leskinen, H.; Rysä, J.; Kerkelä, R.; Janmey, P.; Heljasvaara, R.; et al. Collagen XV is necessary for modeling of the extracellular matrix and its deficiency predisposes to cardiomyopathy. Circ. Res. 2010, 107, 1241–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eklund, L.; Piuhola, J.; Komulainen, J.; Sormunen, R.; Ongvarrasopone, C.; Fässler, R.; Muona, A.; Ilves, M.; Ruskoaho, H.; Takala, T.E.S.; et al. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 1194–1199. [Google Scholar] [CrossRef]
- Walia, A.; Yang, J.F.; Huang, Y.-H.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 2422–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenta, P.S.; Briggs, K.; Xu, K.; Gamboa, E.; Jukkola, A.F.; Li, D.; Myers, J.C. Type XV collagen in human colonic adenocarcinomas has a different distribution than other basement membrane zone proteins. Hum. Pathol. 2000, 31, 359–366. [Google Scholar] [CrossRef]
- Amenta, P.S.; Hadad, S.; Lee, M.T.; Barnard, N.; Li, D.; Myers, J.C. Loss of types XV and XIX collagen precedes basement membrane invasion in ductal carcinoma of the female breast. J. Pathol. 2003, 199, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Fukushige, T.; Kanekura, T.; Ohuchi, E.; Shinya, T.; Kanzaki, T. Immunohistochemical studies comparing the localization of type XV collagen in normal human skin and skin tumours with that of type IV collagen. J. Dermatol. 2005, 32, 74–83. [Google Scholar]
- Karppinen, S.-M.; Honkanen, H.-K.; Heljasvaara, R.; Riihilä, P.; Autio-Harmainen, H.; Sormunen, R.; Harjunen, V.; Väisänen, M.-R.; Väisänen, T.; Hurskainen, T.; et al. Collagens XV and XVIII show different expression and localisation in cutaneous squamous cell carcinoma: Type XV appears in tumor stroma, while XVIII becomes upregulated in tumor cells and lost from microvessels. Exp. Dermatol. 2016, 25, 348–354. [Google Scholar] [CrossRef]
- Kimura, K.; Nakayama, M.; Naito, I.; Komiyama, T.; Ichimura, K.; Asano, H.; Tsukuda, K.; Ohtsuka, A.; Oohashi, T.; Miyoshi, S.; et al. Human collagen XV is a prominent histopathological component of sinusoidal capillarization in hepatocellular carcinogenesis. Int. J. Clin. Oncol. 2015, 21, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Ramchandran, R.; Dhanabal, M.; Volkb, R.; Waterman, M.J.; Segala, M.; Lua, H.; Knebelmanna, B.; Sukhatme, V.P. Antiangiogenic activity of restin, NC10 domain of human collagen XV: Comparison to endostatin. Biochem. Biophys. Res. Commun. 1999, 255, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Larsson, H.; Tisi, D.; Claesson-Welsh, L.; Hohenester, E.; Timpl, R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J. Mol. Biol. 2000, 301, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xin, L.; Fan, Y.; Meng, H.-R.; Li, Z.-P.; Gan, R.-B. Mouse restin inhibits bovine aortic endothelial cell proliferation and causes cell apoptosis. Acta Biochim. Biophys. Sin. 2002, 34, 138–142. [Google Scholar] [PubMed]
- Harris, H. Is Collagen XV a tumor suppressor? DNA Cell Biol. 2003, 22, 225–226. [Google Scholar] [CrossRef]
- Jonasson, J.; Povey, S.; Harris, H. The analysis of malignancy by cell fusion. VII. Cytogenetic analysis of hybrids between malignant and diploid cells and of tumours derived from them. J. Cell Sci. 1977, 24, 217–254. [Google Scholar] [CrossRef]
- Harris, H. Suppression of malignancy in hybrid cells: The mechanism. J. Cell Sci. 1985, 79, 83–94. [Google Scholar] [CrossRef]
- Harris, A.; Harris, H.; Hollingsworth, M.A. Complete suppression of tumor formation by high levels of basement membrane collagen. Mol. Cancer Res. 2007, 5, 1241–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutolo, M.J.; Morris, K.J.; Leir, S.-H.; Caffrey, T.C.; Lewandowska, M.A.; Hollingsworth, M.A.; Harris, A. Tumor suppression by collagen XV is independent of the restin domain. Matrix Biol. 2012, 31, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Clementz, A.G.; Mutolo, M.J.; Leir, S.-H.; Morris, K.J.; Kucybala, K.; Harris, H.; Harris, A. Collagen XV inhibits epithelial to mesenchymal transition in pancreatic adenocarcinoma cells. PLoS ONE 2013, 8, e72250. [Google Scholar] [CrossRef] [Green Version]
- Duivenvoorden, H.M.; Spurling, A.; O’Toole, S.A.; Parker, B.S. Discriminating the earliest stages of mammary carcinoma using myoepithelial and proliferative markers. PLoS ONE 2018, 13, e0201370. [Google Scholar] [CrossRef]
- Rygh, C.B.; Løkka, G.; Heljasvaara, R.; Taxt, T.; Pavlin, T.; Sormunen, R.; Pihlajaniemi, T.; Curry, F.R.; Tenstad, O.; Reed, R.K. Image-based assessment of microvascular function and structure in collagen XV- and XVIII-deficient mice. J. Physiol. 2013, 592, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Blake, S.J.; Smyth, M.J.; Teng, M.W. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin. Transl. Immunol. 2014, 3, e22. [Google Scholar] [CrossRef]
- Guerin, M.V.; Finisguerra, V.; Eynde, B.J.V.D.; Bercovici, N.; Trautmann, A. Preclinical murine tumor models: A structural and functional perspective. eLife 2020, 9, e50740. [Google Scholar] [CrossRef]
- Gyorffy, B.; Surowiak, P.; Budczies, J.; Lanczky, A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE 2013, 8, e82241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hägg, P.M.; Horelli-Kuitunen, N.; Eklund, L.; Palotie, A.; Pihlajaniemi, T. Cloning of mouse type XV collagen sequences and mapping of the corresponding gene to 4B1-3. Genomics 1997, 45, 31–41. [Google Scholar] [CrossRef]
- Pandey, P.R.; Saidou, J.; Watabe, K. Role of myoepithelial cells in breast tumour progression. Front. Biosci. 2010, 15, 226–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudjonsson, T.; Adriance, M.C.; Sternlicht, M.D.; Petersen, O.W.; Bissell, M.J. Myoepithelial cells: Their origin and function in breast morphogenesis and neoplasia. J. Mammary Gland. Biol. Neoplasia 2005, 10, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.C.; Dion, A.S.; Abraham, V.; Amenta, P.S. Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res. 1996, 286, 493–505. [Google Scholar] [CrossRef]
- Sirka, O.K.; Shamir, E.; Ewald, A.J. Myoepithelial cells are a dynamic barrier to epithelial dissemination. J. Cell Biol. 2018, 217, 3368–3381. [Google Scholar] [CrossRef] [Green Version]
- Gudjonsson, T.; Rønnov-Jessen, L.; Villadsen, R.; Rank, F.; Bissell, M.J.; Petersen, O.W. Normal and tumour-derived my-oepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. Cell Sci. 2002, 115, 39–50. [Google Scholar] [CrossRef]
- Hurskainen, M.; Ruggiero, F.; Hägg, P.; Pihlajaniemi, T.; Huhtala, P. Recombinant human collagen XV regulates cell adhesion and migration. J. Biol. Chem. 2010, 285, 5258–5265. [Google Scholar] [CrossRef] [Green Version]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Experientia 2019, 77, 1745–1770. [Google Scholar] [CrossRef] [Green Version]
- Saharinen, P.; Eklund, L.; Pulkki, K.; Bono, P.; Alitalo, K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med. 2011, 17, 347–362. [Google Scholar] [CrossRef]
- Guy, C.T.; Muthuswamy, S.; Cardiff, R.D.; Soriano, P.; Muller, W.J. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 1994, 8, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Liotta, L.A.; Tryggvason, K.; Garbisa, S.; Hart, I.; Foltz, C.M.; Shafie, S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980, 284, 67–68. [Google Scholar] [CrossRef]
- Balbín, M.; Fueyo-Silva, A.; Tester, A.M.; Pendas, A.M.; Pitiot, A.; Astudillo, A.; Overall, C.M.; Shapiro, S.D.; López-Otín, C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 2003, 35, 252–257. [Google Scholar] [CrossRef]
- Brisken, C.; Socolovsky, M.; Lodish, H.F.; Weinberg, R. The signaling domain of the erythropoietin receptor rescues prolactin receptor-mutant mammary epithelium. Proc. Natl. Acad. Sci. USA 2002, 99, 14241–14245. [Google Scholar] [CrossRef] [Green Version]
- Muona, A.; Eklund, L.; Väisänen, T.; Pihlajaniemi, T. Developmentally regulated expression of type XV collagen correlates with abnormalities in Col15a1−/− mice. Matrix Biol. 2002, 21, 89–102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Nieto, G.; Heljasvaara, R.; Heikkinen, A.; Kaski, H.-K.; Devarajan, R.; Rinne, O.; Henriksson, C.; Thomson, E.; von Hertzen, C.; Miinalainen, I.; et al. Deletion of Col15a1 Modulates the Tumour Extracellular Matrix and Leads to Increased Tumour Growth in the MMTV-PyMT Mouse Mammary Carcinoma Model. Int. J. Mol. Sci. 2021, 22, 9978. https://doi.org/10.3390/ijms22189978
Martínez-Nieto G, Heljasvaara R, Heikkinen A, Kaski H-K, Devarajan R, Rinne O, Henriksson C, Thomson E, von Hertzen C, Miinalainen I, et al. Deletion of Col15a1 Modulates the Tumour Extracellular Matrix and Leads to Increased Tumour Growth in the MMTV-PyMT Mouse Mammary Carcinoma Model. International Journal of Molecular Sciences. 2021; 22(18):9978. https://doi.org/10.3390/ijms22189978
Chicago/Turabian StyleMartínez-Nieto, Guillermo, Ritva Heljasvaara, Anne Heikkinen, Hanne-Kaisa Kaski, Raman Devarajan, Otto Rinne, Charlotta Henriksson, Emmi Thomson, Camilla von Hertzen, Ilkka Miinalainen, and et al. 2021. "Deletion of Col15a1 Modulates the Tumour Extracellular Matrix and Leads to Increased Tumour Growth in the MMTV-PyMT Mouse Mammary Carcinoma Model" International Journal of Molecular Sciences 22, no. 18: 9978. https://doi.org/10.3390/ijms22189978
APA StyleMartínez-Nieto, G., Heljasvaara, R., Heikkinen, A., Kaski, H. -K., Devarajan, R., Rinne, O., Henriksson, C., Thomson, E., von Hertzen, C., Miinalainen, I., Ruotsalainen, H., Pihlajaniemi, T., & Karppinen, S. -M. (2021). Deletion of Col15a1 Modulates the Tumour Extracellular Matrix and Leads to Increased Tumour Growth in the MMTV-PyMT Mouse Mammary Carcinoma Model. International Journal of Molecular Sciences, 22(18), 9978. https://doi.org/10.3390/ijms22189978




