How Substitution Combines with Non-Covalent Interactions to Modulate 1,4-Naphthoquinone and Its Derivatives Molecular Features—Multifactor Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Calculation and Analysis of Selected Physico-Chemical Parameters of the Studied 1,4-Naphthoquinone and Its Derivatives on the Basis of Static Density Functional Theory (DFT) Models
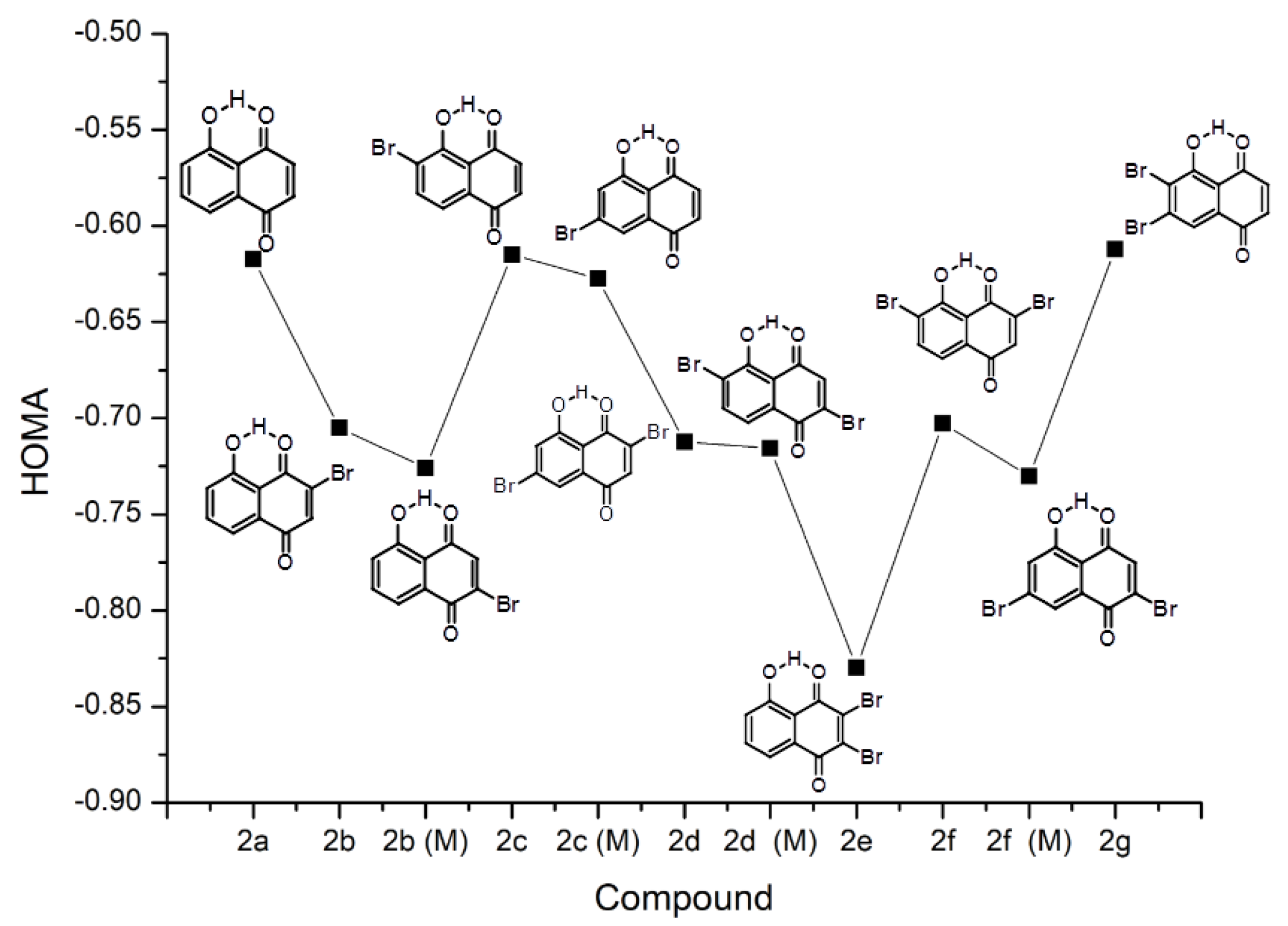
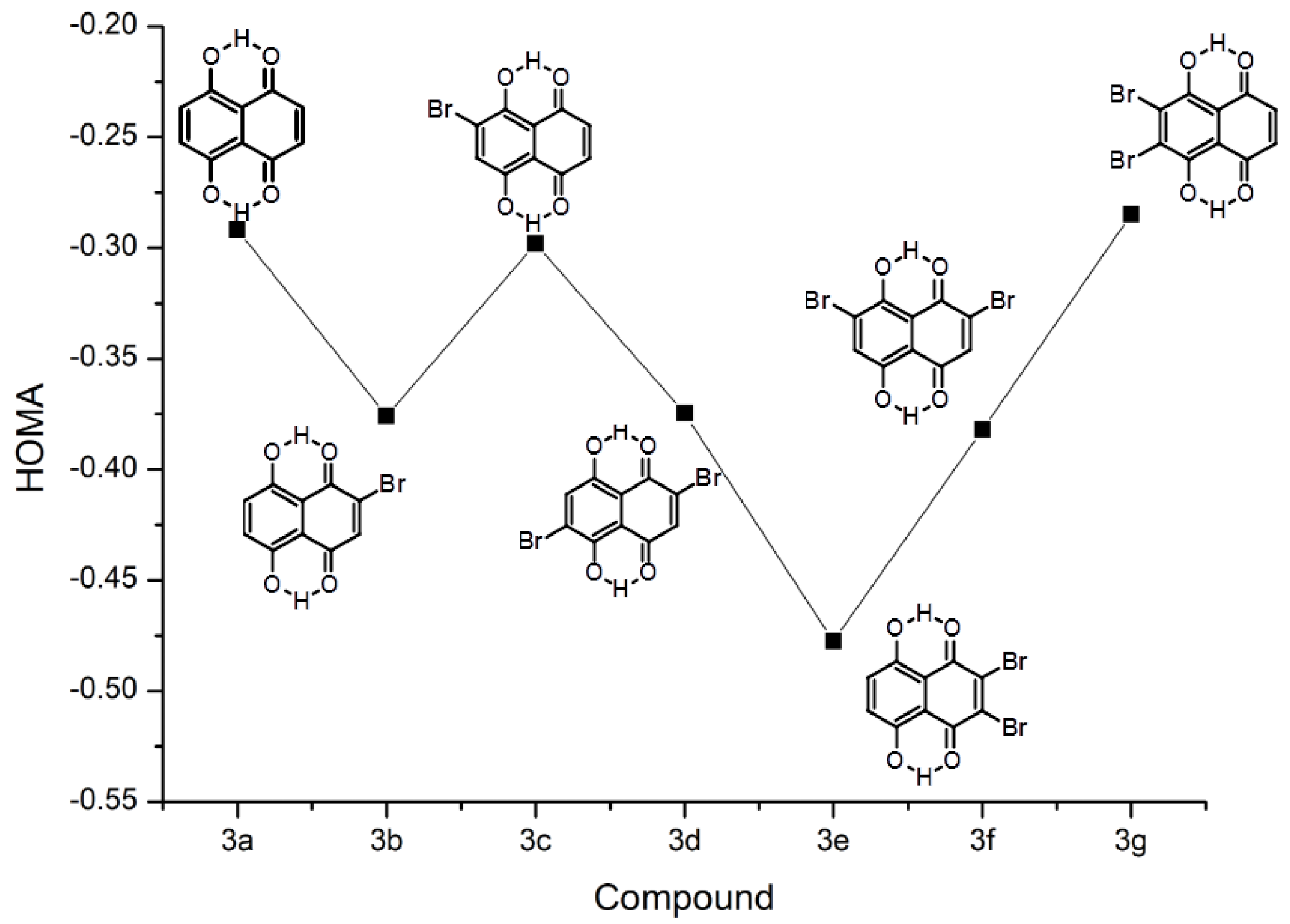
2.1.1. Substituent Effect—Structure and Correlation Analyses
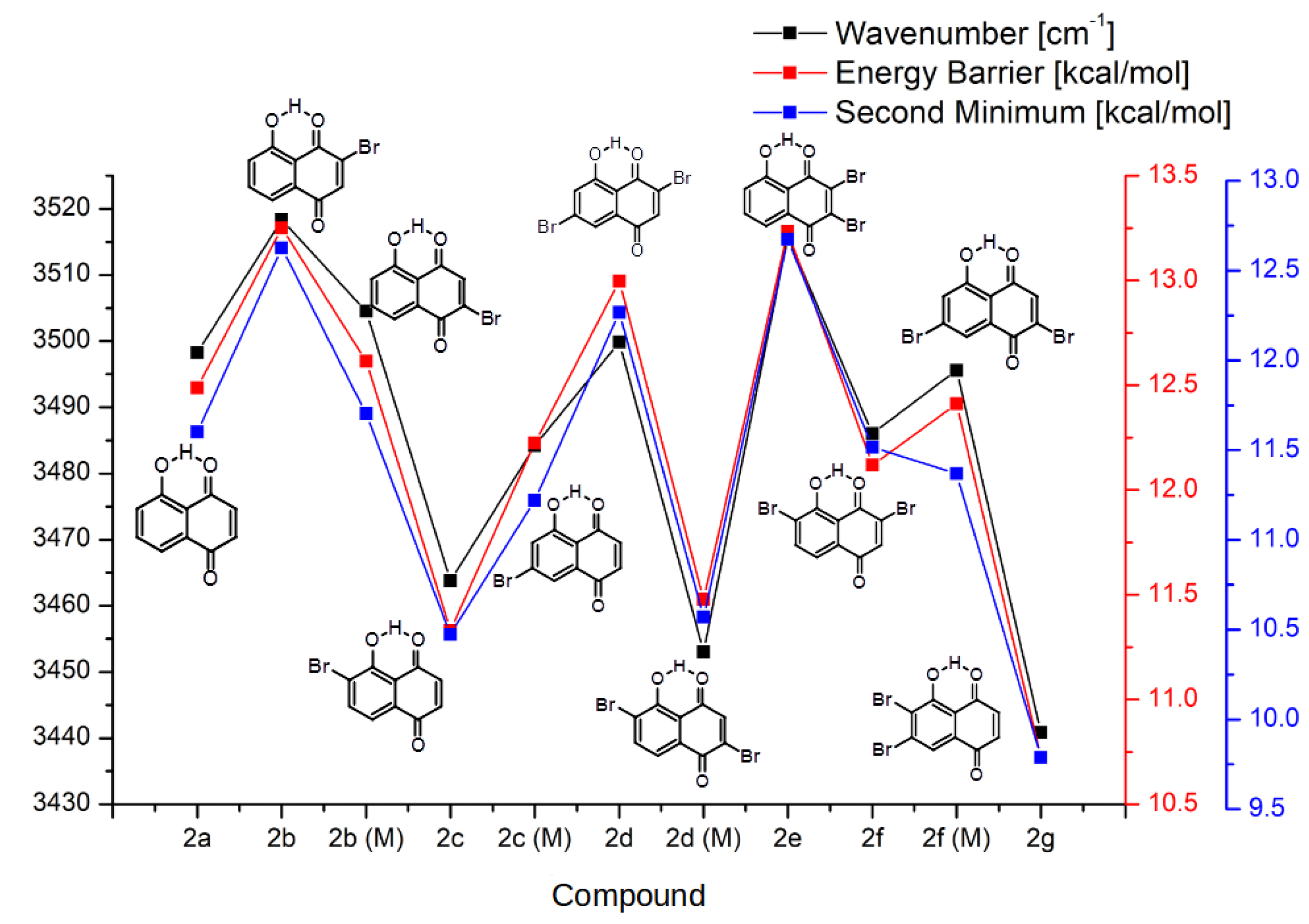
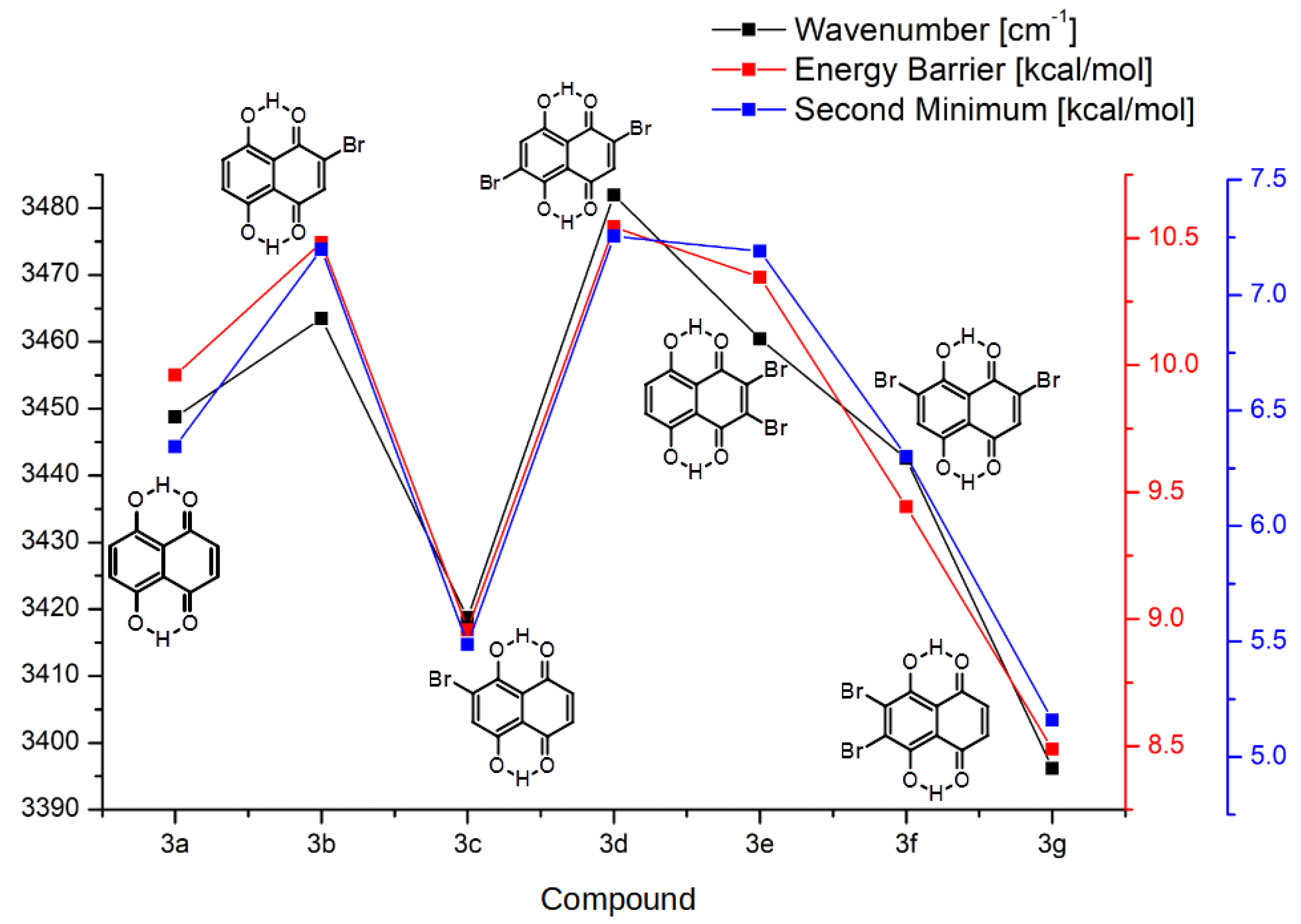
2.1.2. Intramolecular Hydrogen Bond and the Proton Reaction Path
- Both occupied by hydrogens.
- Both occupied by bromine atoms.
2.1.3. Electronic Structure Analysis on the Basis of Hirshfeld Charges Distribution and Substituent Active Region (cSAR) Parameter
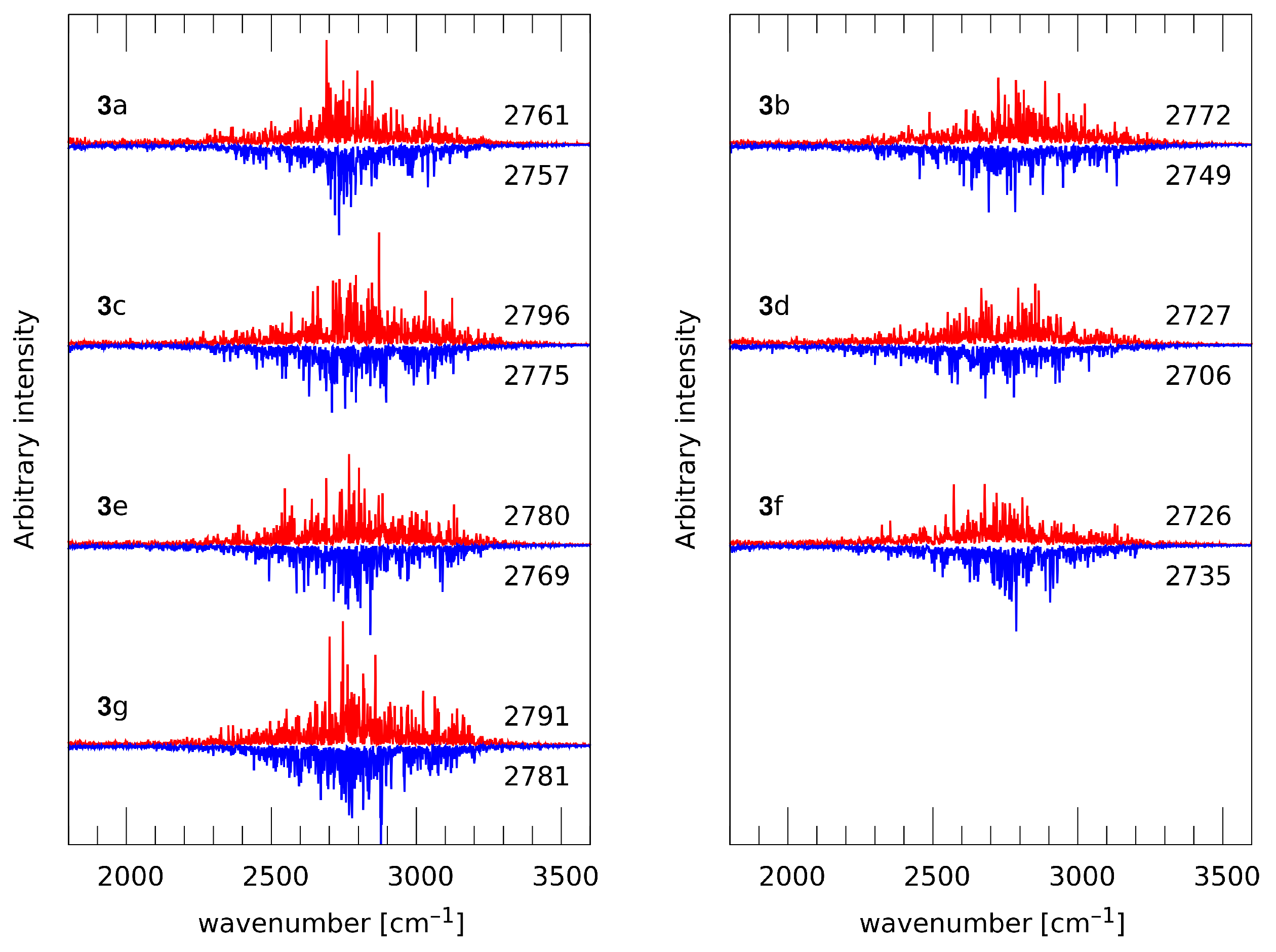
2.2. Time Evolution of Intramolecular Hydrogen Bonds Present in Selected 1,4-Naphthoquinone Derivatives on the Basis of Car–Parrinello Molecular Dynamics (CPMD)
3. Computational Methodology
3.1. Static Density Functional Theory (DFT) Calculations
3.2. Car–Parrinello Molecular Dynamics (CPMD) Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SE | Substituent effect |
| 3D | Three-dimensional |
| DFT | Density Functional Theory |
| HOMA | Harmonic Oscillator Model of Aromaticity |
| CCDC | Cambridge Crystallographic Data Center |
| PES | Potential Energy Surface |
| cSAR | Substituent Active Region parameter |
| PT | Proton transfer |
| CPMD | Car–Parrinello Molecular Dynamics |
| MD | Molecular dynamics |
References
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Cieplak, A.S. Inductive and Resonance Effects of Substituents on π-Face Selection. Chem. Rev. 1999, 99, 1265–1336. [Google Scholar] [CrossRef] [PubMed]
- Brutschy, B. The Structure of Microsolvated Benzene Derivatives and the Role of Aromatic Substituents. Chem. Rev. 2000, 100, 3891–3920. [Google Scholar] [CrossRef] [PubMed]
- Krygowski, T.M.; Cyrański, M.K. Structural Aspects of Aromaticity. Chem. Rev. 2001, 101, 1385–1420. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Stępień, B.T. Sigma- and Pi-Electron Delocalization: Focus on Substituent Effects. Chem. Rev. 2005, 105, 3482–3512. [Google Scholar] [CrossRef]
- Jensen, H.H.; Bols, M. Stereoelectronic Substituent Effects. Acc. Chem. Res. 2006, 39, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Tomsho, J.W.; Pal, A.; Hall, D.G.; Benkovic, S.J. Ring Structure and Aromatic Substituent Effects on the pKa of the Benzoxaborole Pharmacophore. Med. Chem. Lett. 2011, 3, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, S.E. Understanding Substituent Effects in Noncovalent Interactions Involving Aromatic Rings. Acc. Chem. Res. 2012, 46, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Mahadevi, A.S.; Sastry, G.N. Cation-π Interaction: Its Role and Relevance in Chemistry, Biology, and Material Science. Chem. Rev. 2012, 113, 2100–2138. [Google Scholar] [CrossRef]
- Hartley, C.S. Folding of ortho-Phenylenes. Acc. Chem. Res. 2016, 49, 646–654. [Google Scholar] [CrossRef]
- Deb, T.; Tu, J.; Franzini, R.M. Mechanisms and Substituent Effects of Metal-Free Bioorthogonal Reactions. Chem. Rev. 2021, 121, 6850–6914. [Google Scholar] [CrossRef]
- Hammett, L.P. Some Relations between Reaction Rates and Equilibrium Constants. Chem. Rev. 1935, 17, 125–136. [Google Scholar] [CrossRef]
- Hammett, L.P. The Effect of Structure upon the Reactions of Organic Compounds. Benzene Derivatives. J. Am. Chem. Soc. 1937, 59, 96–103. [Google Scholar] [CrossRef]
- Taft, R.W. Linear Free Energy Relationships from Rates of Esterification and Hydrolysis of Aliphatic and Ortho-substituted Benzoate Esters. J. Am. Chem. Soc. 1952, 74, 2729–2732. [Google Scholar] [CrossRef]
- Sadlej-Sosnowska, N. Substituent active region—A gate for communication of substituent charge with the rest of a molecule: Monosubstituted benzenes. Chem. Phys. Lett. 2007, 447, 192–196. [Google Scholar] [CrossRef]
- George, P.; Trachtman, M.; Bock, C.W.; Brett, A.M. Homodesmotic reactions for the assessment of stabilization energies in benzenoid and other conjugated cyclic hydrocarbons. J. Chem. Soc. Perkin Trans. 2 1976, 11, 1222. [Google Scholar] [CrossRef]
- Pross, A.; Radom, L.; Taft, R.W. Theoretical approach to substituent effects. Phenols and phenoxide ions. J. Org. Chem. 1980, 45, 818–826. [Google Scholar] [CrossRef]
- Ozimiński, W.P.; Dobrowolski, J.C. σ- and π-electron contributions to the substituent effect: Natural population analysis. J. Phys. Org. Chem. 2009, 22, 769–778. [Google Scholar] [CrossRef]
- von Ragué Schleyer, P.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J.R. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Kruszewski, J.; Krygowski, T. Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett. 1972, 13, 3839–3842. [Google Scholar] [CrossRef]
- Karpinska, J.; Erxleben, A.; McArdle, P. Applications of Low Temperature Gradient Sublimation in Vacuo: Rapid Production of High Quality Crystals. The First Solvent-Free Crystals of Ethinyl Estradiol. Cryst. Growth Des. 2013, 13, 1122–1130. [Google Scholar] [CrossRef]
- Herbstein, F.H.; Kapon, M.; Reisner, G.M.; Lehman, M.S.; Kress, R.B.; Wilson, R.B.; Shiau, W.I.; Duesler, E.N.; Paul, I.C.; Curtin, D.Y.; et al. Polymorphism of naphthazarin and its relation to solid-state proton transfer. Neutron and X-ray diffraction studies on naphthazarin C. Proc. R. Soc. London A Math. Phys. Sci. 1985, 399, 295–319. [Google Scholar] [CrossRef]
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. Chem. Pharm. Bull. 2020, 68, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandiran, P.; Sheet, S.; Premnath, D.; Kim, A.R.; Yoo, D.J. 1,4-Naphthoquinone Analogues: Potent Antibacterial Agents and Mode of Action Evaluation. Molecules 2019, 24, 1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepetkin, I.A.; Karpenko, A.S.; Khlebnikov, A.I.; Shibinska, M.O.; Levandovskiy, I.A.; Kirpotina, L.N.; Danilenko, N.V.; Quinn, M.T. Synthesis, anticancer activity, and molecular modeling of 1, 4-naphthoquinones that inhibit MKK7 and Cdc25. Eur. J. Med. Chem. 2019, 183, 111719. [Google Scholar] [CrossRef]
- Pekin, G.; Ganzera, M.; Senol, S.; Bedir, E.; Korkmaz, K.; Stuppner, H. Determination of Naphthazarin Derivatives in Endemic Turkish Alkanna Species by Reversed Phase High Performance Liquid Chromatography. Planta Med. 2007, 73, 267–272. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Q.; Yang, C.; Zhu, Y.; Zhang, L.; Wang, L.; Liu, Z.; Zhang, G.; Zhang, C. Assembly Line and Post-PKS Modifications in the Biosynthesis of Marine Polyketide Natural Products. In Comprehensive Natural Products III; Elsevier: Amsterdam, The Netherlands, 2020; pp. 139–197. [Google Scholar] [CrossRef]
- Tchizhova, A.Y.; Anufriev, V.P.; Glazunov, V.P.; Denisenko, V.A. The chemistry of naphthazarin derivatives. Russ. Chem. Bull. 2000, 49, 466–471. [Google Scholar] [CrossRef]
- Sánchez-Calvo, J.M.; Barbero, G.R.; Guerrero-Vásquez, G.; Durán, A.G.; Macías, M.; Rodríguez-Iglesias, M.A.; Molinillo, J.M.G.; Macías, F.A. Synthesis, antibacterial and antifungal activities of naphthoquinone derivatives: A structure—Activity relationship study. Med. Chem. Res. 2016, 25, 1274–1285. [Google Scholar] [CrossRef]
- Masuda, K.; Funayama, S.; Komiyama, K.; Unezawa, I.; Ito, K. Constituents of Tritonia crocosmaeflora, I. Tricrozarin A, a Novel Antimicrobial Naphthazarin Derivative. J. Nat. Prod. 1987, 50, 418–421. [Google Scholar] [CrossRef]
- Rudnicka, M.; Ludynia, M.; Karcz, W. The Effect of Naphthazarin on the Growth, Electrogenicity, Oxidative Stress, and Microtubule Array in Z. mays Coleoptile Cells Treated With IAA. Front. Plant Sci. 2019, 9, 1940. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K.C. The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products. Angew. Chem. Int. Ed. 1999, 38, 270–301. [Google Scholar] [CrossRef]
- Song, G.Y.; Kim, Y.; You, Y.J.; Cho, H.; Kim, S.H.; Sok, D.E.; Ahn, B.Z. Naphthazarin Derivatives (VI): Synthesis, Inhibitory Effect on DNA Topoisomerase-I and Antiproliferative Activity of 2- or 6-(1-Oxyiminoalkyl)-5, 8-dimethoxy-1, 4-naphthoquinones. Arch. Pharm. (Weinheim) 2000, 333, 87–92. [Google Scholar] [CrossRef]
- Park, E.M.; Lee, I.J.; Kim, S.H.; Song, G.Y.; Park, Y.M. Inhibitory effect of a naphthazarin derivative, S64, on heat shock factor (Hsf) activation and glutathione status following hypoxia. Cell Biol. Toxicol. 2003, 19, 273–284. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual Computational Chemistry Laboratory—Design and Description. J. Comput. Aided Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- Court, C.J.; Yildirim, B.; Jain, A.; Cole, J.M. 3-D Inorganic Crystal Structure Generation and Property Prediction via Representation Learning. J. Chem. Inf. Model. 2020, 60, 4518–4535. [Google Scholar] [CrossRef] [PubMed]
- Savko, M.; Kaščáková, S.; Mojzeš, P.; Jancura, D.; Miškovský, P.; Uličný, J. Ground and excited state properties of naphthazarin: Absorption spectroscopy and theoretical modeling study. J. Mol. Struct. Theochem 2007, 803, 79–87. [Google Scholar] [CrossRef]
- Ghahremani, F.A.; Zahedi-Tabrizi, M.; Tayyari, S.F. The nature of intramolecular hydrogen bond in Naphthazarin. Chem. Phys. 2020, 538, 110907. [Google Scholar] [CrossRef]
- Jezierska, A.; Błaziak, K.; Klahm, S.; Lüchow, A.; Panek, J.J. Non-Covalent Forces in Naphthazarin—Cooperativity or Competition in the Light of Theoretical Approaches. Int. J. Mol. Sci. 2021, 22, 8033. [Google Scholar] [CrossRef]
- Sobczyk, L.; Grabowski, S.J.; Krygowski, T.M. Interrelation between H-Bond and Pi-Electron Delocalization. Chem. Rev. 2005, 105, 3513–3560. [Google Scholar] [CrossRef] [PubMed]
- Denisov, G.S.; Mavri, J.; Sobczyk, L. Potential Energy Shape for the Proton Motion in Hydrogen Bonds Reflected in Infraredand NMR Spectra. In Hydrogen Bonding—New Insights, (Challenges and Advances in Computational Chemistry and Physics, 3), 1st ed.; Grabowski, S.J., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 377–416. [Google Scholar]
- Belkova, N.V.; Shubina, E.S.; Epstein, L.M. Diverse World of Unconventional Hydrogen Bonds. Acc. Chem. Res. 2005, 38, 624–631. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. Other weak and non-conventional hydrogen bonds. In The Weak Hydrogen Bond; Oxford University Press: Oxford, UK, 2001; pp. 122–292. [Google Scholar] [CrossRef]
- Scheiner, S. Hydrogen Bonding: A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Jeffrey, G. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Grabowski, S.J. What Is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef]
- Mes, T.; Smulders, M.M.J.; Palmans, A.R.A.; Meijer, E.W. Hydrogen-Bond Engineering in Supramolecular Polymers: Polarity Influence on the Self-Assembly of Benzene-1, 3, 5-tricarboxamides. Macromolecules 2010, 43, 1981–1991. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, M. A TDDFT study on the singlet and triplet excited-state hydrogen bonding and proton transfer of 10-hydroxybenzo[h]quinoline (HBQ) and 7, 9-diiodo-10-hydroxybenzo[h]quinoline (DIHBQ). Chem. Phys. Lett. 2011, 512, 35–39. [Google Scholar] [CrossRef]
- Głowacki, E.D.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Hydrogen-bonds in molecular solids—from biological systems to organic electronics. J. Mater. Chem. B 2013, 1, 3742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, L.D.; Macchi, P. The Role of Hydrogen Bond in Designing Molecular Optical Materials. Crystals 2016, 6, 43. [Google Scholar] [CrossRef]
- Jabłoński, M. A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions. Molecules 2020, 25, 5512. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, J.; Sobolewski, A.L. Modern Theoretical Approaches to Modeling the Excited-State Intramolecular Proton Transfer: An Overview. Molecules 2021, 26, 5140. [Google Scholar] [CrossRef] [PubMed]
- Shiju, J.; Al-Sagheer, F.; Ahmad, Z. Thermal Mechanical Properties of Graphene Nano-Composites with Kevlar-Nomex Copolymer: A Comparison of the Physical and Chemical Interactions. Polymers 2020, 12, 2740. [Google Scholar] [CrossRef]
- Ozgen, B. Physical properties of Kevlar and Nomex plied and covered yarns. Text. Res. J. 2012, 83, 752–760. [Google Scholar] [CrossRef]
- Tanner, D.; Fitzgerald, J.A.; Phillips, B.R. The Kevlar Story–an Advanced Materials Case Study. Angew. Chem. Int. Ed. Engl. 1989, 28, 649–654. [Google Scholar] [CrossRef]
- CCDC Structural Database. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 7 May 2021).
- Wheeler, A.S.; Carson, B.G. Hydroxynaphthoquinone Studies. VII. The Bromination of Naphthazarin. J. Am. Chem. Soc. 1927, 49, 2825–2829. [Google Scholar] [CrossRef]
- Gaultier, J.; Hauw, C. Structure de l’α-naphtoquinone. Acta Cryst. 1965, 18, 179–183. [Google Scholar] [CrossRef]
- Dar, U.A.; Bhand, S.N.; Lande, D.; Rao, S.S.; Patil, Y.P.; Gejji, S.P.; Nethaji, M.; Weyhermüller, T.; Salunke-Gawali, S. Molecular structures of 2-hydroxy-1,4-naphthoqinone derivatives and their zinc(II) complexes: Combining experiment and density functional theory. Polyhedron 2016, 113, 61–72. [Google Scholar] [CrossRef]
- Nowell, H.; Paul Attfield, J. X-Ray and neutron powder diffraction studies of the crystal structure of vitamin K3. New J. Chem. 2004, 28, 406–411. [Google Scholar] [CrossRef]
- Lin, A.J.; Lillis, B.J.; Sartorelli, A.C. Potential bioreductive alkylating agents. 5. Antineoplastic activity of quinoline-5,8-diones, naphthazarins, and naphthoquinones. J. Med. Chem. 1975, 18, 917–921. [Google Scholar] [CrossRef]
- Redaelli, M.; Mucignat-Caretta, C.; Isse, A.A.; Gennaro, A.; Pezzani, R.; Pasquale, R.; Pavan, V.; Crisma, M.; Ribaudo, G.; Zagotto, G. New naphthoquinone derivatives against glioma cells. Eur. J. Med. Chem. 2015, 96, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Gray, D.L.F. Total Synthesis of Hybocarpone and Analogues Thereof. A Facile Dimerization of Naphthazarins to Pentacyclic Systems. J. Am. Chem. Soc. 2004, 126, 607–612. [Google Scholar] [CrossRef]
- Choudhari, D.; Chakravarty, D.; Lande, D.N.; Parveen, S.; Gejji, S.P.; Kodam, K.M.; Salunke-Gawali, S. Crystal structures and biological activity of homologated (N)-n-alkylammonium salts of 2-bromo-3-oxido-1,4-naphthoquinone. Struct. Chem. 2019, 30, 2257–2270. [Google Scholar] [CrossRef]
- Fischer, T.C.; Gosch, C.; Mirbeth, B.; Gselmann, M.; Thallmair, V.; Stich, K. Potent and Specific Bactericidal Effect of Juglone (5-Hydroxy-1,4-naphthoquinone) on the Fire Blight Pathogen Erwinia amylovora. J. Agric. Food Chem. 2012, 60, 12074–12081. [Google Scholar] [CrossRef]
- Mochida, T.; Funasako, Y.; Azumi, H. Charge-transfer complexes from decamethylferrocene and 1,4-quinone derivatives: Neutral–ionic phase diagrams for metallocene complexes. Dalton Trans. 2011, 40, 9221–9228. [Google Scholar] [CrossRef] [Green Version]
- Dias, G.G.; Rogge, T.; Kuniyil, R.; Jacob, C.; Menna-Barreto, R.F.S.; da Silva Júnior, E.N.; Ackermann, L. Ruthenium-catalyzed C–H oxygenation of quinones by weak O-coordination for potent trypanocidal agents. Chem. Commun. 2018, 54, 12840–12843. [Google Scholar] [CrossRef] [Green Version]
- Dessolin, J.; Biot, C.; Davioud-Charvet, E. Bromination Studies of the 2,3-Dimethylnaphthazarin Core Allowing Easy Access to Naphthazarin Derivatives. J. Org. Chem. 2001, 66, 5616–5619. [Google Scholar] [CrossRef]
- Jardim, G.A.M.; da Silva Júnior, E.N.; Bower, J.F. Overcoming naphthoquinone deactivation: Rhodium-catalyzed C-5 selective C–H iodination as a gateway to functionalized derivatives. Chem. Sci. 2016, 7, 3780–3784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonin, M.D.L.; Garden, S.J.; Jotani, M.M.; Wardell, S.M.S.V.; Wardell, J.L.; Tiekink, E.R.T. The 1:1 co-crystal of 2-bromonaphthalene-1,4-dione and 1,8-dihydroxyanthracene-9,10-dione: Crystal structure and Hirshfeld surface analysis. Acta Cryst. Sect. E 2017, 73, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Deng, G.; Zheng, Y.Z.; Xu, J.; Ashraf, H.; Yu, Z.W. Evidences for Cooperative Resonance-Assisted Hydrogen Bonds in Protein Secondary Structure Analogs. Sci. Rep. 2016, 6, 36932. [Google Scholar] [CrossRef]
- Ohno, K.; Okimura, M.; Akai, N.; Katsumoto, Y. The effect of cooperative hydrogen bonding on the OH stretching-band shift for water clusters studied by matrix-isolation infrared spectroscopy and density functional theory. Phys. Chem. Chem. Phys. 2005, 7, 3005. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Car, R.; Parrinello, M. Unified Approach for Molecular Dynamics and Density-Functional Theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cradwick, P.D.; Hall, D. The crystal structure of juglone. Acta Cryst. B 1971, 27, 1468–1470. [Google Scholar] [CrossRef]
- Mercury—Crystal Structure Visualisation. Available online: http://www.ccdc.cam.ac.uk/Solutions/CSDSystem/Pages/Mercury.aspx (accessed on 7 May 2021).
- Schaftenaar, G.; Noordik, J. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput. Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom—Atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615. [Google Scholar] [CrossRef] [Green Version]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11—18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Troullier, N.; Martins, J.L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Hockney, R.W. Potential Calculation and Some Applications. Methods Comput. Phys. 1970, 9, 135–211. [Google Scholar]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD–Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Williams, T.; Kelley, C. Gnuplot 4.4: An Interactive Plotting Program. 2010. Available online: http://gnuplot.sourceforge.net/ (accessed on 12 May 2021).
- CPMD 3.17.1, Copyright IBM Corp. (1990–2004) Copyright MPI für Festkoerperforschung Stuttgart (1997–2001). Available online: http://www.cpmd.org (accessed on 7 May 2021).


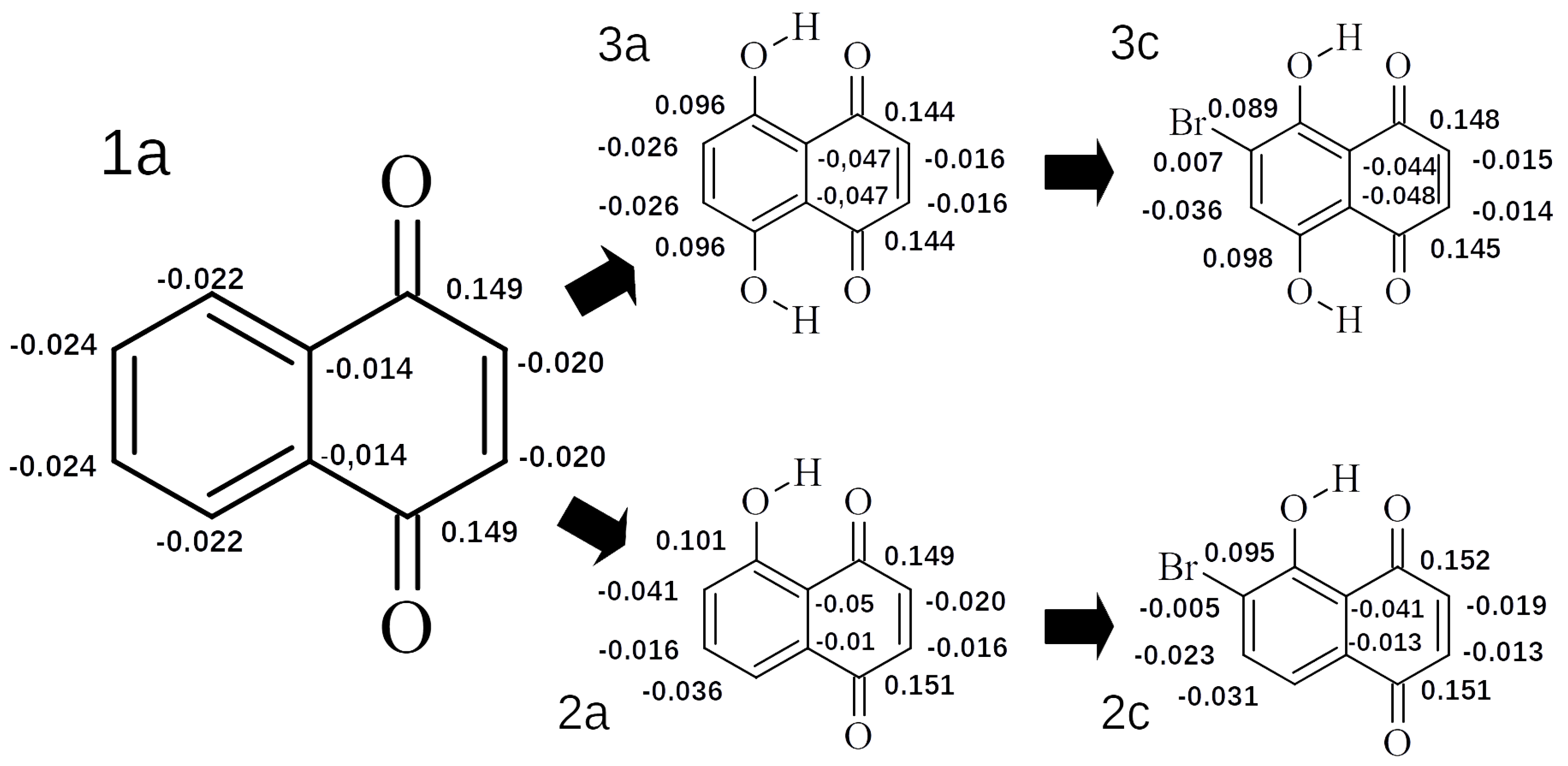

| Compounds | R | R | R | R |
|---|---|---|---|---|
| 1a, 2a, 3a | –H | –H | –H | –H |
| 1b, 2b, 3b | –Br | –H | –H | –H |
| 2b(M) | –H | –Br | –H | –H |
| 1c, 2c, 3c | –H | –H | –H | –Br |
| 2c(M) | –H | –H | –Br | –H |
| 1d, 2d, 3d | –Br | –H | –Br | –H |
| 2d(M) | –H | –Br | –H | –Br |
| 1e, 2e, 3e | –Br | –Br | –H | –H |
| 1f, 2f, 3f | –Br | –H | –H | –Br |
| 2f(M) | –H | –Br | –Br | –H |
| 1g, 2g, 3g | –H | –H | –Br | –Br |
| Compounds | 1a, X-ray [21] | 1a, DFT | 2a, X-ray [76] | 2a, DFT | 3a, X-ray [22] | 3a, DFT |
|---|---|---|---|---|---|---|
| C1=O | 1.222 | 1.211 | 1.257 | 1.228 | 1.247 | 1.231 |
| C4=O | 1.223 | 1.211 | 1.205 | 1.211 | 1.240 | 1.231 |
| C8-O | – | – | 1.329 | 1.332 | 1.508 | 1.331 |
| C5-O | – | – | – | – | 1.341 | 1.331 |
| C1-C2 | 1.474 | 1.484 | 1.437 | 1.479 | 1.478 | 1.477 |
| C2-C3 | 1.332 | 1.331 | 1.350 | 1.331 | 1.343 | 1.332 |
| C3-C4 | 1.473 | 1.484 | 1.478 | 1.484 | 1.478 | 1.447 |
| C4-C10 | 1.486 | 1.491 | 1.500 | 1.492 | 1.476 | 1.459 |
| C10-C5 | 1.393 | 1.389 | 1.339 | 1.378 | 1.402 | 1.391 |
| C5-C6 | 1.384 | 1.385 | 1.413 | 1.396 | 1.417 | 1.412 |
| C6-C7 | 1.388 | 1.390 | 1.389 | 1.376 | 1.384 | 1.364 |
| C7-C8 | 1.382 | 1.385 | 1.416 | 1.400 | 1.430 | 1.412 |
| C8-C9 | 1.394 | 1.389 | 1.388 | 1.404 | 1.309 | 1.391 |
| C9-C10 | 1.404 | 1.397 | 1.380 | 1.408 | 1.433 | 1.421 |
| C1-C9 | 1.490 | 1.491 | 1.477 | 1.463 | 1.461 | 1.459 |
| Compounds | 2b, X-ray [62] | 2b, DFT | 2b(M), X-ray [67] | 2b(M), DFT | |
|---|---|---|---|---|---|
| mol.1 | mol.2 | ||||
| C1=O | 1.224 | 1.231 | 1.222 | 1.240 | 1.228 |
| C4=O | 1.207 | 1.205 | 1.211 | 1.218 | 1.205 |
| C8-O | 1.348 | 1.357 | 1.330 | 1.346 | 1.331 |
| C1-C2 | 1.487 | 1.483 | 1.495 | 1.471 | 1.476 |
| C2-Br | 1.869 | 1.887 | 1.880 | – | – |
| C3-Br | – | – | – | 1.876 | 1.878 |
| C2-C3 | 1.334 | 1.318 | 1.330 | 1.341 | 1.331 |
| C3-C4 | 1.474 | 1.478 | 1.481 | 1.492 | 1.500 |
| C4-C10 | 1.492 | 1.491 | 1.489 | 1.488 | 1.493 |
| C10-C5 | 1.379 | 1.398 | 1.377 | 1.386 | 1.378 |
| C5-C6 | 1.400 | 1.384 | 1.396 | 1.397 | 1.396 |
| C6-C7 | 1.388 | 1.368 | 1.375 | 1.383 | 1.376 |
| C7-C8 | 1.382 | 1.379 | 1.400 | 1.402 | 1.400 |
| C8-C9 | 1.411 | 1.404 | 1.406 | 1.405 | 1.404 |
| C9-C10 | 1.417 | 1.408 | 1.407 | 1.411 | 1.406 |
| C1-C9 | 1.451 | 1.454 | 1.464 | 1.462 | 1.461 |
| Compound | O–H | O–H |
|---|---|---|
| 3a | 66.9 | 67.7 |
| 3b | 79.3 | 77.5 |
| 3c | 2.6 | 2.7 |
| 3d | 44.3 | 47.7 |
| 3e | 96.8 | 96.7 |
| 3f | 53.2 | 51.7 |
| 3g | 3.1 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pocheć, M.; Kułacz, K.; Panek, J.J.; Jezierska, A. How Substitution Combines with Non-Covalent Interactions to Modulate 1,4-Naphthoquinone and Its Derivatives Molecular Features—Multifactor Studies. Int. J. Mol. Sci. 2021, 22, 10357. https://doi.org/10.3390/ijms221910357
Pocheć M, Kułacz K, Panek JJ, Jezierska A. How Substitution Combines with Non-Covalent Interactions to Modulate 1,4-Naphthoquinone and Its Derivatives Molecular Features—Multifactor Studies. International Journal of Molecular Sciences. 2021; 22(19):10357. https://doi.org/10.3390/ijms221910357
Chicago/Turabian StylePocheć, Michał, Karol Kułacz, Jarosław J. Panek, and Aneta Jezierska. 2021. "How Substitution Combines with Non-Covalent Interactions to Modulate 1,4-Naphthoquinone and Its Derivatives Molecular Features—Multifactor Studies" International Journal of Molecular Sciences 22, no. 19: 10357. https://doi.org/10.3390/ijms221910357
APA StylePocheć, M., Kułacz, K., Panek, J. J., & Jezierska, A. (2021). How Substitution Combines with Non-Covalent Interactions to Modulate 1,4-Naphthoquinone and Its Derivatives Molecular Features—Multifactor Studies. International Journal of Molecular Sciences, 22(19), 10357. https://doi.org/10.3390/ijms221910357







