The Bul1/2 Alpha-Arrestins Promote Ubiquitylation and Endocytosis of the Can1 Permease upon Cycloheximide-Induced TORC1-Hyperactivation
Abstract
:1. Introduction
2. Results
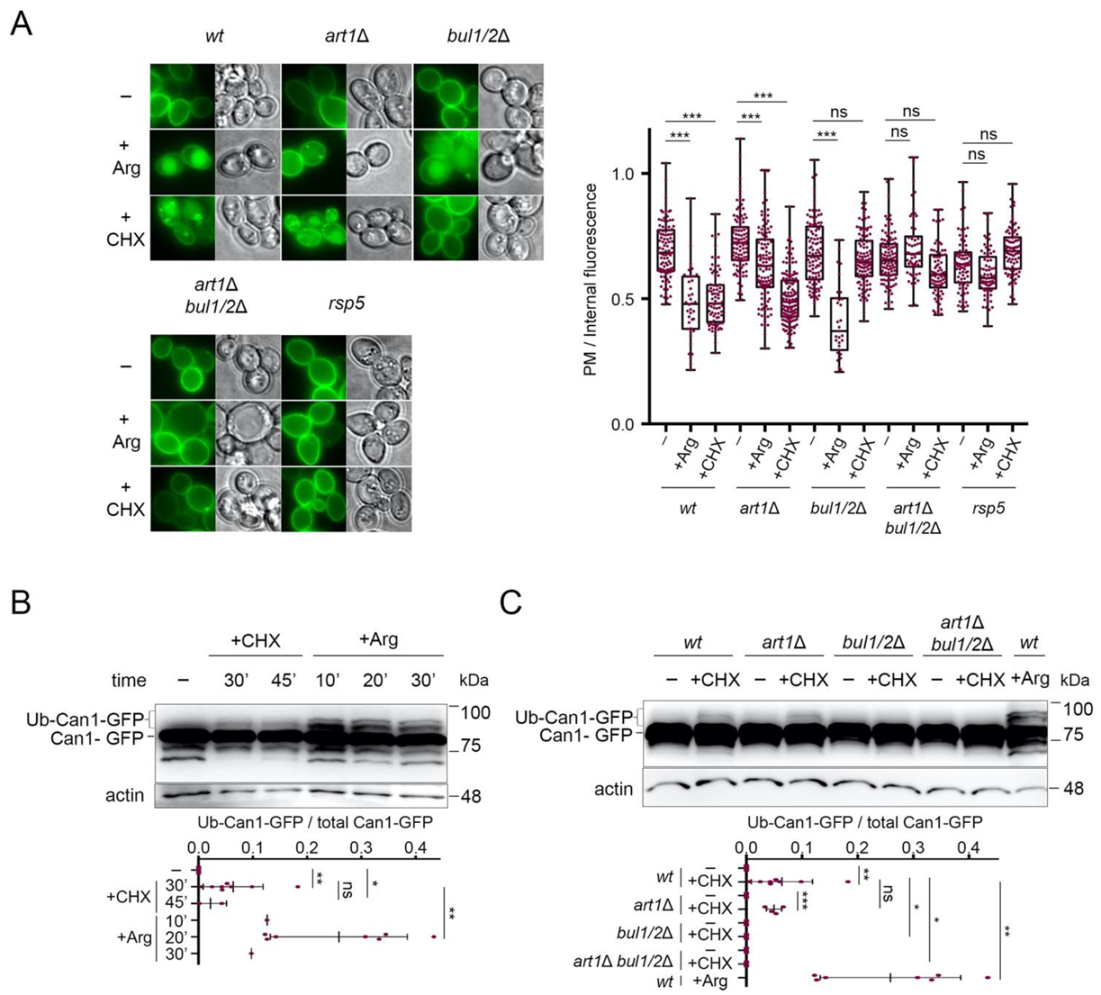
2.1. The Bul α-Arrestins Promote Cycloheximide-Induced Endocytosis of Can1
2.2. Cycloheximide-Induced Endocytosis of Can1 Requires an Intact Putative Bul1/2 Binding Sequence and the Ub-Acceptor Lys42 and Lys45 in the Permease N-Tail
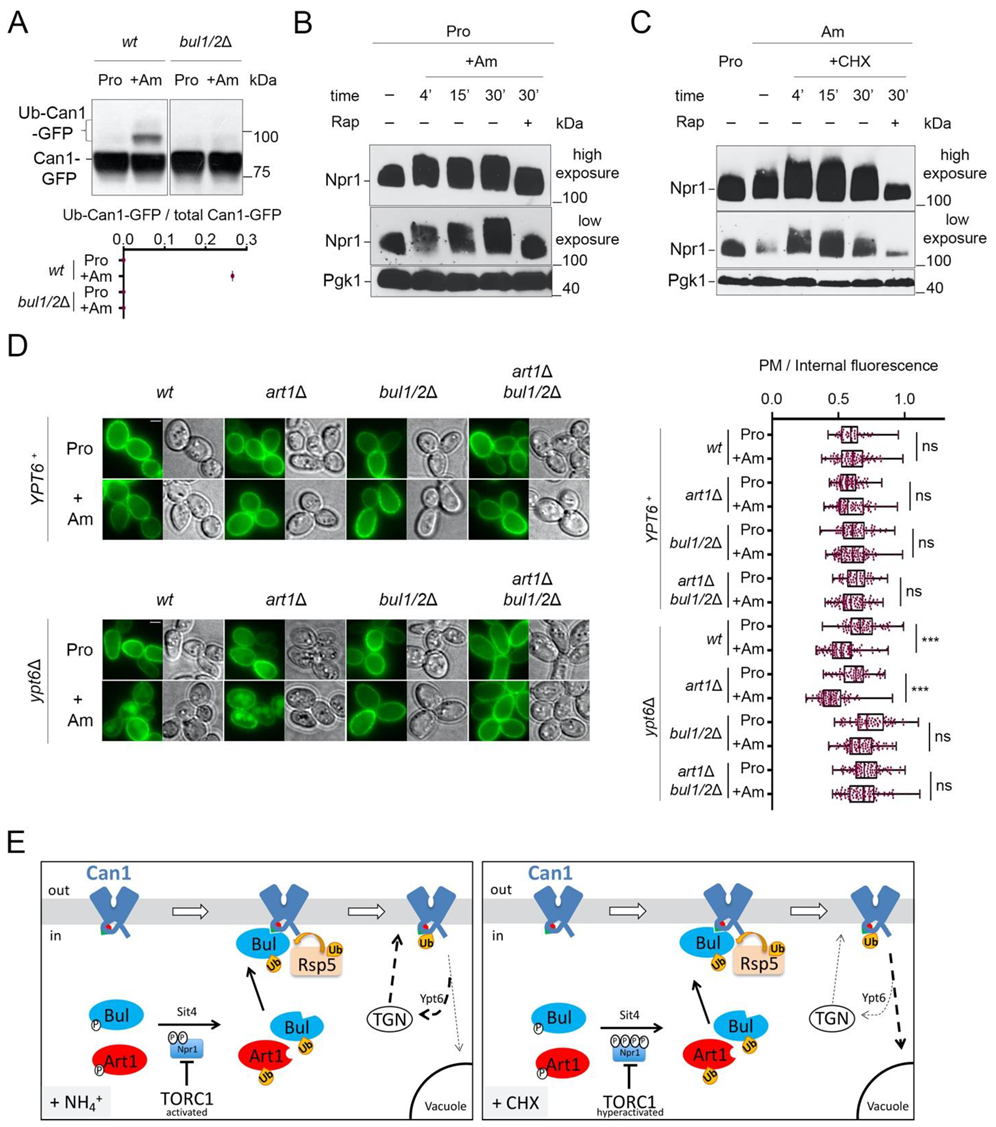
2.3. Cycloheximide Promotes Endocytosis of Can1 via TORC1 Hyperactivation and/or Blockage of Transporter Recycling
2.4. Can1 in the Outward-Facing Conformation Is Not Recognized by TORC1-Activated Art1
3. Discussion
4. Materials and Methods
4.1. Yeast Strains, Plasmids and Growth Conditions
4.2. Epifluorescence Microscopy
4.3. Fluorescence Quantification and Statistical Analysis
4.4. Protein Extracts and Western Blotting
4.5. Quantification and Statistical Analysis of Western Blots
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kaback, H.R.; Smirnova, I.; Kasho, V.; Nie, Y.; Zhou, Y. The Alternating Access Transport Mechanism in LacY. J. Membr. Biol. 2011, 239, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Müller-Hill, B. The Lac Operon: A Short History of a Genetic Paradigm; DE GRUYTER: Berlin, Germany, 2011; ISBN 9783110879476. [Google Scholar]
- Haguenauer-Tsapis, R.; André, B. Membrane trafficking of yeast transporters: Mechanisms and physiological control of downregulation. In Molecular Mechanisms Controlling Transmembrane Transport; Springer: Berlin/Heidelberg, Germany, 2004; pp. 273–323. [Google Scholar]
- Kahlhofer, J.; Leon, S.; Teis, D.; Schmidt, O. The α-arrestin family of ubiquitin ligase adaptors links metabolism with selective endocytosis. Biol. Cell 2021, 113, 183–219. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, E.; Erpapazoglou, Z.; Haguenauer-Tsapis, R.; André, B. The ubiquitin code of yeast permease trafficking. Trends. Cell. Biol. 2010, 20, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Hein, C.; Springael, J.-Y.; Volland, C.; Haguenauer-Tsapis, R.; André, B. NPI1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin—Protein ligase. Mol. Microbiol. 1995, 18, 77–87. [Google Scholar] [CrossRef]
- Kee, Y.; Muñoz, W.; Lyon, N.; Huibregtse, J.M. The deubiquitinating enzyme Ubp2 modulates Rsp5-dependent Lys 63-linked polyubiquitin conjugates in Saccharomyces cerevisiae. J. Biol. Chem. 2006, 281, 36724–36731. [Google Scholar] [CrossRef] [Green Version]
- Lauwers, E.; Jacob, C.; Andre, B. K63-linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J. Cell Biol. 2009, 185, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT Pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; MacGurn, J.A.; Chu, T.; Stefan, C.J.; Emr, S.D. Arrestin-Related Ubiquitin-Ligase Adaptors Regulate Endocytosis and Protein Turnover at the Cell Surface. Cell 2008, 135, 714–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barata-Antunes, C.; Alves, R.; Talaia, G.; Casal, M.; Gerós, H.; Mans, R.; Paiva, S. Endocytosis of nutrient transporters in fungi: The ART of connecting signaling and trafficking. Comput. Struct. Biotechnol. J. 2021, 19, 1713–1737. [Google Scholar] [CrossRef]
- O’donnell, A.F.; Schmidt, M.C. AMPK-mediated regulation of alpha-arrestins and protein trafficking. Int. J. Mol. Sci. 2019, 20, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becuwe, M.; Herrador, A.; Haguenauer-Tsapis, R.; Vincent, O.; Léon, S. Ubiquitin-Mediated Regulation of Endocytosis by Proteins of the Arrestin Family. Biochem. Res. Int. 2012, 2012, 242764. [Google Scholar] [CrossRef] [PubMed]
- Nikko, E.; Pelham, H.R. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic 2009, 10, 1856–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becuwe, M.; Vieira, N.; Lara, D.; Gomes-Rezende, J.; Soares-Cunha, C.; Casal, M.; Haguenauer-Tsapis, R.; Vincent, O.; Paiva, S.; Léon, S. A molecular switch on an arrestin-like protein relays glucose signaling to transporter endocytosis. J. Cell Biol. 2012, 196, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Merhi, A.; André, B. Internal Amino Acids Promote Gap1 Permease Ubiquitylation via TORC1/Npr1/14-3-3-Dependent Control of the Bul Arrestin-Like Adaptors. Mol. Cell. Biol. 2012, 32, 4510–4522. [Google Scholar] [CrossRef] [Green Version]
- Ghaddar, K.; Merhi, A.; Saliba, E.; Krammer, E.-M.; Prévost, M.; André, B. Substrate-Induced Ubiquitylation and Endocytosis of Yeast Amino Acid Permeases. Mol. Cell. Biol. 2014, 34, 4447–4463. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, C.; Shields, S.B.; Williams, C.A.; Winistorfer, S.; Piper, R.C. A Cycle of Ubiquitination Regulates Adaptor Function of the Nedd4-Family Ubiquitin Ligase Rsp5. Curr. Biol. 2020, 30, 465–479.e5. [Google Scholar] [CrossRef]
- Ho, H.C.; MacGurn, J.A.; Emr, S.D. Deubiquitinating enzymes Ubp2 and Ubp15 regulate endocytosis by limiting ubiquitination and degradation of ARTs. Mol. Biol. Cell 2017, 28, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- MacGurn, J.A.A.; Hsu, P.-C.C.; Smolka, M.B.B.; Emr, S.D.D. TORC1 regulates endocytosis via npr1-mediated phosphoinhibition of a ubiquitin ligase adaptor. Cell 2011, 147, 1104–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Márquez, J.Y.; Duncan, M.C. Investigation of Ldb19/Art1 localization and function at the late Golgi. PLoS ONE 2018, 13, e0206944. [Google Scholar] [CrossRef] [Green Version]
- Becuwe, M.; Léon, S. Integrated control of transporter endocytosis and recycling by the arrestin-related protein Rod1 and the ubiquitin ligase Rsp5. Elife 2014, 3, e03307. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.F.; Huang, L.; Thorner, J.; Cyert, M.S. A calcineurin-dependent switch controls the trafficking function of α-arrestin Aly1/Art6. J. Biol. Chem. 2013, 288, 24063–24080. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, Y.; Soejima, S.; Masuda, F.; Saitoh, S. TORC2 inhibition of α-arrestin Aly3 mediates cell surface persistence of S. pombe Ght5 glucose transporter in low glucose. J. Cell Sci. 2021, 134, jcs257485. [Google Scholar] [CrossRef]
- Paiva, S.; Vieira, N.; Nondier, I.; Haguenauer-Tsapis, R.; Casal, M.; Urban-Grimal, D. Glucose-induced ubiquitylation and endocytosis of the yeast Jen1 transporter. Role of lysine 63-linked ubiquitin chains. J. Biol. Chem. 2009, 284, 19228–19236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laidlaw, K.M.E.; Bisinski, D.D.; Shashkova, S.; Paine, K.M.; Veillon, M.A.; Leake, M.C.; MacDonald, C. A glucose-starvation response governs endocytic trafficking and eisosomal retention of surface cargoes in budding yeast. J. Cell Sci. 2021, 134, jcs257733. [Google Scholar] [CrossRef]
- Savocco, J.; Nootens, S.; Afokpa, W.; Bausart, M.; Chen, X.; Villers, J.; Renard, H.F.; Prévost, M.; Wattiez, R.; Morsomme, P. Yeast α-arrestin Art2 is the key regulator of ubiquitylation-dependent endocytosis of plasma membrane vitamin B1 transporters. PLoS Biol. 2019, 17, e3000512. [Google Scholar] [CrossRef] [Green Version]
- Karachaliou, M.; Amillis, S.; Evangelinos, M.; Kokotos, A.C.; Yalelis, V.; Diallinas, G. The arrestin-like protein ArtA is essential for ubiquitination and endocytosis of the UapA transporter in response to both broad-range and specific signals. Mol. Microbiol. 2013, 88, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Gournas, C.; Saliba, E.; Krammer, E.-M.; Barthelemy, C.; Prévost, M.; André, B. Transition of yeast Can1 transporter to the inward-facing state unveils an α-arrestin target sequence promoting its ubiquitylation and endocytosis. Mol. Biol. Cell 2017, 28, 2819–2832. [Google Scholar] [CrossRef] [PubMed]
- Guiney, E.L.; Klecker, T.; Emr, S.D. Identification of the endocytic sorting signal recognized by the Art1-Rsp5 ubiquitin ligase complex. Mol. Biol. Cell. 2016, 27, 4043–4054. [Google Scholar] [CrossRef]
- Fujita, S.; Sato, D.; Kasai, H.; Ohashi, M.; Tsukue, S.; Takekoshi, Y.; Gomi, K.; Shintani, T. The C-terminal region of the yeast monocarboxylate transporter Jen1 acts as a glucose signal–responding degron recognized by the α-arrestin Rod1. J. Biol. Chem. 2018, 293, 10926–10936. [Google Scholar] [CrossRef] [Green Version]
- Ivashov, V.; Zimmer, J.; Schwabl, S.; Kahlhofer, J.; Weys, S.; Gstir, R.; Jakschitz, T.; Kremser, L.; Bonn, G.K.; Lindner, H.; et al. Complementary α-arrestin-ubiquitin ligase complexes control nutrient transporter endocytosis in response to amino acids. Elife 2020, 9, e58246. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ho, H.-C.; Tumolo, J.M.; Hsu, P.-C.; MacGurn, J.A. Methionine triggers Ppz-mediated dephosphorylation of Art1 to promote cargo-specific endocytosis. J. Cell. Biol. 2019, 218, 977–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baile, M.G.; Guiney, E.L.; Sanford, E.J.; Macgurn, J.A.; Smolka, M.B.; Emr, S.D. Activity of a ubiquitin ligase adaptor is regulated by disordered insertions in its arrestin domain. Mol. Biol. Cell 2019, 30, 3057–3072. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Kinner, A.; Kölling, R. The deubiquitinating enzyme Ubp1 affects sorting of the ATP-binding cassette-transporter ste6 in the endocytic pathway. Mol. Biol. Cell 2005, 16, 1319–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, H.G.; Pascoe, N.; Andrews, B. The structure and function of deubiquitinases: Lessons from budding yeast. Open Biol. 2020, 10, 200279. [Google Scholar] [CrossRef] [PubMed]
- Boase, N.A.; Kelly, J.M. A role for creD, a carbon catabolite repression gene from Aspergillus nidulans, in ubiquitination. Mol. Microbiol. 2004, 53, 929–940. [Google Scholar] [CrossRef]
- Lam, M.H.Y.; Urban-Grimal, D.; Bugnicourt, A.; Greenblatt, J.F.; Haguenauer-Tsapis, R.; Emili, A. Interaction of the deubiquitinating enzyme Ubp2 and the e3 ligase Rsp5 is required for transporter/receptor sorting in the multivesicular body pathway. PLoS ONE 2009, 4, e4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikko, E.; Marini, A.-M.M.; André, B. Permease Recycling and Ubiquitination Status Reveal a Particular Role for Bro1 in the Multivesicular Body Pathway. J. Biol. Chem. 2003, 278, 50732–50743. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, G.; Malinsky, J.; Stahlschmidt, W.; Loibl, M.; Weig-Meckl, I.; Frommer, W.B.; Opekarova, M.; Tanner, W.; Opekarová, M.; Tanner, W. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J. Cell Biol. 2008, 183, 1075–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; MacGurn, J.A.; Liu, M.; Emr, S. The ART-Rsp5 ubiquitin ligase network comprises a plasma membrane quality control system that protects yeast cells from proteotoxic stress. Elife 2013, 2, e00459. [Google Scholar] [CrossRef]
- Crapeau, M.; Merhi, A.; André, B. Stress conditions promote yeast Gap1 permease ubiquitylation and down-regulation via the arrestin-like bul and aly proteins. J. Biol. Chem. 2014, 289, 22103–22116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buelto, D.; Hung, C.W.; Aoh, Q.L.; Lahiri, S.; Duncan, M.C. Plasma membrane to vacuole traffic induced by glucose starvation requires Gga2-dependent sorting at the trans-Golgi network. Biol. Cell 2020, 112, 349–367. [Google Scholar] [CrossRef]
- Mikros, E.; Diallinas, G. Tales of tails in transporters. Open Biol. 2019, 9, 190083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keener, J.M.; Babst, M. Quality Control and Substrate-Dependent Downregulation of the Nutrient Transporter Fur4. Traffic 2013, 14, 412–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovsepian, J.; Albanèse, V.; Becuwe, M.; Ivashov, V.; Teis, D.; Léon, S. The yeast arrestin-related protein Bul1 is a novel actor of glucose-induced endocytosis. Mol. Biol. Cell 2018, 29, 1012–1020. [Google Scholar] [CrossRef]
- Talaia, G.; Gournas, C.; Saliba, E.; Barata-Antunes, C.; Casal, M.; André, B.; Diallinas, G.; Paiva, S. The α-Arrestin Bul1p Mediates Lactate Transporter Endocytosis in Response to Alkalinization and Distinct Physiological Signals. J. Mol. Biol. 2017, 429, 3678–3695. [Google Scholar] [CrossRef]
- Binda, M.; Péli-Gulli, M.-P.; Bonfils, G.; Panchaud, N.; Urban, J.; Sturgill, T.W.; Loewith, R.; De Virgilio, C. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol. Cell 2009, 35, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, D.A.; Shi, L.; Tu, B.P.; Weissman, J.S. Cycloheximide can distort measurements of mRNA levels and translation efficiency. Nucleic Acids Res. 2019, 47, 4974–4985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenson, M.; Crabeel, M.; Wiame, J.M.; Béchet, J. Inhibition of protein synthesis and simulation of permease turnover in yeast. Biochem. Biophys. Res. Commun. 1968, 30, 414–419. [Google Scholar] [CrossRef]
- Wiame, J.M.; Grenson, M.; Arst, H.N. Nitrogen Catabolite Repression in Yeasts and Filamentous Fungi. Adv. Microb. Physiol. 1985, 26, 1–88. [Google Scholar] [CrossRef]
- Godard, P.; Urrestarazu, A.; Vissers, S.; Kontos, K.; Bontempi, G.; van Helden, J.; André, B. Effect of 21 Different Nitrogen Sources on Global Gene Expression in the Yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 2007, 27, 3065–3086. [Google Scholar] [CrossRef] [Green Version]
- Magasanik, B.; Kaiser, C.A. Nitrogen regulation in Saccharomyces cerevisiae. Gene 2002, 290, 1–18. [Google Scholar] [CrossRef]
- Roberg, K.J.; Rowley, N.; Kaiser, C.A. Physiological Regulation of Membrane Protein Sorting Late in the Secretory Pathway of Saccharomyces cerevisiae. J. Cell Biol. 1997, 137, 1469–1482. [Google Scholar] [CrossRef] [Green Version]
- Day, K.J.; Casler, J.C.; Glick, B.S. Budding Yeast Has a Minimal Endomembrane System. Dev. Cell 2018, 44, 56–72. [Google Scholar] [CrossRef]
- Schmidt, A.; Beck, T.; Koller, A.; Kunz, J.; Hall, M.N. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 1998, 17, 6924–6931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliba, E.; Evangelinos, M.; Gournas, C.; Corrillon, F.; Georis, I.; André, B. The yeast H+-ATPase Pma1 promotes Rag/Gtr-dependent TORC1 activation in response to H+-coupled nutrient uptake. Elife 2018, 7, e31981. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Hall, M.N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017, 36, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Gallwitz, D. Biochemical and genetic evidence for the involvement of yeast Ypt6-GTPase in protein retrieval to different Golgi compartments. J. Biol. Chem. 2003, 278, 791–799. [Google Scholar] [CrossRef] [Green Version]
- Gournas, C.; Gkionis, S.; Carquin, M.; Twyffels, L.; Tyteca, D.; André, B. Conformation-dependent partitioning of yeast nutrient transporters into starvation-protective membrane domains. Proc. Natl. Acad. Sci. USA 2018, 115, E3145–E3154. [Google Scholar] [CrossRef] [Green Version]
- Grenson, M.; Mousset, M.; Wiame, J.M.; Bechet, J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae: I. Evidence for a specific arginine-transporting system. Biochim. Biophys. Acta 1966, 127, 325–338. [Google Scholar] [CrossRef]
- Malínská, K.; Malínský, J.; Opekarová, M.; Tanner, W. Visualization of Protein Compartmentation within the Plasma Membrane of Living Yeast Cells. Mol. Biol. Cell 2003, 14, 4427–4436. [Google Scholar] [CrossRef]
- Busto, J.V.; Elting, A.; Haase, D.; Spira, F.; Kuhlman, J.; Schäfer-Herte, M.; Wedlich-Söldner, R.; Schäfer-Herte, M.; Wedlich-Söldner, R.; Schäfer-Herte, M.; et al. Lateral plasma membrane compartmentalization links protein function and turnover. EMBO J. 2018, 37, e99473. [Google Scholar] [CrossRef]
- Moharir, A.; Gay, L.; Appadurai, D.; Keener, J.; Babst, M. Eisosomes are metabolically regulated storage compartments for APC-type nutrient transporters. Mol. Biol. Cell 2018, 29, 2113–2127. [Google Scholar] [CrossRef] [PubMed]
- Babst, M. Eisosomes at the Intersection of TORC1 and TORC2 Regulation. Traffic 2019, 20, 543–551. [Google Scholar] [CrossRef]
- Bianchi, F.; Syga, Ł.; Moiset, G.; Spakman, D.; Schavemaker, P.E.; Punter, C.M.; Seinen, A.-B.; van Oijen, A.M.; Robinson, A.; Poolman, B. Steric exclusion and protein conformation determine the localization of plasma membrane transporters. Nat. Commun. 2018, 9, 501. [Google Scholar] [CrossRef]
- Busto, J.V.; Wedlich-Söldner, R. Integration Through Separation—The Role of Lateral Membrane Segregation in Nutrient Uptake. Front. Cell Dev. Biol. 2019, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, A.; André, B.; Sophianopoulou, V.; Gournas, C. Fungal plasma membrane domains. FEMS Microbiol. Rev. 2019, 43, 642–673. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Tanahashi, R.; Takagi, H. The yeast α-arrestin Art3 is a key regulator for arginine-induced endocytosis of the high-affinity proline transporter Put4. Biochem. Biophys. Res. Commun. 2020, 531, 416–421. [Google Scholar] [CrossRef]
- Vandenbol, M.; Jauniaux, J.C.; Grenson, M. Nucleotide sequence of the Saccharomyces cerevisiae PUT4 proline-permease-encoding gene: Similarities between CAN1, HIP1 and PUT4 permeases. Gene 1989, 83, 153–159. [Google Scholar] [CrossRef]
- Jauniaux, J.-C.; Grenson, M. GAP1, the general amino acid permease gene of Saccharomyces cerevisiae Nucleotide sequence, protein similarity with the other bakers yeast amino acid permeases, and nitrogen catabolite repression. Eur. J. Biochem. 1990, 190, 39–44. [Google Scholar] [CrossRef]
- Brach, T.; Specht, T.; Kaksonen, M. Reassessment of the role of plasma membrane domains in the regulation of vesicular traffic in yeast. J. Cell Sci. 2011, 124, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Grenson, M.; Mousset, M.; Wiame, J.M.; Bechet, J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae: II. Evidence for a specific lysine-transporting system. Biochim. Biophys. Acta 1966, 127, 339–346. [Google Scholar] [CrossRef]
- Villers, J.; Savocco, J.; Szopinska, A.; Degand, H.; Nootens, S.; Morsomme, P. Study of the Plasma Membrane Proteome Dynamics Reveals Novel Targets of the Nitrogen Regulation in Yeast. Mol. Cell. Proteom. 2017, 16, 1652–1668. [Google Scholar] [CrossRef] [Green Version]
- Malecki, M.; Kamrad, S.; Ralser, M.; Bähler, J. Mitochondrial respiration is required to provide amino acids during fermentative proliferation of fission yeast. EMBO Rep. 2020, 21, e50845. [Google Scholar] [CrossRef]
- Olin-Sandoval, V.; Yu, J.S.L.; Miller-Fleming, L.; Alam, M.T.; Kamrad, S.; Correia-Melo, C.; Haas, R.; Segal, J.; Peña Navarro, D.A.; Herrera-Dominguez, L.; et al. Lysine harvesting is an antioxidant strategy and triggers underground polyamine metabolism. Nature 2019, 572, 249–253. [Google Scholar] [CrossRef]
- Ruiz, S.J.; van ’t Klooster, J.S.; Bianchi, F.; Poolman, B. Growth inhibition by amino acids in saccharomyces cerevisiae. Microorganisms 2021, 9, 7. [Google Scholar] [CrossRef]
- Hatakeyama, R.; Péli-Gulli, M.P.; Hu, Z.; Jaquenoud, M.; Garcia Osuna, G.M.; Sardu, A.; Dengjel, J.; De Virgilio, C. Spatially Distinct Pools of TORC1 Balance Protein Homeostasis. Mol. Cell 2019, 73, 325–338.e8. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, C.; Piper, R.C. Genetic dissection of early endosomal recycling highlights a TORC1-independent role for Rag GTPases. J. Cell Biol. 2017, 216, 3275–3290. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, P.; Jauniaux, J.-C.; Grenson, M. A cis-dominant regulatory mutation linked to the argB-argC gene cluster in Saccharomyces cerevisiae. J. Mol. Biol. 1980, 139, 691–704. [Google Scholar] [CrossRef]
- Merhi, A.; Gérard, N.; Lauwers, E.; Prévost, M.; André, B. Systematic mutational analysis of the intracellular regions of yeast gap1 permease. PLoS ONE 2011, 6, e18457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megarioti, A.H.; Primo, C.; Kapetanakis, G.C.; Athanasopoulos, A.; Sophianopoulou, V.; André, B.; Gournas, C. The Bul1/2 Alpha-Arrestins Promote Ubiquitylation and Endocytosis of the Can1 Permease upon Cycloheximide-Induced TORC1-Hyperactivation. Int. J. Mol. Sci. 2021, 22, 10208. https://doi.org/10.3390/ijms221910208
Megarioti AH, Primo C, Kapetanakis GC, Athanasopoulos A, Sophianopoulou V, André B, Gournas C. The Bul1/2 Alpha-Arrestins Promote Ubiquitylation and Endocytosis of the Can1 Permease upon Cycloheximide-Induced TORC1-Hyperactivation. International Journal of Molecular Sciences. 2021; 22(19):10208. https://doi.org/10.3390/ijms221910208
Chicago/Turabian StyleMegarioti, Amalia H., Cecilia Primo, George C. Kapetanakis, Alexandros Athanasopoulos, Vicky Sophianopoulou, Bruno André, and Christos Gournas. 2021. "The Bul1/2 Alpha-Arrestins Promote Ubiquitylation and Endocytosis of the Can1 Permease upon Cycloheximide-Induced TORC1-Hyperactivation" International Journal of Molecular Sciences 22, no. 19: 10208. https://doi.org/10.3390/ijms221910208
APA StyleMegarioti, A. H., Primo, C., Kapetanakis, G. C., Athanasopoulos, A., Sophianopoulou, V., André, B., & Gournas, C. (2021). The Bul1/2 Alpha-Arrestins Promote Ubiquitylation and Endocytosis of the Can1 Permease upon Cycloheximide-Induced TORC1-Hyperactivation. International Journal of Molecular Sciences, 22(19), 10208. https://doi.org/10.3390/ijms221910208





