Active Targeted Nanoparticles for Delivery of Poly(ADP-ribose) Polymerase (PARP) Inhibitors: A Preliminary Review
Abstract
:1. Introduction
2. PARP Inhibitors
2.1. Classification
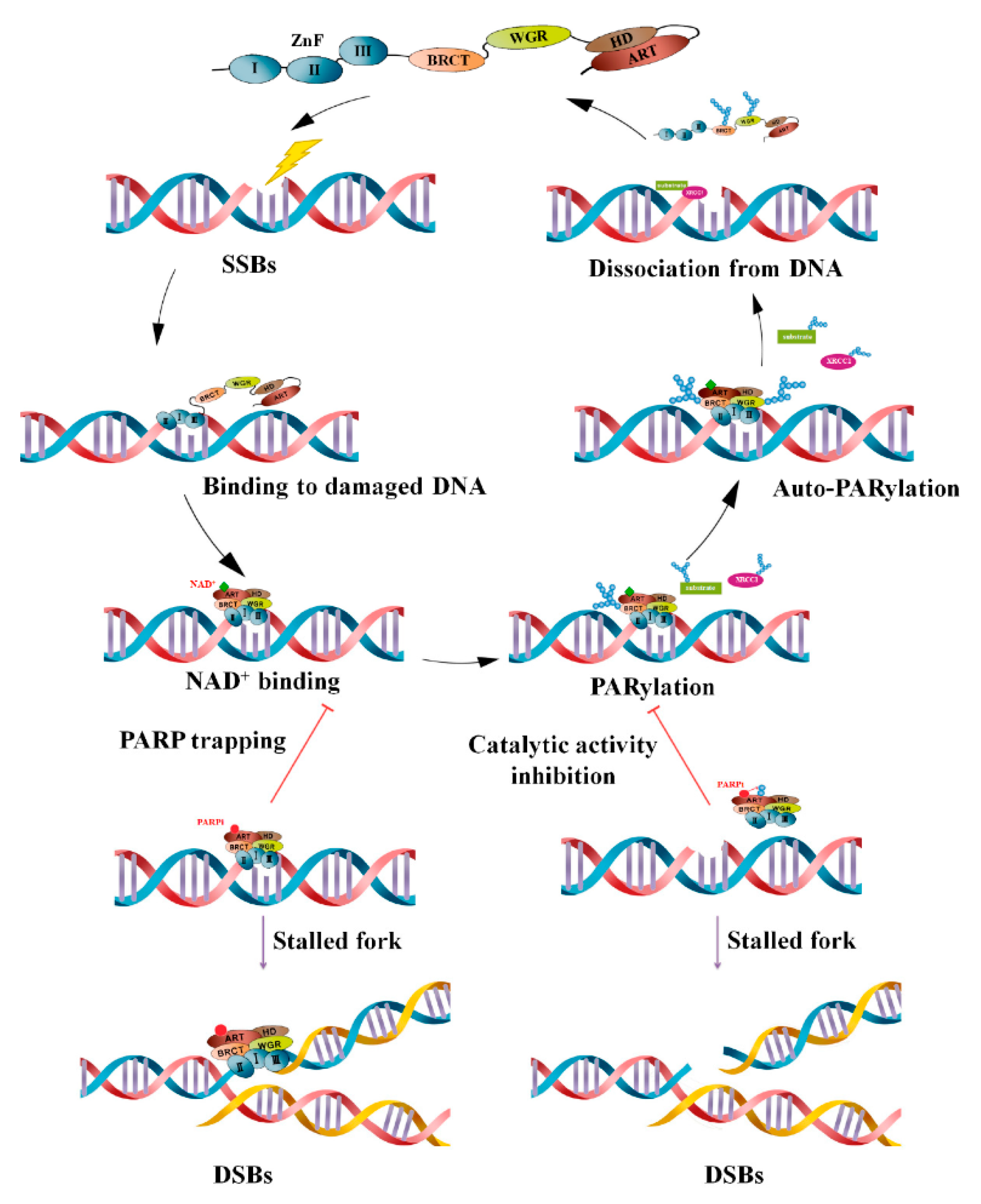
2.2. Molecular Mechanism of Action and Role of NPs
2.3. Clinical Efficacy
3. Nanoformulations for Delivery of PARP Inhibitors to Cancer Cells
3.1. Liposomes
3.2. Polymeric NPs
3.2.1. Poly-(d,l-Lactide-Co-Glycolide) (PLGA)
3.2.2. Methoxy Poly(ethylene glycol)-poly(ε-caprolactone) (MPEG-PCL)
3.2.3. Poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCEC)
3.2.4. Pluronic F127
3.3. Hybrid Nanosystems
3.3.1. Superparamagnetic Iron Oxide (IO)—Hyaluronic Acid (HA)
3.3.2. Metal-Organic Frameworks (MOFs)—PEG
3.4. Self-Assembled NPs
3.4.1. Amphiphilic Peptides
3.4.2. Poloxamer Micelles
3.4.3. Tannic Acid-Docetaxel Self-Assemblies (DSAs)
3.5. Novel Nanosystems
3.5.1. Protein-Based Nanovehicle
3.5.2. Betacaryophyllene (BCP) Carrier
3.5.3. Lipids and Cholesterol Nanoemulsion
3.5.4. Nano-SiO2
3.5.5. (IV) BZP NPs
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current challenges in cancer treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aferni, A.E.; Guettari, M.; Tajouri, T.; Rahdar, A. The confinement of PVP in AOT microemulsions: Effect of water content and PVP concentration regime on electrical percolation phenomenon. J. Mol. Liq. 2020, 318, 114012. [Google Scholar] [CrossRef]
- Arshad, R.; Pal, K.; Sabir, F.; Rahdar, A.; Bilal, M.; Shahnaz, G.; Kyzas, G.Z. A review of the nanomaterials use for the diagnosis and therapy of salmonella typhi. J. Mol. Struct. 2021, 1230, 129928. [Google Scholar] [CrossRef]
- Hakami, T.M.; Davarpanah, A.; Rahdar, A.; Barrett, S. Structural and magnetic study and cytotoxicity evaluation of tetra-metallic nanoparticles of Co0. 5Ni0. 5CrxFe2-xO4 prepared by co-precipitation. J. Mol. Struct. 2018, 1165, 344–348. [Google Scholar] [CrossRef]
- Hasanein, P.; Rahdar, A.; Bahabadi, S.E.; Kumar, A.; Kyzas, G.Z. Manganese/cerium nanoferrites: Synthesis and toxicological effects by intraperitoneal administration in rats. Inorg. Chem. Commun. 2021, 125, 108433. [Google Scholar] [CrossRef]
- Heydari, M.; Yousefi, A.R.; Rahdar, A.; Nikfarjam, N.; Jamshidi, K.; Bilal, M.; Taboada, P. Microemulsions of tribenuron-methyl using Pluronic F127: Physico-chemical characterization and efficiency on wheat weed. J. Mol. Liq. 2021, 326, 115263. [Google Scholar] [CrossRef]
- Mohammadi, L.; Pal, K.; Bilal, M.; Rahdar, A.; Fytianos, G.; Kyzas, G.Z. Green nanoparticles to treat patients from Malaria disease: An overview. J. Mol. Struct. 2021, 129857. [Google Scholar] [CrossRef]
- Nikazar, S.; Sivasankarapillai, V.S.; Rahdar, A.; Gasmi, S.; Anumol, P.; Shanavas, M.S. Revisiting the cytotoxicity of quantum dots: An in-depth overview. Biophys. Rev. 2020, 12, 703–718. [Google Scholar] [CrossRef]
- Rahdar, A.; Aliahmad, M.; Samani, M.; HeidariMajd, M.; Susan, M.A.B.H. Synthesis and characterization of highly efficacious Fe-doped ceria nanoparticles for cytotoxic and antifungal activity. Ceramics Int. 2019, 45, 7950–7955. [Google Scholar] [CrossRef]
- Zou, Q.; Xing, P.; Wei, L.; Liu, B. Gene2vec: Gene subsequence embedding for prediction of mammalian N6-methyladenosine sites from mRNA. RNA 2019, 25, 205–218. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhou, X. A CRISPR-based and Post-amplification Coupled SARS-CoV-2 Detection with a Portable Evanescent Wave Biosensor. Biosens. Bioelectron. 2021, 190, 113418. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, L.; Sun, Y. Nanotechnology applied to overcome tumor drug resistance. J. Control. Release 2012, 162, 45–55. [Google Scholar] [CrossRef]
- Wang, X.-F.; Gao, P.; Liu, Y.-F.; Li, H.-F.; Lu, F. Predicting thermophilic proteins by machine learning. Curr. Bioinform. 2020, 15, 493–502. [Google Scholar] [CrossRef]
- Niu, M.; Lin, Y.; Zou, Q. sgRNACNN: Identifying sgRNA on-target activity in four crops using ensembles of convolutional neural networks. Plant Mol. Biol. 2021, 105, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, L.; Zou, Q.; Wang, G. BP4RNAseq: A babysitter package for retrospective and newly generated RNA-seq data analyses using both alignment-based and alignment-free quantification method. Bioinform. 2021, 37, 1319–1321. [Google Scholar] [CrossRef]
- Sheervalilou, R.; Shirvaliloo, M.; Sargazi, S.; Ghaznavi, H. Recent advances in iron oxide nanoparticles for brain cancer theranostics: From in vitro to clinical applications. Exp. Opin. Drug Deliv. 2021, 1–29. [Google Scholar]
- Shirvalilou, S.; Khoei, S.; Esfahani, A.J.; Kamali, M.; Shirvaliloo, M.; Sheervalilou, R.; Mirzaghavami, P. Magnetic Hyperthermia as an adjuvant cancer therapy in combination with radiotherapy versus radiotherapy alone for recurrent/progressive glioblastoma: A systematic review. J. Neurooncol. 2021, 1–10. [Google Scholar]
- Tila, D.; Yazdani-Arazi, S.N.; Ghanbarzadeh, S.; Arami, S.; Pourmoazzen, Z. pH-sensitive, polymer modified, plasma stable niosomes: Promising carriers for anti-cancer drugs. EXCLI J. 2015, 14, 21. [Google Scholar] [PubMed]
- Chang, D.-K.; Chiu, C.-Y.; Kuo, S.-Y.; Lin, W.-C.; Lo, A.; Wang, Y.-P.; Li, P.-C.; Wu, H.-C. Antiangiogenic targeting liposomes increase therapeutic efficacy for solid tumors. J. Biol. Chem. 2009, 284, 12905–12916. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, R.; Bhuvaneshwar, G.; Sharma, C.P. Hemocompatible curcumin–dextran micelles as pH sensitive pro-drugs for enhanced therapeutic efficacy in cancer cells. Carbohydr. Polym. 2016, 137, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. European J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Wang, L.; Wang, Z.; He, S.; Zhou, D.; Jing, X.; Huang, Y. Enhancing therapeutic efficacy of cisplatin by blocking DNA damage repair. ACS Med. Chem. Lett. 2016, 7, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Hosseinikhah, S.M.; Barani, M.; Rahdar, A.; Madry, H.; Arshad, R.; Mohammadzadeh, V.; Cucchiarini, M. Nanomaterials for the Diagnosis and Treatment of Inflammatory Arthritis. Int. J. Mol. Sci. 2021, 22, 3092. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Sarani, M.; Khatami, M. Nickel-doped cerium oxide nanoparticles: Biosynthesis, cytotoxicity and UV protection studies. RSC Adv. 2020, 10, 3967–3977. [Google Scholar] [CrossRef]
- Nazaripour, E.; Mousazadeh, F.; Moghadam, M.D.; Najafi, K.; Borhani, F.; Sarani, M.; Ghasemi, M.; Rahdar, A.; Iravani, S.; Khatami, M. Biosynthesis of lead oxide and cerium oxide nanoparticles and their cytotoxic activities against colon cancer cell line. Inorg. Chem. Commun. 2021, 131, 108800. [Google Scholar] [CrossRef]
- Farooq, M.A.; Aquib, M.; Farooq, A.; Haleem Khan, D.; Joelle Maviah, M.B.; Sied Filli, M.; Kesse, S.; Boakye-Yiadom, K.O.; Mavlyanova, R.; Parveen, A. Recent progress in nanotechnology-based novel drug delivery systems in designing of cisplatin for cancer therapy: An overview. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1674–1692. [Google Scholar] [CrossRef]
- Panzarini, E.; Dini, L. Nanomaterial-induced autophagy: A new reversal MDR tool in cancer therapy? Mol. Pharm. 2014, 11, 2527–2538. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Cheng, Y.; Wei, Y.; Wei, X. Immune checkpoint blockade and its combination therapy with small-molecule inhibitors for cancer treatment. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 199–224. [Google Scholar] [CrossRef]
- Prieto-Peña, D.; Dasgupta, B. Biologic agents and small-molecule inhibitors in systemic autoimmune conditions: An update. Pol. Arch. Intern. Med. 2020, 131, 171–181. [Google Scholar] [CrossRef]
- Smithgall, T.E.; Thomas, G. Small molecule inhibitors of the HIV-1 virulence factor, Nef. Drug Disc. Today Technol. 2013, 10, e523–e529. [Google Scholar] [CrossRef]
- Watanabe, M.; Uesugi, M. Small-molecule inhibitors of SREBP activation–potential for new treatment of metabolic disorders. MedChemComm 2013, 4, 1422–1433. [Google Scholar] [CrossRef]
- Kannt, A.; Rajagopal, S.; Kadnur, S.V.; Suresh, J.; Bhamidipati, R.K.; Swaminathan, S.; Hallur, M.S.; Kristam, R.; Elvert, R.; Czech, J. A small molecule inhibitor of Nicotinamide N-methyltransferase for the treatment of metabolic disorders. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-X.; Zhang, Z.-Y. Targeting PTPs with small molecule inhibitors in cancer treatment. Cancer Metastasis Rev. 2008, 27, 263–272. [Google Scholar] [CrossRef]
- Yap, J.L.; Worlikar, S.; MacKerell, A.D., Jr.; Shapiro, P.; Fletcher, S. Small Molecule Inhibitors of the ERK Signalling Pathway: Towards Novel Anti-Cancer Therapeutics. ChemMedChem 2011, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Ivy, S.P.; Wick, J.Y.; Kaufman, B.M. An overview of small-molecule inhibitors of VEGFR signaling. Nat. Rev. Clin. Oncol. 2009, 6, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Phillip Bowen, J. Recent advances in small molecule inhibitors of VEGFR and EGFR signaling pathways. Curr. Top. Med. Chem. 2011, 11, 1571–1590. [Google Scholar] [CrossRef]
- Peukert, S.; Miller-Moslin, K. Small-molecule inhibitors of the hedgehog signaling pathway as cancer therapeutics. ChemMedChem: Chem. Enabling Drug Discov. 2010, 5, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Grande, F.; Neamati, N. Small molecule inhibitors of Stat3 signaling pathway. Curr. Cancer Drug Targ. 2007, 7, 91–107. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Degterev, A. Small-molecule inhibitors of the PI3K signaling network. Future Med. Chem. 2011, 3, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Segerström, L.; Baryawno, N.; Sveinbjörnsson, B.; Wickström, M.; Elfman, L.; Kogner, P.; Johnsen, J.I. Effects of small molecule inhibitors of PI3K/Akt/mTOR signaling on neuroblastoma growth in vitro and in vivo. Int. J. Cancer 2011, 129, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, A.; Krauss, S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr. Pharm. Des. 2013, 19, 634–664. [Google Scholar] [CrossRef]
- Akhurst, R.J. Large-and small-molecule inhibitors of transforming growth factor-ß signaling. Curr. Opin. Investig. Drugs 2006, 7, 513–521. [Google Scholar] [PubMed]
- Gable, K.L.; Maddux, B.A.; Penaranda, C.; Zavodovskaya, M.; Campbell, M.J.; Lobo, M.; Robinson, L.; Schow, S.; Kerner, J.A.; Goldfine, I.D. Diarylureas are small-molecule inhibitors of insulin-like growth factor I receptor signaling and breast cancer cell growth. Mol. Cancer Ther. 2006, 5, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Gold, B. Small-molecule inhibitors of DNA damage-repair pathways: An approach to overcome tumor resistance to alkylating anticancer drugs. Future Med. Chem. 2012, 4, 1093–1111. [Google Scholar] [CrossRef]
- Steffen, J.D.; Brody, J.R.; Armen, R.S.; Pascal, J.M. Structural implications for selective targeting of PARPs. Front. Oncol. 2013, 3, 301. [Google Scholar] [CrossRef]
- Xue, C.; You, J.; Zhang, H.; Xiong, S.; Yin, T.; Huang, Q. Capacity of myofibrillar protein to adsorb characteristic fishy-odor compounds: Effects of concentration, temperature, ionic strength, pH and yeast glucan addition. Food Chem. 2021, 130304. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, S.; Wu, J.; Zou, Q. An in silico approach to identification, categorization and prediction of nucleic acid binding proteins. Briefings Bioinform. 2021, 22, bbaa171. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.B.; Nakhaee, A.; Saravani, R.; Sargazi, S. Significant association of LXRβ (NR1H2) polymorphisms (rs28514894, rs2303044) with type 2 diabetes mellitus and laboratory characteristics. J. Diabetes Metab. Disord. 2021, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Aslam, H.M.; Anwar, S. PARP inhibitors. Hered. Cancer Clin. Pract. 2015, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Romero, R.; Martínez-Lara, E.; Aguilar-Quesada, R.; Peralta, A.; Oliver, F.J.; Siles, E. PARP-1 modulates deferoxamine-induced HIF-1α accumulation through the regulation of nitric oxide and oxidative stress. J. Cell. Biochem. 2008, 104, 2248–2260. [Google Scholar] [CrossRef]
- Patel, M.; Nowsheen, S.; Maraboyina, S.; Xia, F. The role of poly (ADP-ribose) polymerase inhibitors in the treatment of cancer and methods to overcome resistance: A review. Cell Biosci. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Dedes, K.J.; Wilkerson, P.M.; Wetterskog, D.; Weigelt, B.; Ashworth, A.; Reis-Filho, J.S. Synthetic lethality of PARP inhibition in cancers lacking BRCA1 and BRCA2 mutations. Cell Cycle 2011, 10, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A.; Kassem, L.; Azim, H., Jr. Integrating PARP inhibitors into the management of breast cancer: Where are we? Chin. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Bogliolo, S.; Cassani, C.; Dominoni, M.; Musacchi, V.; Venturini, P.L.; Spinillo, A.; Ferrero, S.; Gardella, B. Veliparib for the treatment of ovarian cancer. Expert Opin. Investig. Drugs 2016, 25, 367–374. [Google Scholar] [CrossRef]
- Murai, J.; Pommier, Y. Classification of PARP inhibitors based on PARP trapping and catalytic inhibition, and rationale for combinations with topoisomerase I inhibitors and alkylating agents. In PARP Inhibitors for Cancer Therapy; Springer: Berlin, Germany, 2015; pp. 261–274. [Google Scholar]
- Min, A.; Im, S.-A. PARP inhibitors as therapeutics: Beyond modulation of PARylation. Cancers 2020, 12, 394. [Google Scholar] [CrossRef]
- Hopkins, T.A.; Shi, Y.; Rodriguez, L.E.; Solomon, L.R.; Donawho, C.K.; DiGiammarino, E.L.; Panchal, S.C.; Wilsbacher, J.L.; Gao, W.; Olson, A.M. Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol. Cancer Res. 2015, 13, 1465–1477. [Google Scholar] [CrossRef]
- Mehta, I.S.; Kulashreshtha, M.; Chakraborty, S.; Kolthur-Seetharam, U.; Rao, B.J. Chromosome territories reposition during DNA damage-repair response. Genome Biol. 2013, 14, 1–15. [Google Scholar] [CrossRef]
- Yan, S.; Sorrell, M.; Berman, Z. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cell. Mol. Life Sci. 2014, 71, 3951–3967. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, H.; Wu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Nickel carcinogenesis mechanism: DNA damage. Int. J. Mol. Sci. 2019, 20, 4690. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Price, B.D.; Day, T.A. Multiple roles for mono-and poly (ADP-ribose) in regulating stress responses. Trends Genet. 2019, 35, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yu, X. Functions of PARylation in DNA damage repair pathways. Genom. Proteom. Bioinform. 2016, 14, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Houl, J.H.; Ye, Z.; Brosey, C.A.; Balapiti-Modarage, L.P.; Namjoshi, S.; Bacolla, A.; Laverty, D.; Walker, B.L.; Pourfarjam, Y.; Warden, L.S. Selective small molecule PARG inhibitor causes replication fork stalling and cancer cell death. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef] [PubMed]
- Virág, L.; Szabó, C. The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 2002, 54, 375–429. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Munshi, A.; Ramesh, R. Polymeric nanoparticle-mediated gene delivery for lung cancer treatment. Polym. Gene Delivery Syst. 2017, 233–255. [Google Scholar]
- Ventola, C. LProgress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742. [Google Scholar]
- Di Zhang, P.B.; Leal, A.S.; Carapellucci, S.; Sridhar, S.; Liby, K.T. A nano-liposome formulation of the PARP inhibitor Talazoparib enhances treatment efficacy and modulates immune cell populations in mammary tumors of BRCA-deficient mice. Theranostics 2019, 9, 6224. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, M.J.; DuRoss, A.N.; Landry, M.R.; Winter, H.; Goforth, A.M.; Sun, C. Co-delivery of PARP and PI3K inhibitors by nanoscale metal–organic frameworks for enhanced tumor chemoradiation. Nano Res. 2019, 12, 3003–3017. [Google Scholar] [CrossRef]
- Gonzales, J.; Kossatz, S.; Roberts, S.; Pirovano, G.; Brand, C.; Pérez-Medina, C.; Donabedian, P.; de la Cruz, M.J.; Mulder, W.J.; Reiner, T. Nanoemulsion-based delivery of fluorescent PARP inhibitors in mouse models of small cell lung cancer. Bioconjug. Chem. 2018, 29, 3776–3782. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Yang, S.; Krishna, A.; Sridhar, S. Nanoparticle formulations of poly (ADP-ribose) polymerase inhibitors for cancer therapy. Front. Chem. 2020, 8, 1129. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Ohman, A.W.; Tangutoori, S.; Dinulescu, D.M.; Sridhar, S. Intraperitoneal delivery of NanoOlaparib for disseminated late-stage cancer treatment. Int. J. Nanomed. 2018, 13, 8063. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Majera, D.; Skrott, Z.; Bouchal, J.; Bartkova, J.; Simkova, D.; Gachechiladze, M.; Steigerova, J.; Kurfurstova, D.; Gursky, J.; Korinkova, G. Targeting genotoxic and proteotoxic stress-response pathways in human prostate cancer by clinically available PARP inhibitors, vorinostat and disulfiram. Prostate 2019, 79, 352–362. [Google Scholar] [CrossRef]
- Setton, J.S.; Powell, S.N. Moving beyond PARP Inhibition in ATM-Deficient Prostate Cancer. Cancer Res. 2020, 80, 2085–2086. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Zhang, Y.; Morris, J.; Ji, J.; Takeda, S.; Doroshow, J.H.; Pommier, Y. Rationale for poly (ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J. Pharmacol. Exp. Ther. 2014, 349, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Löser, D.A.; Shibata, A.; Shibata, A.K.; Woodbine, L.J.; Jeggo, P.A.; Chalmers, A.J. Sensitization to radiation and alkylating agents by inhibitors of poly (ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol. Cancer Ther. 2010, 9, 1775–1787. [Google Scholar] [CrossRef]
- Sargazi, S.; Saravani, R.; Reza, J.Z.; Jaliani, H.Z.; Mirinejad, S.; Rezaei, Z.; Zarei, S. Induction of apoptosis and modulation of homologous recombination DNA repair pathway in prostate cancer cells by the combination of AZD2461 and valproic acid. EXCLI J. 2019, 18, 485. [Google Scholar]
- Plummer, R.; Lorigan, P.; Steven, N.; Scott, L.; Middleton, M.R.; Wilson, R.H.; Mulligan, E.; Curtin, N.; Wang, D.; Dewji, R. A phase II study of the potent PARP inhibitor, Rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemother. Pharmacol. 2013, 71, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Wasyluk, W.; Zwolak, A. PARP Inhibitors: An Innovative Approach to the Treatment of Inflammation and Metabolic Disorders in Sepsis. J. Inflamm. Res. 2021, 14, 1827. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J.; Bourgeois, M.; Harbison, R.D. Poly (ADP-ribose) polymerase (PARP) and PARP inhibitors: Mechanisms of action and role in cardiovascular disorders. Cardiovasc. Toxicol. 2018, 18, 493–506. [Google Scholar] [CrossRef]
- Celik-Ozenci, C.; Kuscu, N.; Gungor-Ordueri, N.; Tasatargil, A.; Sahin, P.; Durmus, H. Inhibition of poly (ADP-ribose) polymerase may have preventive potential for varicocoele-associated testicular damage in rats. Andrology 2017, 5, 362–369. [Google Scholar] [CrossRef]
- Zakaria, E.M.; El-Maraghy, N.N.; Ahmed, A.F.; Ali, A.A.; El-Bassossy, H.M. PARP inhibition ameliorates nephropathy in an animal model of type 2 diabetes: Focus on oxidative stress, inflammation, and fibrosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 621. [Google Scholar] [CrossRef]
- Fritz, C.; Portwood, S.M.; Przespolewski, A.; Wang, E.S. PARP goes the weasel! Emerging role of PARP inhibitors in acute leukemias. Blood Rev. 2021, 45, 100696. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Praetorius, N.P.; Mandal, T.K. Engineered nanoparticles in cancer therapy. Recent Patents on Drug Delivery & Formulation 2007, 1, 37–51. [Google Scholar]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Awasthi, R.; Roseblade, A.; Hansbro, P.M.; Rathbone, M.J.; Dua, K.; Bebawy, M. Nanoparticles in cancer treatment: Opportunities and obstacles. Curr. Drug Targets 2018, 19, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Mensah, L.B.; Morton, S.W.; Li, J.; Xiao, H.; Quadir, M.A.; Elias, K.M.; Penn, E.; Richson, A.K.; Ghoroghchian, P.P.; Liu, J. Layer-by-layer nanoparticles for novel delivery of cisplatin and PARP inhibitors for platinum-based drug resistance therapy in ovarian cancer. Bioeng. Transl. Med. 2019, 4, e10131. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Tangutoori, S.; Sridhar, S. In vitro analysis of PARP inhibitor nanoformulations. Int. J. Nanomed. 2018, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Novohradsky, V.; Zajac, J.; Vrana, O.; Kasparkova, J.; Brabec, V. Simultaneous delivery of olaparib and carboplatin in PEGylated liposomes imparts this drug combination hypersensitivity and selectivity for breast tumor cells. Oncotarget 2018, 9, 28456. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, A.L.; Tangutoori, S.; Baldwin, P.; Qiao, J.; Gharagouzloo, C.; Seitzer, N.; Clohessy, J.G.; Makrigiorgos, G.M.; Cormack, R.; Pandolfi, P.P. Nanoformulation of olaparib amplifies PARP inhibition and sensitizes PTEN/TP53-deficient prostate cancer to radiation. Mol. Cancer Ther. 2017, 16, 1279–1289. [Google Scholar] [CrossRef]
- Pathade, A.D.; Kommineni, N.; Bulbake, U.; Thummar, M.M.; Samanthula, G.; Khan, W. Preparation and comparison of oral bioavailability for different nano-formulations of olaparib. AAPS PharmSciTech 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Eetezadi, S. Nanomedicines and Combination Therapy of Doxorubicin and Olaparib for Treatment of Ovarian Cancer. Ph.D. Thesis, University of Toronto, Toronto, Canada, March 2016. [Google Scholar]
- Caster, J.M.; Sethi, M.; Kowalczyk, S.; Wang, E.; Tian, X.; Hyder, S.N.; Wagner, K.T.; Zhang, Y.-A.; Kapadia, C.; Au, K.M. Nanoparticle delivery of chemosensitizers improve chemotherapy efficacy without incurring additional toxicity. Nanoscale 2015, 7, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Misra, S.; Rodriguez, N.S.; Vulugundam, G.; Oh, A.L.; Senyuk, V.; Mahmud, N.; Rondelli, D.; Pan, D. Combined nanoparticle delivery of PARP and DNA-PK inhibition for multiple myeloma. Blood 2017, 130, 1809. [Google Scholar]
- Dasa, S.S.K.; Diakova, G.; Suzuki, R.; Mills, A.M.; Gutknecht, M.F.; Klibanov, A.L.; Slack-Davis, J.K.; Kelly, K.A. Plectin-targeted liposomes enhance the therapeutic efficacy of a PARP inhibitor in the treatment of ovarian cancer. Theranostics 2018, 8, 2782. [Google Scholar] [CrossRef] [PubMed]
- Belz, J.E.; Kumar, R.; Baldwin, P.; Ojo, N.C.; Leal, A.S.; Royce, D.B.; Zhang, D.; van de Ven, A.L.; Liby, K.T.; Sridhar, S. Sustained release talazoparib implants for localized treatment of BRCA1-deficient breast cancer. Theranostics 2017, 7, 4340. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, Y.; Liu, N.; Suo, Y. Nanoparticles Loading Porphyrin Sensitizers in Improvement of Photodynamic Therapy for Ovarian Cancer. Photodiagnosis Photodyn. Ther. 2020, 33, 102156. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.A.; Arruda, D.C.; Baptista, M.S.; Tada, D.B. Co-Encapsulation of Methylene Blue and PARP-Inhibitor into Poly (Lactic-Co-Glycolic Acid) Nanoparticles for Enhanced PDT of Cancer. Nanomaterials 2021, 11, 1514. [Google Scholar] [CrossRef]
- Misra, R.; Patra, B.; Varadharaj, S.; Verma, R.S. Establishing the promising role of novel combination of triple therapeutics delivery using polymeric nanoparticles for Triple negative breast cancer therapy. BioImpacts: BI 2021, 11, 199. [Google Scholar] [CrossRef]
- Wu, M.; Liu, J.; Hu, C.; Li, D.; Yang, J.; Wu, Z.; Yang, L.; Chen, Y.; Fu, S.; Wu, J. Olaparib nanoparticles potentiated radiosensitization effects on lung cancer. Int. J. Nanomed. 2018, 13, 8461. [Google Scholar] [CrossRef]
- Li, D.; Hu, C.; Yang, J.; Liao, Y.; Chen, Y.; Fu, S.Z.; Wu, J.B. Enhanced Anti-Cancer Effect of Folate-Conjugated Olaparib Nanoparticles Combined with Radiotherapy in Cervical Carcinoma. Int. J. Nanomed. 2020, 15, 10045. [Google Scholar] [CrossRef]
- Zhang, D.; Baldwin, P.; Sridhar, S.; Liby, K. Nanoformulated Talazoparib enhances the efficacy and reduces the toxicity of this PARP inhibitor in a preClin. model of BRCA-deficient breast cancer. FASEB J. 2018, 32, 565.10. [Google Scholar]
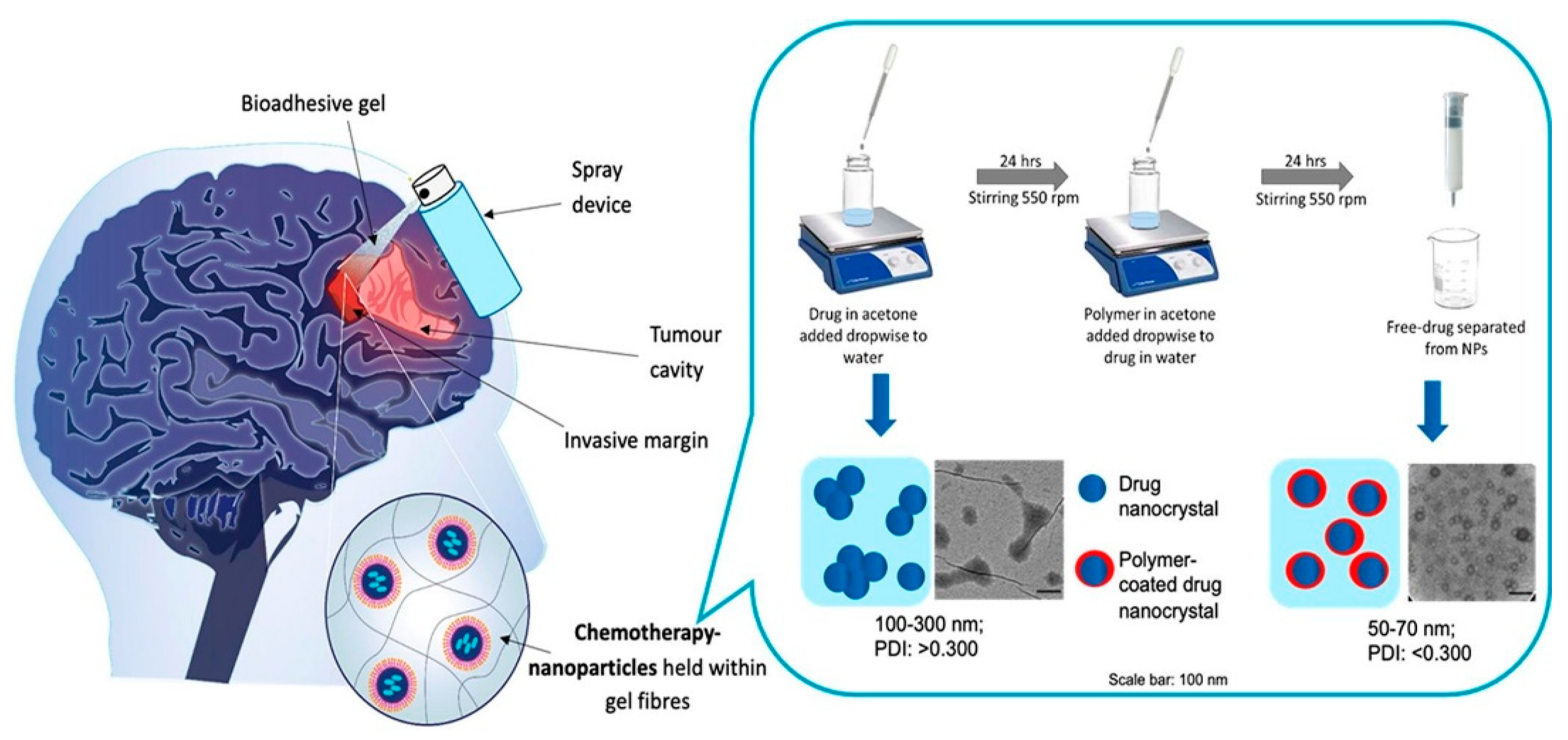
- McCrorie, P.; Mistry, J.; Taresco, V.; Lovato, T.; Fay, M.; Ward, I.; Ritchie, A.A.; Clarke, P.A.; Smith, S.J.; Marlow, M. Etoposide and olaparib polymer-coated nanoparticles within a bioadhesive sprayable hydrogel for post-surgical localised delivery to brain tumours. Eur. J. Pharm. Biopharm. 2020, 157, 108–120. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, H.; Dong, P.; Zhang, T.; Lu, C.; Jin, T.; Chai, K.Y. Pegylated azelaic acid: Synthesis, tyrosinase inhibitory activity, antibacterial activity and cytotoxic studies. J. Mol. Struct. 2021, 1224, 129234. [Google Scholar] [CrossRef]
- Cheng, H.-W.; Chiang, C.-S.; Ho, H.-Y.; Chou, S.-H.; Lai, Y.-H.; Shyu, W.-C.; Chen, S.-Y. Dextran-modified Quercetin-Cu (II)/hyaluronic acid nanoMed. with natural poly (ADP-ribose) polymerase inhibitor and dual targeting for programmed synthetic lethal therapy in triple-negative breast cancer. J. Control. Release 2021, 329, 136–147. [Google Scholar] [CrossRef] [PubMed]
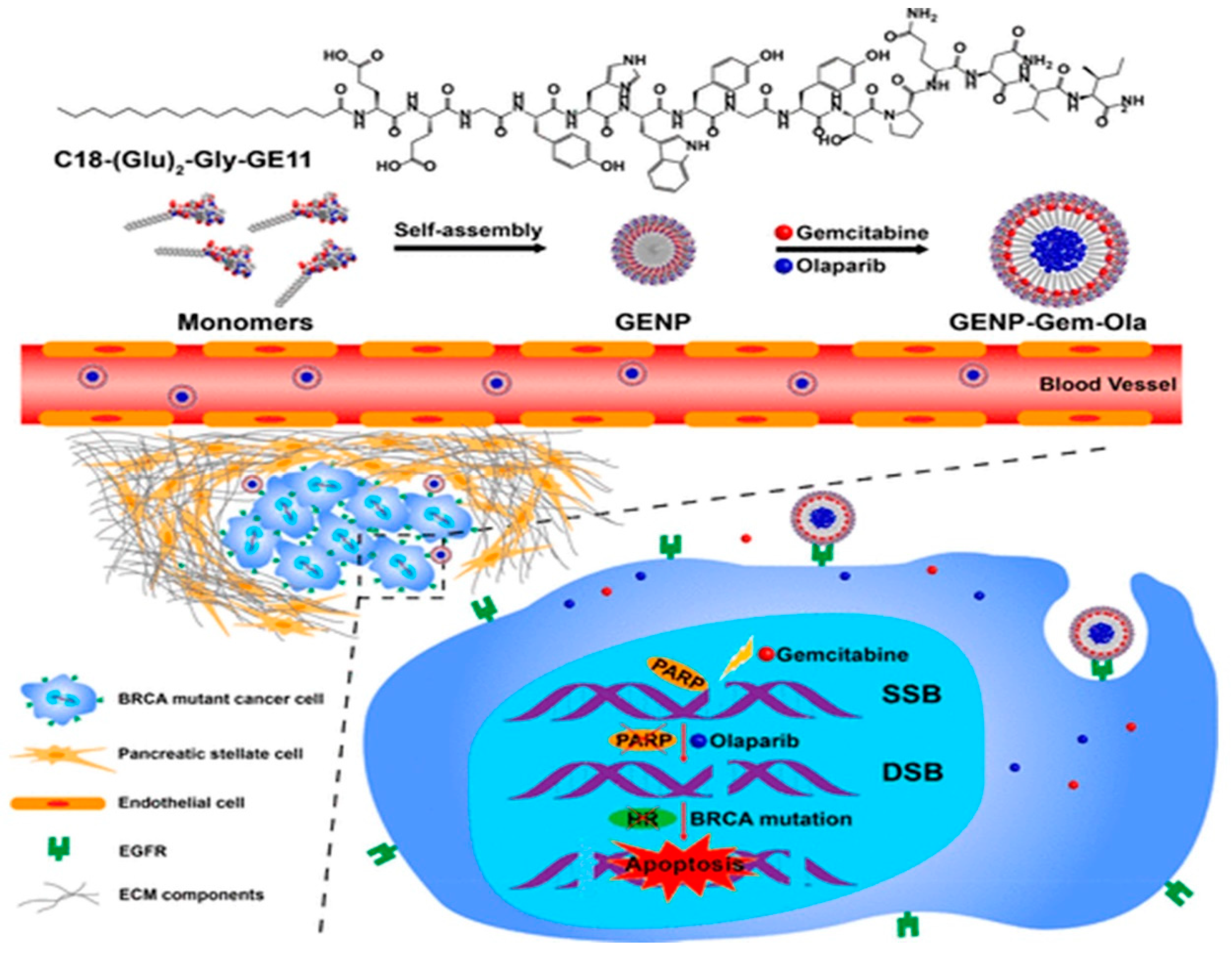
- Du, C.; Qi, Y.; Zhang, Y.; Wang, Y.; Zhao, X.; Min, H.; Han, X.; Lang, J.; Qin, H.; Shi, Q. Epidermal growth factor receptor-targeting peptide nanoparticles simultaneously deliver gemcitabine and olaparib to treat pancreatic cancer with breast cancer 2 (BRCA2) mutation. ACS Nano 2018, 12, 10785–10796. [Google Scholar] [CrossRef]
- DuRoss, A.N.; Neufeld, M.J.; Landry, M.R.; Rosch, J.G.; Eaton, C.T.; Sahay, G.; Thomas Jr, C.R.; Sun, C. Micellar formulation of talazoparib and buparlisib for enhanced DNA damage in breast cancer chemoradiotherapy. ACS Appl. Mater. Interfaces 2019, 11, 12342–12356. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.; Chowdhury, P.; Hatami, E.; Kumari, S.; Kashyap, V.K.; Tripathi, M.K.; Wagh, S.; Meibohm, B.; Chauhan, S.C.; Jaggi, M. Cross-Linked Polyphenol-Based Drug Nano-Self-Assemblies Engineered to Blockade Prostate Cancer Senescence. ACS Appl. Mater. Interfaces 2019, 11, 38537–38554. [Google Scholar] [CrossRef] [PubMed]
- Jitariu, A.-A.; Cîmpean, A.M.; Ribatti, D.; Raica, M. Triple negative breast cancer: The kiss of death. Oncotarget 2017, 8, 46652. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Truffi, M.; Baccarini, F.; Beretta, M.; Sorrentino, L.; Bellini, M.; Rizzuto, M.; Ottria, R.; Ravelli, A.; Ciuffreda, P. H-Ferritin-nanocaged olaparib: A promising choice for both BRCA-mutated and sporadic triple negative breast cancer. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Dufour, R.; Daumar, P.; Mounetou, E.; Aubel, C.; Kwiatkowski, F.; Abrial, C.; Vatoux, C.; Penault-Llorca, F.; Bamdad, M. BCRP and P-gp relay overexpression in triple negative basal-like breast cancer cell line: A prospective role in resistance to Olaparib. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Mehra, N.K.; Tekmal, R.R.; Palakurthi, S. Development and evaluation of talazoparib nanoemulsion for systemic therapy of BRCA1-mutant cancer. Anticancer Res. 2018, 38, 4493–4503. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafeez, B.B.; Meibohm, B.; Chauhan, S.C. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wang, H.; Huang, X.; Chen, Z.; Zhu, J.; Wang, X. Digital image colorimetry detection of carbaryl in food samples based on liquid phase microextraction coupled with a microfluidic thread-based analytical device. Food Chem. 2021, 337, 127971. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Tao, G.; Yang, L.; Liu, J.; Liu, Q.; Li, W.; Zhuang, Z. Methylation of PARP-1 promoter involved in the regulation of nano-SiO2-induced decrease of PARP-1 mRNA expression. Toxicol. Lett. 2012, 209, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.M.; El-Karim, A.; Somaia, S.; Mahmoud, A.H.; Amr, A.E.-G.E.; Al-Omar, M.A. A Comparative Study of the Anticancer Activity and PARP-1 Inhibiting Effect of Benzofuran–Pyrazole Scaffold and Its Nano-Sized Particles in Human Breast Cancer Cells. Molecules 2019, 24, 2413. [Google Scholar] [CrossRef] [PubMed]


| Nanocarrier | Type | Refs |
|---|---|---|
| Liposomes | Phospholipids | [75,79,97,98,99,100,101,102,103,104,105] |
| Polymeric NPs | Poly-(d,l-lactide-co-glycolide) (PLGA) | [106,108] |
| Methoxy poly (ethylene glycol)-poly (e-caprolac-304 tone) (MPEG-PCL) | [110] | |
| Poly (ε-caprolactone)-poly (ethyleneglycol)-poly (ε-caprolactone) (PCEC) | [111] | |
| Pluronic F127 | [113] | |
| Hybrid Nanosystems | Quercetin (Q), dextran-aldehyde (DA), Cu(II) and superparamagnetic iron oxide (IO) | [115] |
| Metal-organic frameworks (MOFs) and PEG | [76] | |
| Self-Assembled NPs | Amphiphilic peptides | [116] |
| Poloxamer micelle (MPM) | [117] | |
| Tannic acid-docetaxel self-assemblies | [118] | |
| Novel Nanosystems | Protein-based nanovehicle | [121] |
| Betacaryophyllene (BCP) carrier | [122] | |
| Lipids and cholesterol nanoemulsion | [77] | |
| Nano-SiO2 | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargazi, S.; Mukhtar, M.; Rahdar, A.; Barani, M.; Pandey, S.; Díez-Pascual, A.M. Active Targeted Nanoparticles for Delivery of Poly(ADP-ribose) Polymerase (PARP) Inhibitors: A Preliminary Review. Int. J. Mol. Sci. 2021, 22, 10319. https://doi.org/10.3390/ijms221910319
Sargazi S, Mukhtar M, Rahdar A, Barani M, Pandey S, Díez-Pascual AM. Active Targeted Nanoparticles for Delivery of Poly(ADP-ribose) Polymerase (PARP) Inhibitors: A Preliminary Review. International Journal of Molecular Sciences. 2021; 22(19):10319. https://doi.org/10.3390/ijms221910319
Chicago/Turabian StyleSargazi, Saman, Mahwash Mukhtar, Abbas Rahdar, Mahmood Barani, Sadanad Pandey, and Ana M. Díez-Pascual. 2021. "Active Targeted Nanoparticles for Delivery of Poly(ADP-ribose) Polymerase (PARP) Inhibitors: A Preliminary Review" International Journal of Molecular Sciences 22, no. 19: 10319. https://doi.org/10.3390/ijms221910319
APA StyleSargazi, S., Mukhtar, M., Rahdar, A., Barani, M., Pandey, S., & Díez-Pascual, A. M. (2021). Active Targeted Nanoparticles for Delivery of Poly(ADP-ribose) Polymerase (PARP) Inhibitors: A Preliminary Review. International Journal of Molecular Sciences, 22(19), 10319. https://doi.org/10.3390/ijms221910319










