Enteric Microbiota-Mediated Serotonergic Signaling in Pathogenesis of Irritable Bowel Syndrome
Abstract
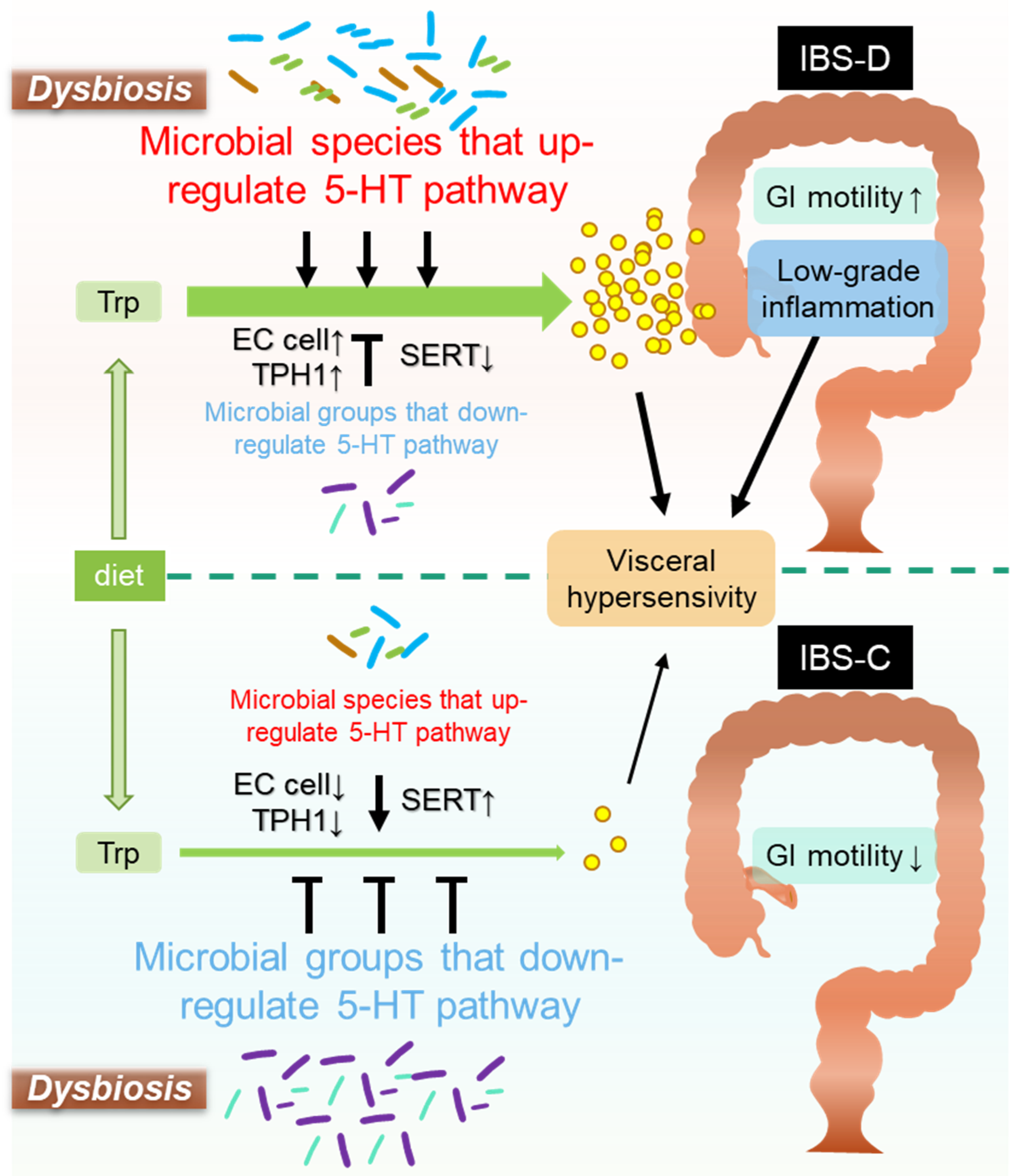
:1. Background
2. Brain–Gut–Microbiome Interactions in IBS
3. Microbiota-Mediated Serotonergic Signaling in Intestines
- (1)
- EC cells
- (2)
- TPH
- (3)
- SERT
- (4)
- 5-HT receptors
- (5)
- Quorum sensing (QS)
4. Microbiota-Mediated Serotonergic Signaling in IBS Pathology
- (1)
- Role of Gut Microbe-Mediated 5-HT Signaling in GI Motility
- (2)
- Role of Gut Microbe-Mediated 5-HT Signaling in Visceral Pain Sensation
- (3)
- Role of Gut-Microbe-Mediated 5-HT Signaling in Mucosal Inflammation and Immune Response
5. Microbiota Mediation of Serotonergic Signaling Outside GI Tract
6. Treatment of IBS by Modulating Microbiota-Mediated Serotonergic Pathways
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable bowel syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.-Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Schmulson, M.J.; Drossman, D.A. What is new in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Hasler, W.L. Rome IV-functional GI disorders: Disorders of gut-brain interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Tack, J. New treatments and therapeutic targets for IBS and other functional bowel disorders. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 589–605. [Google Scholar] [CrossRef]
- Staller, K.; Olén, O.; Söderling, J.; Roelstraete, B.; Törnblom, H.; Khalili, H.; Joshi, A.D.; Nguyen, L.H.; Song, M.; Kuo, B.; et al. Mortality risk in irritable bowel syndrome: Results from a nationwide prospective cohort study. Am. J. Gastroenterol. 2020, 115, 746–755. [Google Scholar] [CrossRef]
- Mönnikes, H. Quality of life in patients with irritable bowel syndrome. J. Clin. Gastroenterol. 2011, 45, S98–S101. [Google Scholar] [CrossRef]
- Levy, R.L.; Von Korff, M.; Whitehead, W.E.; Stang, P.; Saunders, K.; Jhingran, P.; Barghout, V.; Feld, A.D. Costs of care for irritable bowel syndrome patients in a health maintenance organization. Am. J. Gastroenterol. 2001, 96, 3122–3129. [Google Scholar] [CrossRef]
- Doshi, J.A.; Cai, Q.; Buono, J.L.; Spalding, W.M.; Sarocco, P.; Tan, H.; Stephenson, J.J.; Carson, R.T. Economic burden of irritable bowel syndrome with constipation: A retrospective analysis of health care costs in a commercially insured population. J. Manag. Care Spec. Pharm. 2014, 20, 382–390. [Google Scholar] [CrossRef]
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.-Y.; Fang, X.-C.; Li, X.-Q.; Fei, G.-J. Ethnic differences in genetic polymorphism associated with irritable bowel syndrome. World J. Gastroenterol. 2020, 26, 2049–2063. [Google Scholar] [CrossRef]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Barbara, G.; Grover, M.; Bercik, P.; Corsetti, M.; Ghoshal, U.C.; Ohman, L.; Rajilić-Stojanović, M. Rome foundation working team report on post-infection irritable bowel syndrome. Gastroenterology 2019, 156, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter dysfunction in irritable bowel syndrome: Emerging approaches for management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef] [PubMed]
- Mihindukulasuriya, K.A.; Mars, R.A.T.; Johnson, A.J.; Ward, T.; Priya, S.; Lekatz, H.R.; Kalari, K.R.; Droit, L.; Zheng, T.; Blekhman, R.; et al. Multi-omics analyses show disease, diet, and transcriptome interactions with the virome. Gastroenterology 2021. [Google Scholar] [CrossRef]
- Mars, R.A.T.; Yang, Y.; Ward, T.; Houtti, M.; Priya, S.; Lekatz, H.R.; Tang, X.; Sun, Z.; Kalari, K.R.; Korem, T.; et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 2020, 182, 1460–1473. [Google Scholar] [CrossRef] [PubMed]
- Putignani, L.; Del Chierico, F.; Vernocchi, P.; Cicala, M.; Cucchiara, S.; Dallapiccola, B. Gut Microbiota Dysbiosis as risk and premorbid factors of IBD and IBS along the childhood-adulthood transition. Inflamm. Bowel Dis. 2016, 22, 487–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishima, Y.; Ishihara, S. Molecular mechanisms of microbiota-mediated pathology in irritable bowel syndrome. Int. J. Mol. Sci. 2020, 21, 8664. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hellström, P.M. Pathophysiology of the irritable bowel syndrome—Reflections of today. Best Pract. Res. Clin. Gastroenterol. 2019, 40, 101620. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Bruta, K.; Vanshika; Bhasin, K.; Bhawana. The role of serotonin and diet in the prevalence of irritable bowel syndrome: A systematic review. Transl. Med. Commun. 2021, 6, 1. [Google Scholar] [CrossRef]
- Crowell, M.D. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br. J. Pharmacol. 2004, 141, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Brandt, L.J.; Young, C.; Chey, W.D.; Foxx-Orenstein, A.E.; Moayyedi, P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: Systematic review and meta-analysis. Am. J. Gastroenterol. 2009, 104, 1831–1843. [Google Scholar] [CrossRef]
- Chang, L.; Di Lorenzo, C.; Farrugia, G.; Hamilton, F.A.; Mawe, G.M.; Pasricha, P.J.; Wiley, J.W. Functional bowel disorders: A roadmap to guide the next generation of research. Gastroenterology 2018, 154, 723–735. [Google Scholar] [CrossRef]
- Qin, H.-Y.; Cheng, C.-W.; Tang, X.-D.; Bian, Z.-X. Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 14126–14131. [Google Scholar] [CrossRef]
- Barbara, G. IBS: Biomarkers for IBS: Ready for prime time? Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 9–10. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Evangelista, S. Experimental models of irritable bowel syndrome and the role of the enteric neurotransmission. J. Clin. Med. 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.-Y.; Wu, J.C.Y.; Tong, X.-D.; Sung, J.J.Y.; Xu, H.-X.; Bian, Z.-X. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J. Gastroenterol. 2011, 46, 164–174. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Chang, C.; Lin, H. Dysbiosis in gastrointestinal disorders. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 3–15. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Mu, A.; Haslam, N.; Schwartz, O.S.; Simmons, J.G. Feeling down? A systematic review of the gut microbiota in anxiety/depression and irritable bowel syndrome. J. Affect. Disord. 2020, 266, 429–446. [Google Scholar] [CrossRef]
- Amato, K.R.; Arrieta, M.-C.; Azad, M.B.; Bailey, M.T.; Broussard, J.L.; Bruggeling, C.E.; Claud, E.C.; Costello, E.K.; Davenport, E.R.; Dutilh, B.E.; et al. The human gut microbiome and health inequities. Proc. Natl. Acad. Sci. USA 2021, 118, e2017947118. [Google Scholar] [CrossRef]
- Integrative HMP (iHMP); Proctor, L.M.; Creasy, H.H.; Fettweis, J.M.; Lloyd-Price, J.; Mahurkar, A.; Zhou, W.; Buck, G.A.; Snyder, M.P.; Strauss, J.F., III; et al. Research network consortium the integrative human microbiome project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The gut-brain axis and the microbiome: Mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.; Menees, S. The gut microbiome and irritable bowel syndrome. F1000Research 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, A.E.; Singh, R.; Ayoub, Y.K.; Khairy, A.M.; Mullin, G.E. The gut microbiome and irritable bowel syndrome: State of art review. Arab J. Gastroenterol. 2018, 19, 136–141. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. In Proceedings of the Nutrition Society; Cambridge University Press: Cambridge, UK, 2016; Volume 75, pp. 306–318. [Google Scholar]
- Enck, P.; Mazurak, N. Microbiota and irritable bowel syndrome: A critical inventory. Z. Gastroenterol. 2019, 57, 859–870. [Google Scholar] [CrossRef]
- Collins, S.M. A role for the gut microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Valentina, C.; Fabio, P. Gut microbiota, dysbiosis and colon lavage. Dig. Liver Dis. 2019, 51, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiong, L.; Li, L.; Li, M.; Chen, M. Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2017, 32, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Rodiño-Janeiro, B.K.; Vicario, M.; Alonso-Cotoner, C.; Pascua-García, R.; Santos, J. A review of microbiota and irritable bowel syndrome: Future in therapies. Adv. Ther. 2018, 35, 289–310. [Google Scholar] [CrossRef] [Green Version]
- Duan, R.; Zhu, S.; Wang, B.; Duan, L. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing. Clin. Transl. Gastroenterol. 2019, 10, e00012. [Google Scholar] [CrossRef]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Song, Y.; Chaudhary, R.; Mullin, G.E. Gut microbial dysbiosis in the irritable bowel syndrome: A systematic review and meta-analysis of case-control studies. J. Acad. Nutr. Diet. 2020, 120, 565–586. [Google Scholar] [CrossRef] [Green Version]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut microbiota in patients with irritable bowel syndrome-A systematic review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, M.; Lang, M.; Holley, H.; Crepaz, D.; Hausmann, B.; Pjevac, P.; Moser, D.; Haller, F.; Hof, F.; Beer, A.; et al. Mucosal biofilms are an endoscopic feature of irritable bowel syndrome and ulcerative colitis. Gastroenterology 2021. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Qin, X.; Huang, S.; Wang, B.; Cao, H. The potential role of gut mycobiome in irritable bowel syndrome. Front. Microbiol. 2019, 10, 1894. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic review with meta-analysis: The prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Koloski, N.A.; Jones, M.; Talley, N.J. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: A 1-year population-based prospective study. Aliment. Pharmacol. Ther. 2016, 44, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Sibelli, A.; Chalder, T.; Everitt, H.; Workman, P.; Windgassen, S.; Moss-Morris, R. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol. Med. 2016, 46, 3065–3080. [Google Scholar] [CrossRef] [PubMed]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Kułak-Bejda, A.; Bejda, G.; Waszkiewicz, N. Antidepressants for irritable bowel syndrome—A systematic review. Pharmacol. Rep. 2017, 69, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Pachnis, V. The effect of microbiota and the immune system on the development and Organization of the Enteric Nervous System. Gastroenterology 2016, 151, 836–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Spichak, S.; Guzzetta, K.E.; O’Leary, O.F.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Without a bug’s life: Germ-free rodents to interrogate microbiota-gut-neuroimmune interactions. Drug Discov. Today Dis. Model. 2018, 28, 79–93. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Toth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [Green Version]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boziki, M.K.; Kesidou, E.; Theotokis, P.; Mentis, A.-F.A.; Karafoulidou, E.; Melnikov, M.; Sviridova, A.; Rogovski, V.; Boyko, A.; Grigoriadis, N. Microbiome in multiple sclerosis: Where are we, what we know and do not know. Brain Sci. 2020, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Liao, J.; Liu, X.; Zhong, Y.; Cai, X.; Long, L. Review: The role of intestinal dysbiosis in Parkinson’s disease. Front. Cell. Infect. Microbiol. 2021, 11, 615075. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Kazmi, S.A.; Jameson, K.G.; Hsiao, E.Y. The microbiome as a modifier of neurodegenerative disease risk. Cell Host Microbe 2020, 28, 201–222. [Google Scholar] [CrossRef]
- Sasmita, A.O. Modification of the gut microbiome to combat neurodegeneration. Rev. Neurosci. 2019, 30, 795–805. [Google Scholar] [CrossRef]
- König, J.; Brummer, R.J. Faecal microbiota transplantation in IBS—New evidence for success? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical guideline: Management of irritable bowel syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lembo, A.; Chey, W.D.; Zakko, S.; Ringel, Y.; Yu, J.; Mareya, S.M.; Shaw, A.L.; Bortey, E.; Forbes, W.P.; et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 2011, 364, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C.; Rainey, J.F.; Szurszewski, J.H.; et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Cremon, C.; Carini, G.; Wang, B.; Vasina, V.; Cogliandro, R.F.; De Giorgio, R.; Stanghellini, V.; Grundy, D.; Tonini, M.; De Ponti, F.; et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am. J. Gastroenterol. 2011, 106, 1290–1298. [Google Scholar] [CrossRef]
- Gershon, M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roshchina, V.V. Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells. In Microbial Endocrinology; Springer: New York, NY, USA, 2010; pp. 17–52. [Google Scholar]
- Mandić, A.D.; Woting, A.; Jaenicke, T.; Sander, A.; Sabrowski, W.; Rolle-Kampcyk, U.; von Bergen, M.; Blaut, M. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci. Rep. 2019, 9, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021, 12, 3597–3610. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef]
- Luqman, A.; Nega, M.; Nguyen, M.-T.; Ebner, P.; Götz, F. SadA-Expressing Staphylococci in the human gut show increased cell adherence and internalization. Cell Rep. 2018, 22, 535–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nzakizwanayo, J.; Dedi, C.; Standen, G.; Macfarlane, W.M.; Patel, B.A.; Jones, B.V. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci. Rep. 2015, 5, 17324. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef]
- Singhal, M.; Turturice, B.A.; Manzella, C.R.; Ranjan, R.; Metwally, A.A.; Theorell, J.; Huang, Y.; Alrefai, W.A.; Dudeja, P.K.; Finn, P.W.; et al. Serotonin transporter deficiency is associated with dysbiosis and changes in metabolic function of the mouse intestinal microbiome. Sci. Rep. 2019, 9, 2138. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.-E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef] [Green Version]
- Modasia, A.; Parker, A.; Jones, E.; Stentz, R.; Brion, A.; Goldson, A.; Defernez, M.; Wileman, T.; Ashley Blackshaw, L.; Carding, S.R. Regulation of enteroendocrine cell networks by the major human gut symbiont Bacteroides thetaiotaomicron. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luck, B.; Visuthranukul, C.; Ihekweazu, F.D.; Engevik, A.C.; Shi, Z.; Danhof, H.A.; Chang-Graham, A.L.; Hall, A.; Endres, B.T.; et al. Human-derived Bifidobacterium dentium modulates the mammalian serotonergic system and gut–brain axis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Ranuh, R.; Athiyyah, A.F.; Darma, A.; Risky, V.P.; Riawan, W.; Surono, I.S.; Sudarmo, S.M. Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: Role of intestinal microbiota on the gut-brain axis. Iran J. Microbiol. 2019, 11, 145–150. [Google Scholar] [CrossRef]
- Chen, C.-M.; Wu, C.-C.; Huang, C.-L.; Chang, M.-Y.; Cheng, S.-H.; Lin, C.-T.; Tsai, Y.-C. Lactobacillus plantarum PS128 promotes intestinal motility, mucin production, and serotonin signaling in mice. Probiotics Antimicrob. Proteins 2021, 1–11. [Google Scholar] [CrossRef]
- Cao, Y.-N.; Feng, L.-J.; Liu, Y.-Y.; Jiang, K.; Zhang, M.-J.; Gu, Y.-X.; Wang, B.-M.; Gao, J.; Wang, Z.-L.; Wang, Y.-M. Effect of Lactobacillus rhamnosus GG supernatant on serotonin transporter expression in rats with post-infectious irritable bowel syndrome. World J. Gastroenterol. 2018, 24, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Wheatcroft, J.; Wakelin, D.; Smith, A.; Mahoney, C.R.; Mawe, G.; Spiller, R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol. Motil. 2005, 17, 863–870. [Google Scholar] [CrossRef]
- Cao, Y.-N.; Feng, L.-J.; Wang, B.-M.; Jiang, K.; Li, S.; Xu, X.; Wang, W.-Q.; Zhao, J.-W.; Wang, Y.-M. Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi J. Gastroenterol. 2018, 24, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tatsuoka, M.; Osaki, Y.; Ohsaka, F.; Tsuruta, T.; Kadota, Y.; Tochio, T.; Hino, S.; Morita, T.; Sonoyama, K. Consumption of indigestible saccharides and administration of Bifidobacterium pseudolongum reduce mucosal serotonin in murine colonic mucosa. Br. J. Nutr. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Uribe, A.; Alam, M.; Johansson, O.; Midtvedt, T.; Theodorsson, E. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology 1994, 107, 1259–1269. [Google Scholar] [CrossRef]
- Yang, M.; Fukui, H.; Eda, H.; Kitayama, Y.; Hara, K.; Kodani, M.; Tomita, T.; Oshima, T.; Watari, J.; Miwa, H. Involvement of gut microbiota in the association between gastrointestinal motility and 5-HT expression/M2 macrophage abundance in the gastrointestinal tract. Mol. Med. Rep. 2017, 16, 3482–3488. [Google Scholar] [CrossRef] [Green Version]
- Swami, T.; Weber, H.C. Updates on the biology of serotonin and tryptophan hydroxylase. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 12–21. [Google Scholar] [CrossRef]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The ever-changing roles of serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Beaulieu, J.-M.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 2004, 305, 217. [Google Scholar] [CrossRef] [PubMed]
- McKinney, J.; Knappskog, P.M.; Haavik, J. Different properties of the central and peripheral forms of human tryptophan hydroxylase. J. Neurochem. 2005, 92, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Kohen, R.; Cain, K.C.; Jarrett, M.E.; Heitkemper, M.M. Associations of tryptophan hydroxylase gene polymorphisms with irritable bowel syndrome. Neurogastroenterol. Motil. 2011, 23, 233-e116. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.-E.; Kohen, R.; Cain, K.C.; Jarrett, M.E.; Heitkemper, M.M. TPH gene polymorphisms are associated with disease perception and quality of life in women with irritable bowel syndrome. Biol. Res. Nurs. 2014, 16, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Katsumata, R.; Shiotani, A.; Murao, T.; Ishii, M.; Fujita, M.; Matsumoto, H.; Haruma, K. The TPH1 rs211105 gene polymorphism affects abdominal symptoms and quality of life of diarrhea-predominant irritable bowel syndrome. J. Clin. Biochem. Nutr. 2018, 62, 270–276. [Google Scholar] [CrossRef]
- Grasberger, H.; Chang, L.; Shih, W.; Presson, A.P.; Sayuk, G.S.; Newberry, R.D.; Karagiannides, I.; Pothoulakis, C.; Mayer, E.; Merchant, J.L. Identification of a functional TPH1 polymorphism associated with irritable bowel syndrome bowel habit subtypes. Am. J. Gastroenterol. 2013, 108, 1766–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerckhoffs, A.P.M.; ter Linde, J.J.M.; Akkermans, L.M.A.; Samsom, M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1053–G1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiotani, A.; Kusunoki, H.; Ishii, M.; Imamura, H.; Manabe, N.; Kamada, T.; Hata, J.; Merchant, J.L.; Haruma, K. Pilot study of Biomarkers for predicting effectiveness of ramosetron in diarrhea-predominant irritable bowel syndrome: Expression of S100A10 and polymorphisms of TPH1. Neurogastroenterol. Motil. 2015, 27, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, D.-C.; Cao, H.-L.; Xu, M.-Q.; Wang, S.-N.; Wang, Y.-M.; Yan, F.; Wang, B.-M. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J. Gastroenterol. 2016, 22, 8137–8148. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Ishihara, S.; Kawashima, K.; Fukuba, N.; Sonoyama, H.; Kusunoki, R.; Oka, A.; Mishima, Y.; Oshima, N.; Moriyama, I.; et al. Downregulation of serotonin reuptake transporter gene expression in healing colonic mucosa in presence of remaining low-grade inflammation in ulcerative colitis. J. Gastroenterol. Hepatol. 2016, 31, 1443–1452. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, S.; Meng, Y.; Feng, Y.; Wang, Y.; Wang, E.; Pan, X.; Zhu, R.; Fan, H.; Pang, S.; et al. Female serotonin transporter-knockout rat: A potential model of irritable bowel syndrome. FASEB J. 2021, 35, e21701. [Google Scholar] [CrossRef]
- Coates, M.D.; Mahoney, C.R.; Linden, D.R.; Sampson, J.E.; Chen, J.; Blaszyk, H.; Crowell, M.D.; Sharkey, K.A.; Gershon, M.D.; Mawe, G.M. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004, 126, 1657–1664. [Google Scholar] [CrossRef]
- Del Colle, A.; Israelyan, N.; Gross Margolis, K. Novel aspects of enteric serotonergic signaling in health and brain-gut disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G130–G143. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, D.; Martin, G. 5-HT receptor classification and nomenclature: Towards a harmonization with the human genome. Neuropharmacology 1997, 36, 419–428. [Google Scholar] [CrossRef]
- Gershon, M.D. Review article: Serotonin receptors and transporters—Roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 2004, 20, 3–14. [Google Scholar] [CrossRef]
- Binienda, A.; Storr, M.; Fichna, J.; Salaga, M. Efficacy and safety of serotonin receptor ligands in the treatment of irritable bowel syndrome: A review. Curr. Drug Targets 2018, 19, 1774–1781. [Google Scholar] [CrossRef]
- De Vadder, F.; Grasset, E.; Mannerås Holm, L.; Karsenty, G.; Macpherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe 2018, 23, 775–785.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, C.M.; Bassler, B.L. QUORUM SENSING: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlig, F.; Grundy, L.; Garcia-Caraballo, S.; Brierley, S.M.; Foster, S.J.; Grundy, D. Identification of a quorum sensing-dependent communication pathway mediating bacteria-gut-brain cross talk. iScience 2020, 23, 101695. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.-S.; He, S.-H.; Zheng, P.-Y.; Wu, L.; Yang, P.-C. Mast cells play a crucial role in Staphylococcus aureus peptidoglycan-induced diarrhea. Am. J. Pathol. 2007, 171, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Knecht, L.D.; O’Connor, G.; Mittal, R.; Liu, X.Z.; Daftarian, P.; Deo, S.K.; Daunert, S. Serotonin activates bacterial quorum sensing and enhances the virulence of Pseudomonas aeruginosa in the host. EBioMedicine 2016, 9, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Terry, N.; Margolis, K.G. Serotonergic mechanisms regulating the GI tract: Experimental evidence and therapeutic relevance. Handb. Exp. Pharmacol. 2017, 239, 319–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, M.; Chambers, J.D.; Gwynne, R.M.; Bornstein, J.C. Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am. J. Physiol. Liver Physiol. 2013, 304, G749–G761. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Chalazonitis, A.; Huang, Y.-Y.; Mann, J.J.; Margolis, K.G.; Yang, Q.M.; Kim, D.O.; Cote, F.; Mallet, J.; Gershon, M.D. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 2011, 31, 8998–9009. [Google Scholar] [CrossRef]
- Margolis, K.G.; Li, Z.; Stevanovic, K.; Saurman, V.; Israelyan, N.; Anderson, G.M.; Snyder, I.; Veenstra-VanderWeele, J.; Blakely, R.D.; Gershon, M.D. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J. Clin. Invest. 2016, 126, 2221–2235. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.M.; Young, R.L.; Leong, L.; Rogers, G.B.; Spencer, N.J.; Jessup, C.F.; Keating, D.J. The diverse metabolic roles of peripheral serotonin. Endocrinology 2017, 158, 1049–1063. [Google Scholar] [CrossRef]
- Miyata, K.; Ito, H.; Fukudo, S. Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. Am. J. Physiol. 1998, 274, G827–G831. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.; Ding, C.; Zhao, W.; Xu, L.; Tian, H.; Gong, J.; Zhu, M.; Li, J.; Li, N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waclawiková, B.; Bullock, A.; Schwalbe, M.; Aranzamendi, C.; Nelemans, S.A.; van Dijk, G.; El Aidy, S. Gut bacteria-derived 5-hydroxyindole is a potent stimulant of intestinal motility via its action on L-type calcium channels. PLoS Biol. 2021, 19, e3001070. [Google Scholar] [CrossRef] [PubMed]
- Golubeva, A.V.; Joyce, S.A.; Moloney, G.; Burokas, A.; Sherwin, E.; Arboleya, S.; Flynn, I.; Khochanskiy, D.; Moya-Pérez, A.; Peterson, V.; et al. Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine 2017, 24, 166–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaki, S.; Mitsui, R.; Hayashi, H.; Kato, I.; Sugiya, H.; Iwanaga, T.; Furness, J.B.; Kuwahara, A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006, 324, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar] [CrossRef] [Green Version]
- McVey Neufeld, K.A.; Mao, Y.K.; Bienenstock, J.; Foster, J.A.; Kunze, W.A. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013, 25, 183-e88. [Google Scholar] [CrossRef]
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Microbiota regulates visceral pain in the mouse. eLife 2017, 6, e25887. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.-H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Thiel, I.A.M.; Botschuijver, S.; de Jonge, W.J.; Seppen, J. Painful interactions: Microbial compounds and visceral pain. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165534. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [Green Version]
- Crouzet, L.; Gaultier, E.; Del’Homme, C.; Cartier, C.; Delmas, E.; Dapoigny, M.; Fioramonti, J.; Bernalier-Donadille, A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol. Motil. 2013, 25, e272–e282. [Google Scholar] [CrossRef]
- Browning, K.N. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front. Neurosci. 2015, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Cangemi, D.J.; Lacy, B.E. Management of irritable bowel syndrome with diarrhea: A review of nonpharmacological and pharmacological interventions. Therap. Adv. Gastroenterol. 2019, 12, 1756284819878950. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. Role of serotonin 5-HT3 receptors in intestinal inflammation. Biol. Pharm. Bull. 2013, 36, 1406–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Lasch, K.; Zhou, W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. AJP Gastrointest. Liver Physiol. 2012, 303, G775–G785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keszthelyi, D.; Troost, F.J.; Jonkers, D.M.; van Eijk, H.M.; Lindsey, P.J.; Dekker, J.; Buurman, W.A.; Masclee, A.A.M. Serotonergic reinforcement of intestinal barrier function is impaired in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2014, 40, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Shajib, M.S.; Khan, W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Herr, N.; Bode, C.; Duerschmied, D. The effects of serotonin in immune cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Ghia, J.; Li, N.; Wang, H.; Collins, M.; Deng, Y.; El–Sharkawy, R.T.; Côté, F.; Mallet, J.; Khan, W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 2009, 137, 1649–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Ghia, J.-E.; Wang, H.; McClemens, J.; Cote, F.; Suehiro, Y.; Mallet, J.; Khan, W.I. Serotonin activates dendritic cell function in the context of gut inflammation. Am. J. Pathol. 2011, 178, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, S.C.; Mailer, R.; Pabst, O.; Weier, G.; Sedlik, W.; Li, Z.; Chen, J.J.; Murphy, D.L.; Gershon, M.D. Role of serotonin in intestinal inflammation: Knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol. Liver Physiol. 2009, 296, G685–G695. [Google Scholar] [CrossRef]
- Margolis, K.G.; Stevanovic, K.; Li, Z.; Yang, Q.M.; Oravecz, T.; Zambrowicz, B.; Jhaver, K.G.; Diacou, A.; Gershon, M.D. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 2014, 63, 928–937. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 139–148. [Google Scholar] [CrossRef]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Lim, D.Y.; Yeo, W.-S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, S.; Tada, Y.; Fukuba, N.; Oka, A.; Kusunoki, R.; Mishima, Y.; Oshima, N.; Moriyama, I.; Yuki, T.; Kawashima, K.; et al. Pathogenesis of irritable bowel syndrome—Review regarding associated infection and immune activation. Digestion 2013, 87, 204–211. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Hou, X. Mast cells and irritable bowel syndrome: From the bench to the bedside. J. Neurogastroenterol. Motil. 2016, 22, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.J.; Sharkey, K.A.; McKay, D.M. Modulation of the immune response by helminths: A role for serotonin? Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, K.; Lo, M.W.; Hosoya, T.; Tashima, K.; Takayama, H.; Murayama, T.; Horie, S. Experimental colitis alters expression of 5-HT receptors and transient receptor potential vanilloid 1 leading to visceral hypersensitivity in mice. Lab. Investig. 2012, 92, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Coldwell, J.R.; Phillis, B.D.; Sutherland, K.; Howarth, G.S.; Blackshaw, L.A. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J. Physiol. 2007, 579, 203–213. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Qian, M.; He, N.; Cao, Y.; Liu, Y.; Wu, K.; He, S. The comprehensive pathophysiological changes in a novel rat model of postinflammatory visceral hypersensitivity. FASEB J. 2019, 33, 13560–13571. [Google Scholar] [CrossRef] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; de Roos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Charnay, Y.; Léger, L. Brain serotonergic circuitries. Dialogues Clin. Neurosci. 2010, 12, 471–487. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Nutt, D. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef] [Green Version]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Franke, L.; Schewe, H.-J.; Uebelhack, R.; Berghöfer, A.; Müller-Oerlinghausen, B. Platelet-5HT uptake and gastrointestinal symptoms in patients suffering from major depression. Life Sci. 2003, 74, 521–531. [Google Scholar] [CrossRef]
- Martin, C.B.; Martin, V.S.; Trigo, J.M.; Chevarin, C.; Maldonado, R.; Fink, L.H.; Cunningham, K.A.; Hamon, M.; Lanfumey, L.; Mongeau, R. 5-HT2C Receptor desensitization moderates anxiety in 5-HTT deficient mice: From behavioral to cellular evidence. Int. J. Neuropsychopharmacol. 2015, 18, pyu056. [Google Scholar] [CrossRef] [Green Version]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.-L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Mathee, K.; Cickovski, T.; Deoraj, A.; Stollstorff, M.; Narasimhan, G. The gut microbiome and neuropsychiatric disorders: Implications for attention deficit hyperactivity disorder (ADHD). J. Med. Microbiol. 2020, 69, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-F.; Zhu, Y.-L.; Zhou, Z.-L.; Jia, X.-B.; Xu, Y.-D.; Yang, Q.; Cui, C.; Shen, Y.-Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Dekel, R.; Drossman, D.A.; Sperber, A.D. The use of psychotropic drugs in irritable bowel syndrome. Expert Opin. Investig. Drugs 2013, 22, 329–339. [Google Scholar] [CrossRef] [PubMed]
- North, C.S.; Hong, B.A.; Alpers, D.H. Relationship of functional gastrointestinal disorders and psychiatric disorders: Implications for treatment. World J. Gastroenterol. 2007, 13, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Yabut, J.M.; Choo, J.M.; Page, A.J.; Sun, E.W.; Jessup, C.F.; Wesselingh, S.L.; Khan, W.I.; Rogers, G.B.; Steinberg, G.R.; et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc. Natl. Acad. Sci. USA 2019, 116, 19802–19804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Niu, K.; Momma, H.; Kobayashi, Y.; Chujo, M.; Otomo, A.; Fukudo, S.; Nagatomi, R. Irritable bowel syndrome is positively related to metabolic syndrome: A population-based cross-sectional study. PLoS ONE 2014, 9, e112289. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.A.; Baffy, N. Modulation of the gut microbiota: A focus on treatments for irritable bowel syndrome. Postgrad. Med. 2017, 129, 872–888. [Google Scholar] [CrossRef] [PubMed]

| Upregulation of 5-HT in Microbiota | Mechanisms of Action and Observations | Ref. |
| Akkermansia muciniphila (Amuc_1100) | Promote intestinal 5-HT biosynthesis and extracellular availability through TLR2 signaling. | [88] |
| Akkermansiamuciniphila (extracellular vesicles) | Increase expression of the Htr4 gene, and decreases that of the Htr2B, Htr3B, and Htr7 genes. | [89] |
| Bacteriodes thetaiotaomicron | Restore 5-HT+ EC cells and shape EC networks in the GI tract of GF mice by producing SCFAs. | [96] |
| Bifidobacterium dentium | Increase intestinal 5-HT level, expressions of 5-HTra receptors 2a and 4, and SERT by producing acetate. | [97] |
| Clostridium ramosum | Promote 5-HT synthesis in colonic EC cells and program differentiation of intestinal stem progenitors toward a secretory 5-HT-producing lineage. | [85,87] |
| Corynebacterium spp., Enterococcus spp., Streptococcus spp. | Enable the direct production of 5-HT. | [86] |
| Escherichia coli Nissle 1917 | Enhance 5-HT bioavailability in ileal tissue through interaction with compounds secreted from host tissue. | [91] |
| Indigenous spore-forming bacteria | Enhance colonic 5-HT pathway by upregulation of Htr4. | [87] |
| Lactobacillus plantarum IS-10506 | Increase gut 5-HT production along with brain 5-HTT, neurotrophin, and brain-derived neurotrophic factor. | [98] |
| Lactobacillus plantarum PS128 | Increase 5-HT+ cells in the gut and alter expression levels of Tph1, Chga, Slc6a4, and Htr4. | [99] |
| SadA-expressing Staphylococci | Promote converting 5-HTP into 5-HT. | [90] |
| Trichinella spiralis and Campylobacter jejuni (pathogens) | Increase EC cell number and reduce SERT expression. | [100,101] |
| Downregulation of 5-HT in Microbiota | Mechanisms of action and observations | Ref. |
| Bifidobacterium longum and Lactobacillus acidophilus | Upregulate SERT expression. | [102] |
| Bifidobacterium pseudolongum | Diminish EC cells. | [103] |
| Lactobacillus rhamnosus | Upregulate gene and protein level of SERT. | [92,100] |
| 5-HT Pathway-Induced Specific Dysbiosis | Mechanisms of Action and Observations | Ref. |
|---|---|---|
| Turicibacter sanguinis | Reduce sporulation factors and membrane transporters by 5-HT supplementation and in SERT−/− mice. | [93] |
| Bacilli spp. (Lactobacillus, Streptococcus, Enterococcus, and Listeria) | Increase in SERT−/− mice. | [94] |
| Bifidobacterium spp. | Decrease in SERT−/− mice. | [94] |
| Akkermansia muciniphila | Increase in Tph1–/– mice and decrease in SERT−/− mice. | [94,95] |
| Two distinct Bacteroidales OTUs | Decrease in Tph1–/– mice. | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishima, Y.; Ishihara, S. Enteric Microbiota-Mediated Serotonergic Signaling in Pathogenesis of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2021, 22, 10235. https://doi.org/10.3390/ijms221910235
Mishima Y, Ishihara S. Enteric Microbiota-Mediated Serotonergic Signaling in Pathogenesis of Irritable Bowel Syndrome. International Journal of Molecular Sciences. 2021; 22(19):10235. https://doi.org/10.3390/ijms221910235
Chicago/Turabian StyleMishima, Yoshiyuki, and Shunji Ishihara. 2021. "Enteric Microbiota-Mediated Serotonergic Signaling in Pathogenesis of Irritable Bowel Syndrome" International Journal of Molecular Sciences 22, no. 19: 10235. https://doi.org/10.3390/ijms221910235
APA StyleMishima, Y., & Ishihara, S. (2021). Enteric Microbiota-Mediated Serotonergic Signaling in Pathogenesis of Irritable Bowel Syndrome. International Journal of Molecular Sciences, 22(19), 10235. https://doi.org/10.3390/ijms221910235






