Dietary Isothiocyanates, Sulforaphane and 2-Phenethyl Isothiocyanate, Effectively Impair Vibrio cholerae Virulence
Abstract
:1. Introduction
2. Results and Discussion
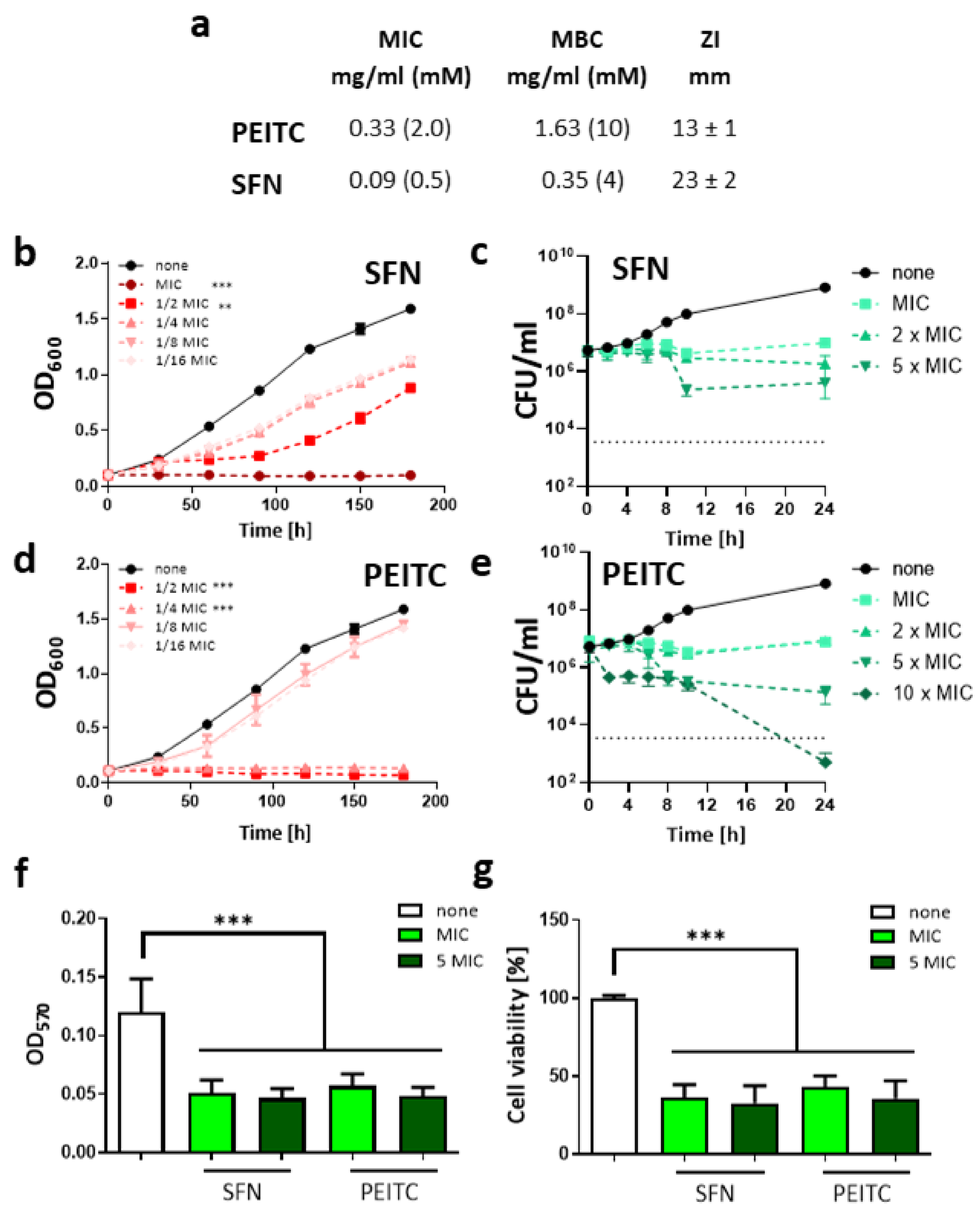
2.1. Sulforaphane and 2-Phenethyl Isothiocyanate Impair the Growth of V. cholerae in a Dose-Dependent Manner
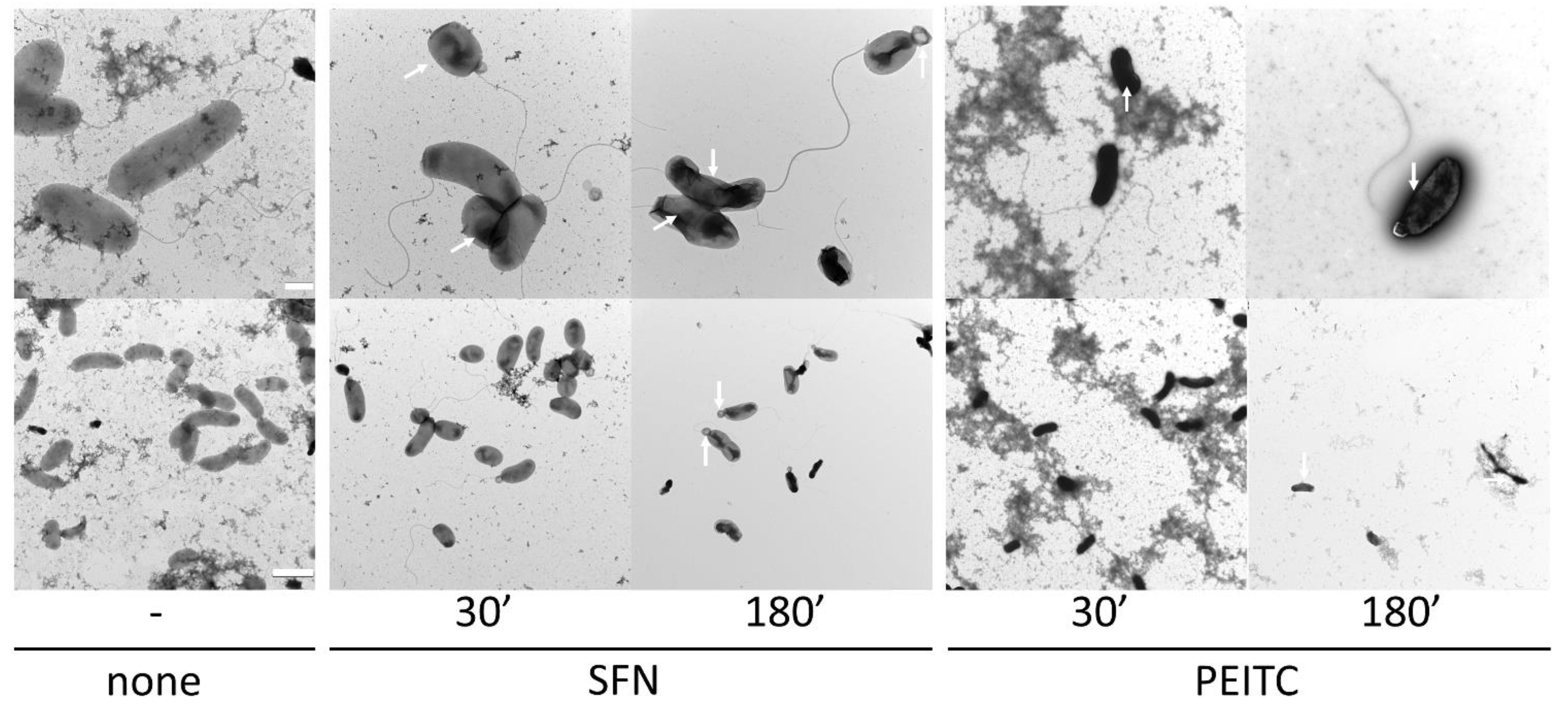
2.2. ITC Affects the Cell Morphology of V. cholerae
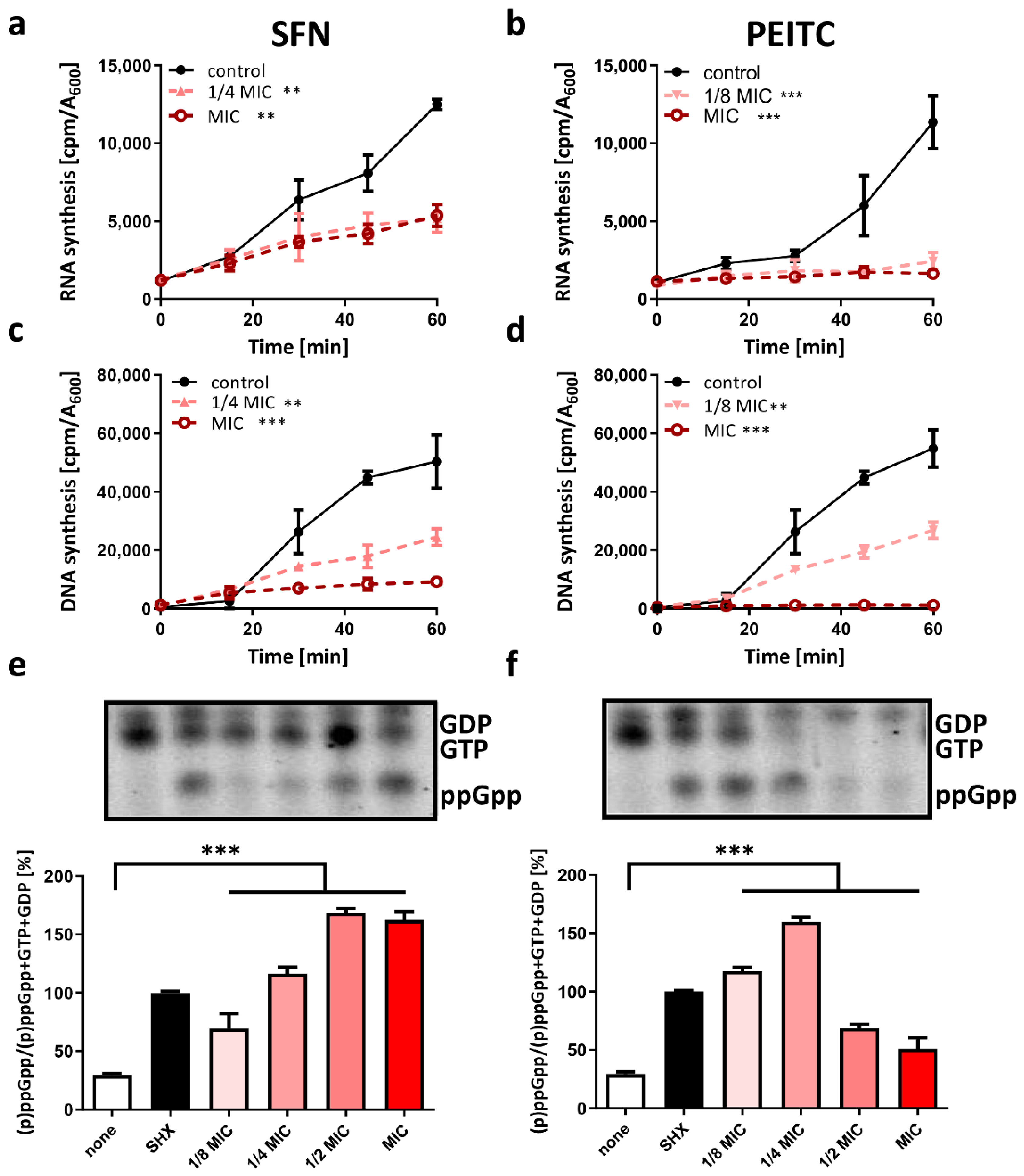
2.3. Starvation as a Mode of ITC Action against V. cholerae
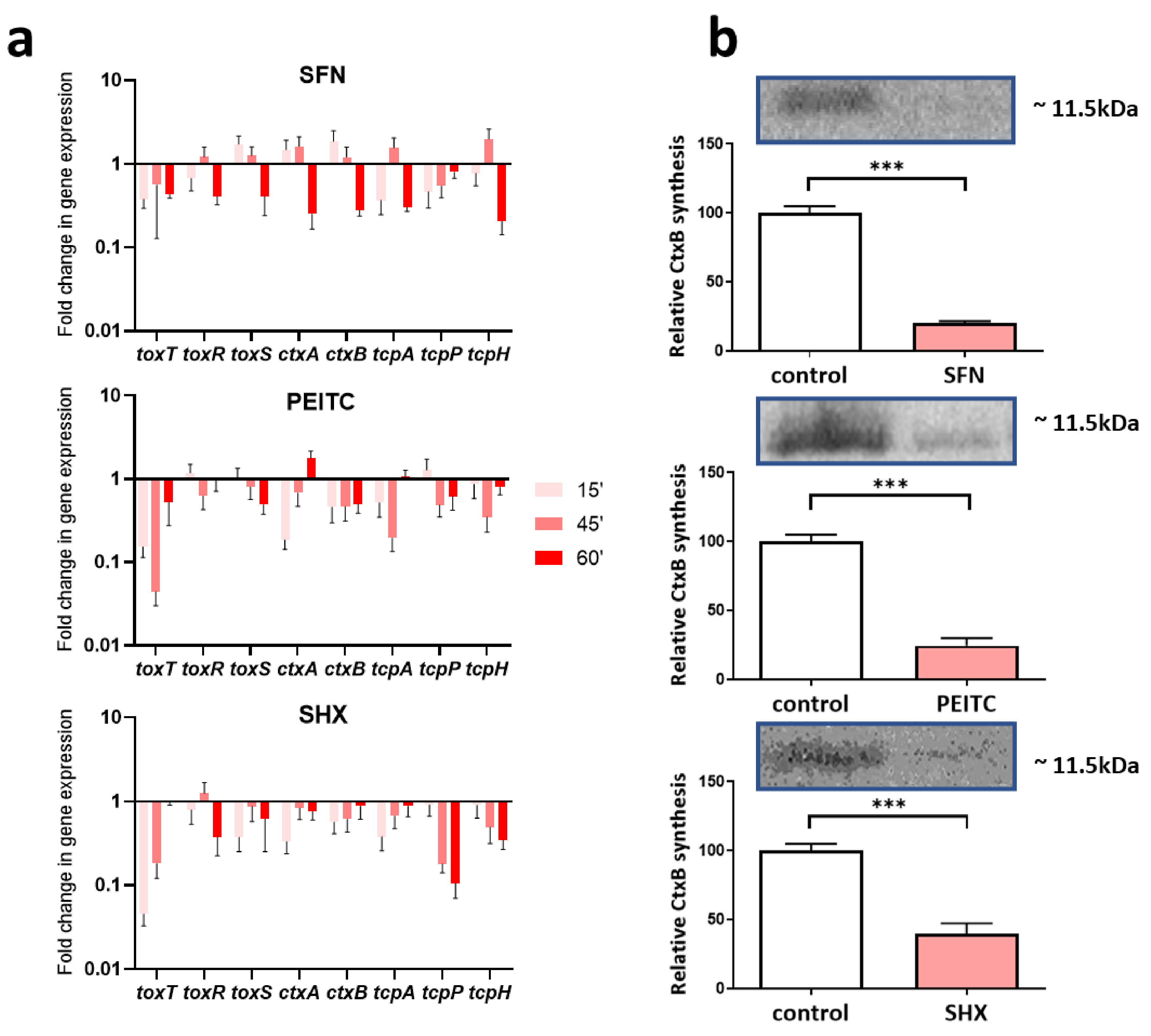
2.4. The SFN and PEITC Treatment Controls Expression of Virulence Factors of V. cholerae
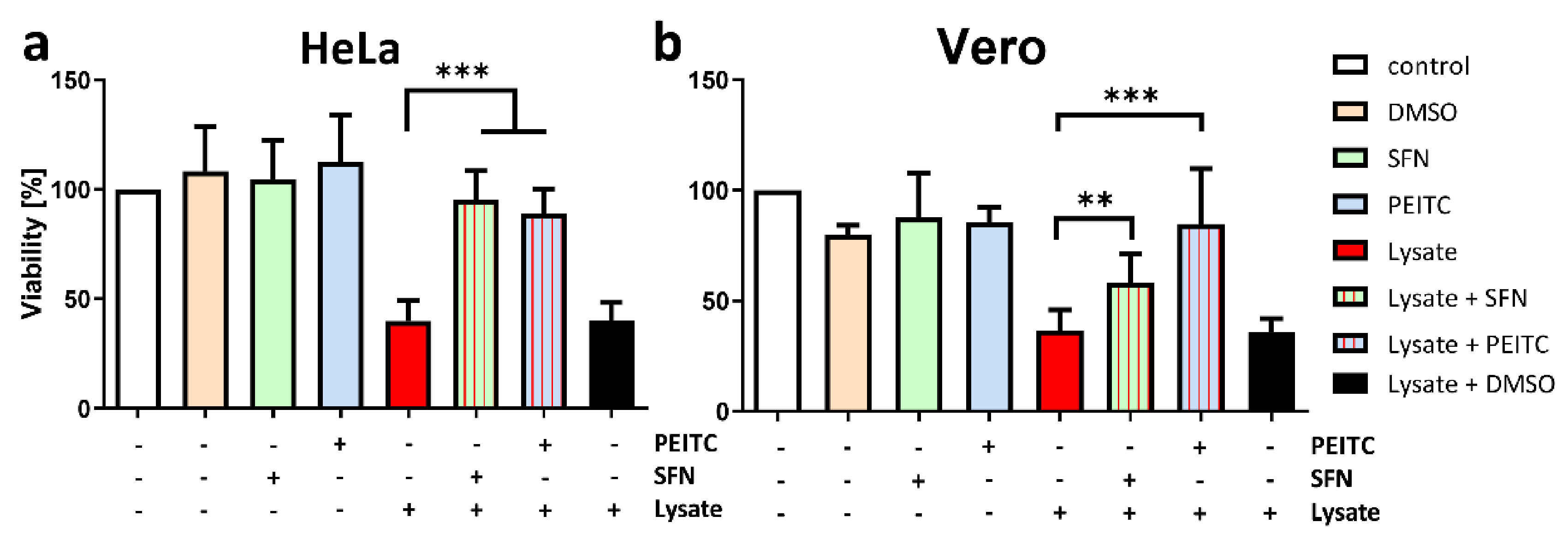
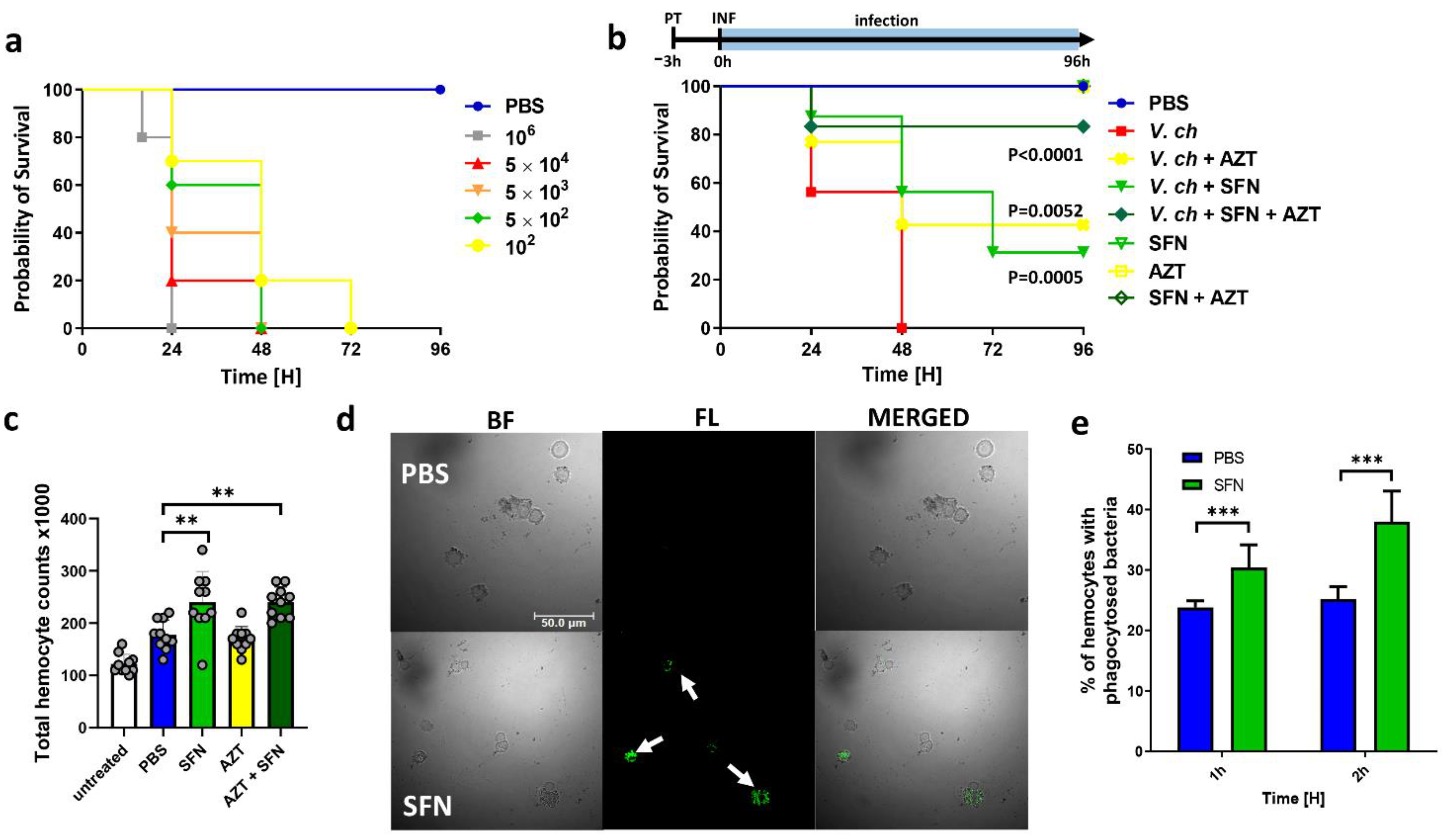
2.5. ITC Reduces the Toxicity of V. cholera In Vitro and In Vivo
3. Materials and Methods
3.1. Bacterial Isolates and Growth Conditions
3.2. Determination of Bacterial Growth Inhibition
3.3. Time-Kill Assay
3.4. Biofilm Eradication
3.5. Measurement of DNA and RNA Synthesis
3.6. RNA Extraction, Reverse Transcription, Primers, and qPCR Analysis
3.7. In Vitro Cultures Toxicity Assay
3.8. Assessment of (p)ppGpp Accumulation Levels in Cells after ITC’s Treatment
3.9. Galleria Mellonella Model of Infection Procedures
3.9.1. Virulence and Treatment of V. cholerae Infection in G. mellonella
3.9.2. FITC Labeling of Bacteria
3.9.3. In Vivo Phagocytosis Assay
3.10. Transmission Electron Microscopy (TEM)
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kaper, J.B.; Morris, J.G.; Levine, M.M. Cholera. Clin. Microbiol. Rev. 1995, 8, 48–86. [Google Scholar] [CrossRef]
- Sack, D.A.; Sack, R.B.; Nair, G.B.; Siddique, A. Cholera. Lancet 2004, 363, 223–233. [Google Scholar] [CrossRef]
- Deen, J.; Mengel, M.A.; Clemens, J.D. Epidemiology of cholera. Vaccine 2020, 38, A31–A40. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.D.; Nair, G.B.; Ahmed, T.; Qadri, F.; Holmgren, J. Cholera. Lancet 2017, 390, 1539–1549. [Google Scholar] [CrossRef]
- Chatterjee, S.; Asakura, M.; Chowdhury, N.; Neogi, S.B.; Sugimoto, N.; Haldar, S.; Awasthi, S.P.; Hinenoya, A.; Aoki, S.; Yamasaki, S. Capsaicin, a potential inhibitor of cholera toxin production in Vibrio cholerae. FEMS Microbiol. Lett. 2010, 306, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, J.; Bag, S.; Saha, B.; Kumar, P.; Ghosh, S.; Dayal, M.; Senapati, T.; Mehra, S.; Dey, P.; Desigamani, A.; et al. Genomic plasticity associated with antimicrobial resistance in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2019, 116, 6226–6231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, D.; Kaushik, A.; Kumar, D.; Bag, S. Foodborne Pathogenic Vibrios: Antimicrobial Resistance. Front. Microbiol. 2021, 12, 638331. [Google Scholar] [CrossRef]
- Kaunitz, J.D. Oral Defense: How Oral Rehydration Solutions Revolutionized the Treatment of Toxigenic Diarrhea. Dig. Dis. Sci. 2020, 65, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Zahid, M.S.H.; Awasthi, S.P.; Asakura, M.; Chatterjee, S.; Hinenoya, A.; Faruque, S.M.; Yamasaki, S. Suppression of virulence of toxigenic vibrio cholerae by anethole through the cyclic AMP (cAMP)-cAMP receptor protein signaling system. PLoS ONE 2015, 10, e0137529. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.J.; Benitez, J.A. Vibrio cholerae Biofilms and Cholera Pathogenesis. PLoS Negl. Trop. Dis. 2016, 10, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Moorthy, S.; Watnick, P.I. Identification of novel stage-specific genetic requirements through whole genome transcription profiling of Vibrio cholerae biofilm development. Mol. Microbiol. 2005, 57, 1623–1635. [Google Scholar] [CrossRef] [Green Version]
- Gallego-Hernandez, A.L.; Depas, W.H.; Park, J.H.; Teschler, J.K.; Hartmann, R.; Jeckel, H.; Drescher, K.; Beyhan, S.; Newman, D.K.; Yildiz, F.H.; et al. Upregulation of virulence genes promotes Vibrio cholerae biofilm hyperinfectivity. Proc. Natl. Acad. Sci. USA 2020, 117, 11010–11017. [Google Scholar] [CrossRef] [PubMed]
- Faruque, S.M.; Biswas, K.; Nashir Udden, S.M.; Ahmad, Q.S.; Sack, D.A.; Balakrish Nair, G. Transmissibility of cholera: In vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc. Natl. Acad. Sci. USA 2006, 103, 6350–6355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teschler, J.K.; Zamorano-Sánchez, D.; Utada, A.S.; Warner, C.J.A.; Wong, G.C.L.; Linington, R.G.; Yildiz, F.H. Living in the matrix: Assembly and control of Vibrio cholerae biofilms. Nat. Rev. Microbiol. 2015, 13, 255–268. [Google Scholar] [CrossRef] [Green Version]
- Papenfort, K.; Silpe, J.E.; Schramma, K.R.; Cong, J.P.; Seyedsayamdost, M.R.; Bassler, B.L. A Vibrio cholerae autoinducer-receptor pair that controls biofilm formation. Nat. Chem. Biol. 2017, 13, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aires, A.; Mota, V.R.; Saavedra, M.J.; Rosa, E.A.S.; Bennett, R.N. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Drobnica, L.; Zemanová, M.; Nemec, P.; Antos, K.; Kristián, P.; Stullerová, A.; Knoppová, V.; Nemec, P. Antifungal activity of isothiocyanates and related compounds. I. Naturally occurring isothiocyanates and their analogues. Appl. Microbiol. 1967, 15, 701–709. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [Green Version]
- Coscueta, E.R.; Reis, C.A.; Pintado, M. Phenylethyl isothiocyanate extracted from watercress by-products with aqueous micellar systems: Development and optimisation. Antioxidants 2020, 9, 698. [Google Scholar] [CrossRef]
- Palliyaguru, D.L.; Yuan, J.-M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62, 1700965. [Google Scholar] [CrossRef]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Bogucka, K.; Szalewska-Pałasz, A.; Herman-Antosiewicz, A. Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Nowicki, D.; Macia̧g-Dorszyńska, M.; Kobiela, W.; Herman-Antosiewicz, A.; Wȩgrzyn, A.; Szalewska-Pałasz, A.; Wȩgrzyn, G. Phenethyl isothiocyanate inhibits Shiga toxin production in enterohemorrhagic Escherichia coli by stringent response induction. Antimicrob. Agents Chemother. 2014, 58, 2304–2315. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, S.J.; Mutters, N.T.; Blessing, B.; Günther, F. Natural isothiocyanates express antimicrobial activity against developing and mature biofilms of Pseudomonas aeruginosa. Fitoterapia 2017, 119, 57–63. [Google Scholar] [CrossRef]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, D.; Rodzik, O.; Herman-Antosiewicz, A.; Szalewska-Pałasz, A. Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: Insight to the mode of action. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Barlow, G. Clinical challenges in antimicrobial resistance. Nat. Microbiol. 2018. [Google Scholar] [CrossRef]
- Kolter, R.; Van Wezel, G.P. Goodbye to brute force in antibiotic discovery? Nat. Microbiol. 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Jang, M.; Hong, E.; Kim, G.-H. Evaluation of antibacterial activity of 3-butenyl, 4-pentenyl, 2-phenylethyl, and benzyl isothiocyanate in Brassica vegetables. J. Food Sci. 2010, 75, M412–M416. [Google Scholar] [CrossRef]
- Aires, A.; Mota, V.R.; Saavedra, M.J.; Monteiro, A.A.; Simões, M.; Rosa, E.A.S.; Bennett, R.N. Initial in vitro evaluations of the antibacterial activities of glucosinolate enzymatic hydrolysis products against plant pathogenic bacteria. J. Appl. Microbiol. 2009, 106, 2096–2105. [Google Scholar] [CrossRef]
- Dufour, V.; Alazzam, B.; Thepaut, M.; Ermel, G.; Baysse, C. Antimicrobial Activities of Isothiocyanates Against Campylobacter jejuni Isolates. Front. Cell. Infect. Microbiol. 2012, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Pujante, P.J.; Borja-Martínez, M.; Pedreño, M.Á.; Almagro, L. Biosynthesis and bioactivity of glucosinolates and their production in plant in vitro cultures. Planta 2017, 246, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, T.-X.; Wang, X.-N.; Wu, H.-Y.; Bi, J.-R.; Hao, H.-S.; Hou, H.-M.; Zhang, G.-L. Transcriptomic Analysis, Motility and Biofilm Formation Characteristics of Salmonella typhimurium Exposed to Benzyl Isothiocyanate Treatment. Int. J. Mol. Sci. 2020, 21, 1025. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Simoes, L.C.; Saavedra, M.J.; Simoes, M. The action of selected isothiocyanates on bacterial biofilm prevention and control. Int. Biodeterior. Biodegrad. 2014, 86, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, T.H.; Bragason, S.K.; Phipps, R.K.; Christensen, L.D.; van Gennip, M.; Alhede, M.; Skindersoe, M.; Larsen, T.O.; Høiby, N.; Bjarnsholt, T.; et al. Food as a source for quorum sensing inhibitors: Iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2012, 78, 2410–2421. [Google Scholar] [CrossRef] [Green Version]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L.; Hayashi, M.A.F.; Knapp, C. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Muscolino, D.; Giarratana, F.; Beninati, C.; Ziino, G.; Giuffrida, A.; Panebianco, A. Effects of allyl isothiocyanate on the shelf-life of gilthead sea bream (Sparus aurata) fillets. Czech J. Food Sci. 2016, 34, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Saleh, N.M.; Mabrouk, M.I.; Salem-Bekhit, M.M.; Hafez, E.H.; Salem-Bekhit, M. Challenge of Moringa peregrina Forssk as an antimicrobial agent against multi-drug-resistant Salmonella sp. Biotechnol. Biotechnol. Equip. 2016, 21, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Westfall, C.S.; Kao, J.; Odermatt, P.D.; Anderson, S.E.; Cesar, S.; Sievert, M.; Moore, J.; Gonzalez, C.G.; Zhang, L.; et al. Starvation induces shrinkage of the bacterial cytoplasm. Proc. Natl. Acad. Sci. USA 2021, 118, e2104686118. [Google Scholar] [CrossRef]
- Brooks, A.; Yau, J.; Pham, S. Stringent Response Changes Cell Membrane Permeability in Escherichia coli but does not Develop Cross Tolerance to Kanamycin, Tetracycline and Ampicillin. J. Exp. Microbiol. Immunol. 2011, 15, 30–35. [Google Scholar]
- Gitter, B.; Diefenbach, R.; Keweloh, H.; Riesenberg, D. Influence of stringent and relaxed response on excretion of recombinant proteins and fatty acid composition in Escherichia coli. Appl. Microbiol. Biotechnol. 1995, 43, 89–92. [Google Scholar] [CrossRef]
- Potrykus, K.; Cashel, M. (p)ppGpp: Still magical? Annu. Rev. Microbiol. 2008, 62, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Dennis, P.P.; Ehrenberg, M.; Bremer, H. Control of rRNA synthesis in Escherichia coli: A systems biology approach. Microbiol. Mol. Biol. Rev. 2004, 68, 639–668. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, D.; Krause, K.; Szamborska, P.; Żukowska, A.; Cech, G.M.; Szalewska-Pałasz, A. Induction of the Stringent Response Underlies the Antimicrobial Action of Aliphatic Isothiocyanates. Front. Microbiol. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Karlsson, I.; Samuelsson, K.; Ponting, D.J.; Törnqvist, M.; Ilag, L.L.; Nilsson, U. Peptide Reactivity of Isothiocyanates--Implications for Skin Allergy. Sci. Rep. 2016, 6, 21203. [Google Scholar] [CrossRef]
- Cejpek, K.; Valusek, J.; Velísek, J. Reactions of allyl isothiocyanate with alanine, glycine, and several peptides in model systems. J. Agric. Food Chem. 2000, 48, 3560–3565. [Google Scholar] [CrossRef]
- Lin, C.M.; Preston, J.F., 3rd; Wei, C.I. Antibacterial mechanism of allyl isothiocyanate. J. Food Prot. 2000, 63, 727–734. [Google Scholar] [CrossRef]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157:H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef]
- Bina, J.; Zhu, J.; Dziejman, M.; Faruque, S.; Calderwood, S.; Mekalanos, J. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci. USA 2003, 100, 2801–2806. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.J.; Benitez, J.A. A Vibrio cholerae relaxed (relA) mutant expresses major virulence factors, exhibits biofilm formation and motility, and colonizes the suckling mouse intestine. J. Bacteriol. 2006, 188, 794–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Coll, L.; Cashel, M. Possible Roles for Basal Levels of (p)ppGpp: Growth Efficiency Vs. Surviving Stress. Front. Microbiol. 2020, 11, 592718. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Nagano, T.; Kumagai, H.; Okamoto, Y.; Otani, S. Destruction of cholera toxin receptor on HeLa cell membrane using microbial endoglycoceramidase. Arch. Biochem. Biophys. 1996, 328, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Tugba Artun, F.; Karagoz, A.; Ozcan, G.; Melikoglu, G.; Anil, S.; Kultur, S.; Sutlupinar, N. In vitro anticancer and cytotoxic activities of some plant extracts on HeLa and Vero cell lines. JBUON 2016, 21, 720–725. [Google Scholar]
- Majoul, I.; Schmidt, T.; Pomasanova, M.; Boutkevich, E.; Kozlov, Y.; Söling, H.-D. Differential expression of receptors for Shiga and Cholera toxin is regulated by the cell cycle. J. Cell Sci. 2002, 115, 817–826. [Google Scholar] [CrossRef]
- Samuel, J.E.; Perera, L.P.; Ward, S.; Brien, A.D.O.; Ginsburg, V.; Krivan, H.C.; Al, S.E.T. Comparison of the Glycolipid Receptor Specificities of Shiga-Like Toxin Type II and Shiga-Like Toxin Type II Variants. Infect. Immunol. 1990, 58, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Bokhari, H.; Ali, A.; Noreen, Z.; Thomson, N.; Wren, B.W. Galleria mellonella is low cost and suitable surrogate host for studying virulence of human pathogenic Vibrio cholerae. Gene 2017, 628, 1–7. [Google Scholar] [CrossRef]
- Nuidate, T.; Tansila, N.; Saengkerdsub, S.; Kongreung, J.; Bakkiyaraj, D.; Vuddhakul, V. Role of Indole Production on Virulence of Vibrio cholerae Using Galleria mellonella Larvae Model. Indian J. Microbiol. 2016, 56, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Schepici, G.; Bramanti, P.; Mazzon, E. Efficacy of Sulforaphane in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8637. [Google Scholar] [CrossRef]
- Ambrecht, L.A.; Perlman, J.I.; McDonnell, J.F.; Zhai, Y.; Qiao, L.; Bu, P. Protection of retinal function by sulforaphane following retinal ischemic injury. Exp. Eye Res. 2015, 138, 66–69. [Google Scholar] [CrossRef]
- Kaushik, J.S.; Gupta, P.; Faridi, M.M.; Das, S. Single dose azithromycin versus ciprofloxacin for cholera in children: A randomized controlled trial. Indian Pediatr. 2010, 47, 309–315. [Google Scholar] [CrossRef]
- Mahn, A.; Castillo, A. Potential of Sulforaphane as a Natural Immune System Enhancer: A Review. Molecules 2021, 26, 752. [Google Scholar] [CrossRef]
- Ali, M.; Bonay, M.; Vanhee, V.; Vinit, S.; Deramaudt, T.B. Comparative effectiveness of 4 natural and chemical activators of Nrf2 on inflammation, oxidative stress, macrophage polarization, and bactericidal activity in an in vitro macrophage infection model. PLoS ONE 2020, 15, e0234484. [Google Scholar] [CrossRef]
- Deramaudt, T.B.; Ali, M.; Vinit, S.; Bonay, M. Sulforaphane reduces intracellular survival of Staphylococcus aureus in macrophages through inhibition of JNK and p38 MAPK-induced inflammation. Int. J. Mol. Med. 2020, 45, 1927–1941. [Google Scholar] [CrossRef] [Green Version]
- Wojda, I.; Koperwas, K.; Jakubowicz, T. Activation of MAP kinase pathways in Galleria mellonella infected with Bacillus thuringiensis. Acta Biochim. Pol. 2014, 61, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Grizanova, E.V.; Dubovskiy, I.M.; Whitten, M.M.A.; Glupov, V. V Contributions of cellular and humoral immunity of Galleria mellonella larvae in defence against oral infection by Bacillus thuringiensis. J. Invertebr. Pathol. 2014, 119, 40–46. [Google Scholar] [CrossRef]
- Häse, C.C.; Mekalanos, J.J. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1998, 95, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Häse, C.C.; Mekalanos, J.J. Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1999, 96, 3183–3187. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.-Y.; Ye, X.-G.; He, L.-T.; Zhang, S.-R.; Wang, R.-L.; Zhou, J.; He, Z.-S. In vitro characterization and inhibition of the interaction between ciprofloxacin and berberine against multidrug-resistant Klebsiella pneumoniae. J. Antibiot. 2016, 69, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Nejman-Faleńczyk, B.; Bloch, S.; Januszkiewicz, A.; Węgrzyn, A.; Węgrzyn, G. A simple and rapid procedure for the detection of genes encoding shiga toxins and other specific DNA sequences. Toxins 2015, 7, 4745–4757. [Google Scholar] [CrossRef]
- Mechold, U.; Potrykus, K.; Murphy, H.; Murakami, K.S.; Cashel, M. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res. 2013, 41, 6175–6189. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Ding, Y.; Yi, Y. Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva: Cell types and their role in the innate immunity. Tissue Cell 2016, 48, 297–304. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krause, K.; Pyrczak-Felczykowska, A.; Karczewska, M.; Narajczyk, M.; Herman-Antosiewicz, A.; Szalewska-Pałasz, A.; Nowicki, D. Dietary Isothiocyanates, Sulforaphane and 2-Phenethyl Isothiocyanate, Effectively Impair Vibrio cholerae Virulence. Int. J. Mol. Sci. 2021, 22, 10187. https://doi.org/10.3390/ijms221910187
Krause K, Pyrczak-Felczykowska A, Karczewska M, Narajczyk M, Herman-Antosiewicz A, Szalewska-Pałasz A, Nowicki D. Dietary Isothiocyanates, Sulforaphane and 2-Phenethyl Isothiocyanate, Effectively Impair Vibrio cholerae Virulence. International Journal of Molecular Sciences. 2021; 22(19):10187. https://doi.org/10.3390/ijms221910187
Chicago/Turabian StyleKrause, Klaudyna, Agnieszka Pyrczak-Felczykowska, Monika Karczewska, Magdalena Narajczyk, Anna Herman-Antosiewicz, Agnieszka Szalewska-Pałasz, and Dariusz Nowicki. 2021. "Dietary Isothiocyanates, Sulforaphane and 2-Phenethyl Isothiocyanate, Effectively Impair Vibrio cholerae Virulence" International Journal of Molecular Sciences 22, no. 19: 10187. https://doi.org/10.3390/ijms221910187






