A Systematic Review and Meta-Analysis on the Significance of TIGIT in Solid Cancers: Dual TIGIT/PD-1 Blockade to Overcome Immune-Resistance in Solid Cancers
Abstract
:1. Introduction
2. Material and Methods
2.1. The Strategy of the Systematic Search
2.2. Study Selection and Data Extraction
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Assessing the Potential Risk of Bias among the Included Studies
2.6. Statistical Analysis
3. Results
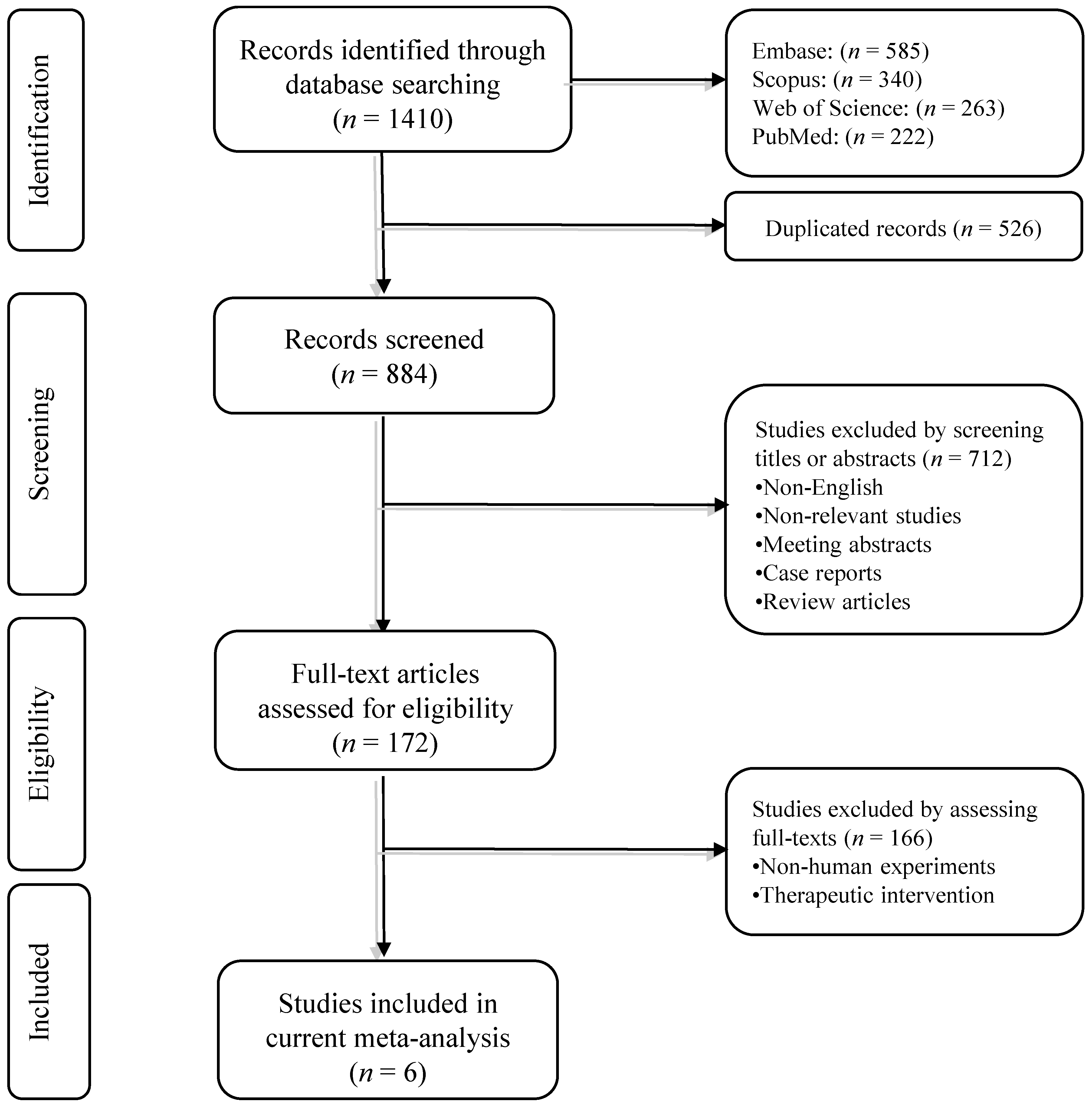
3.1. Systematic Search
3.2. The Characteristic of Included Studies
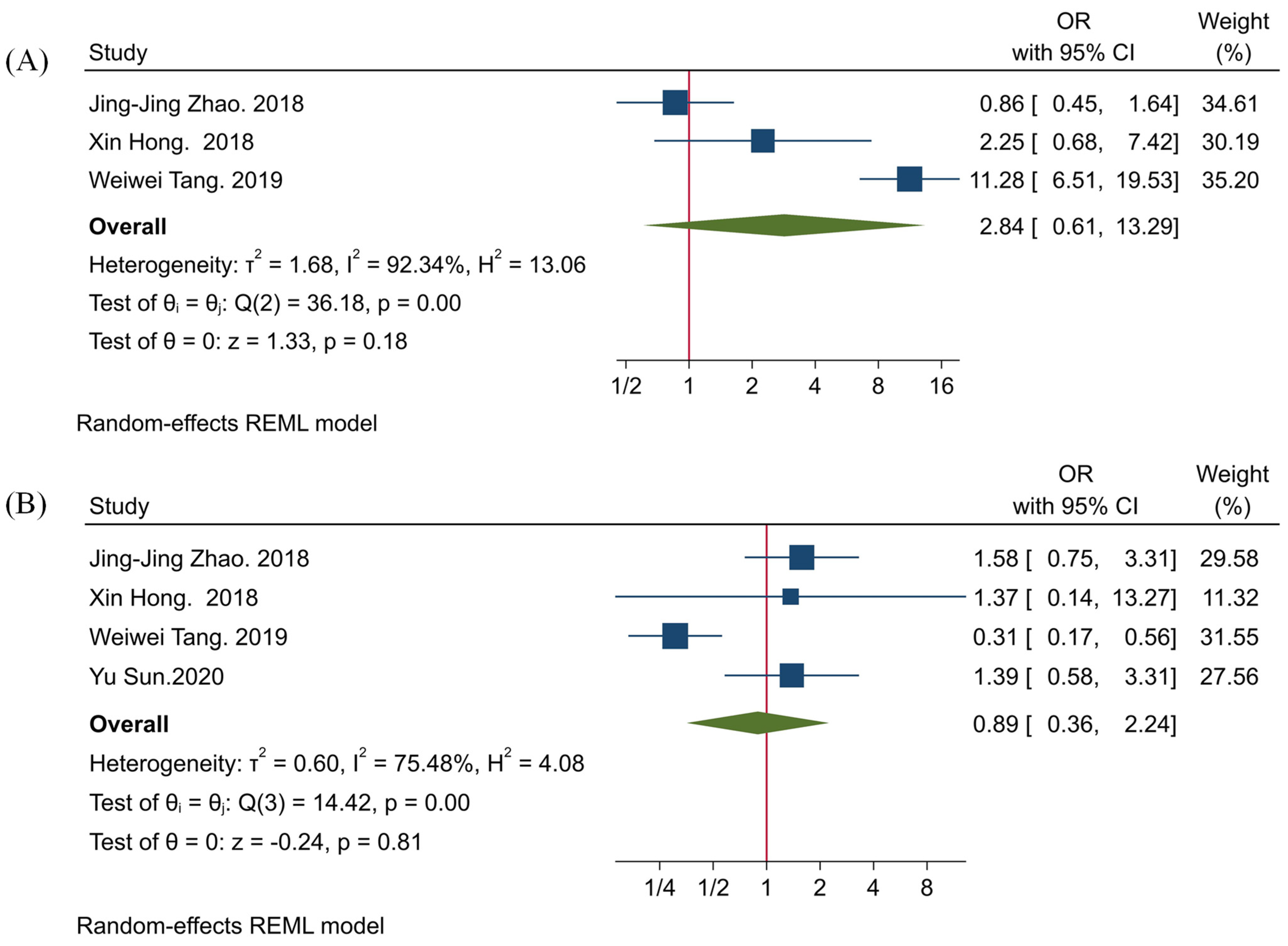
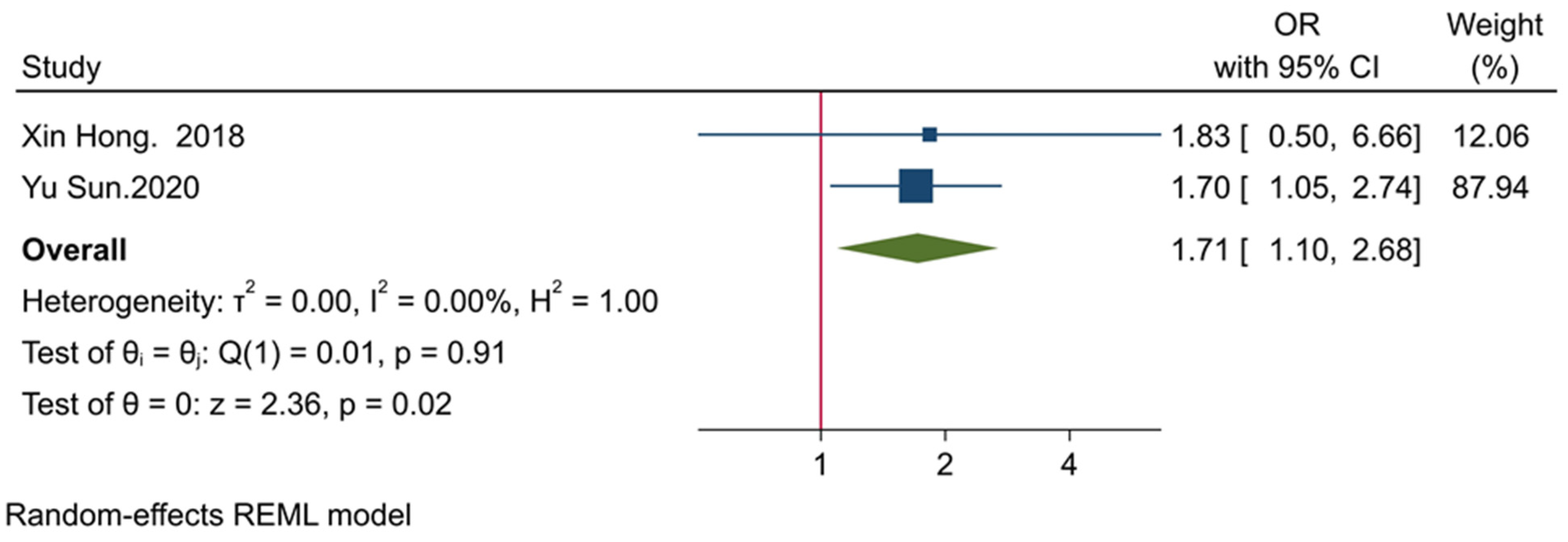
3.3. The Clinicopathological Significance of TIGIT
3.4. The Association between TIGIT and PD-1
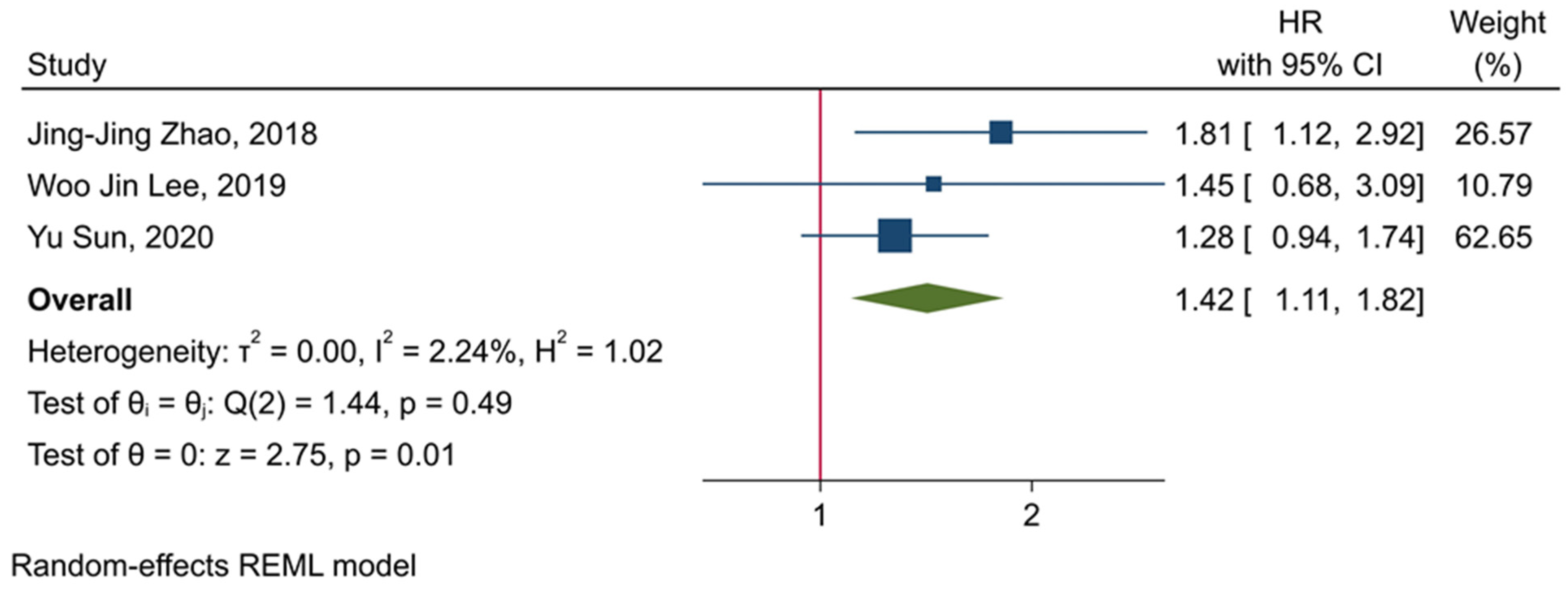
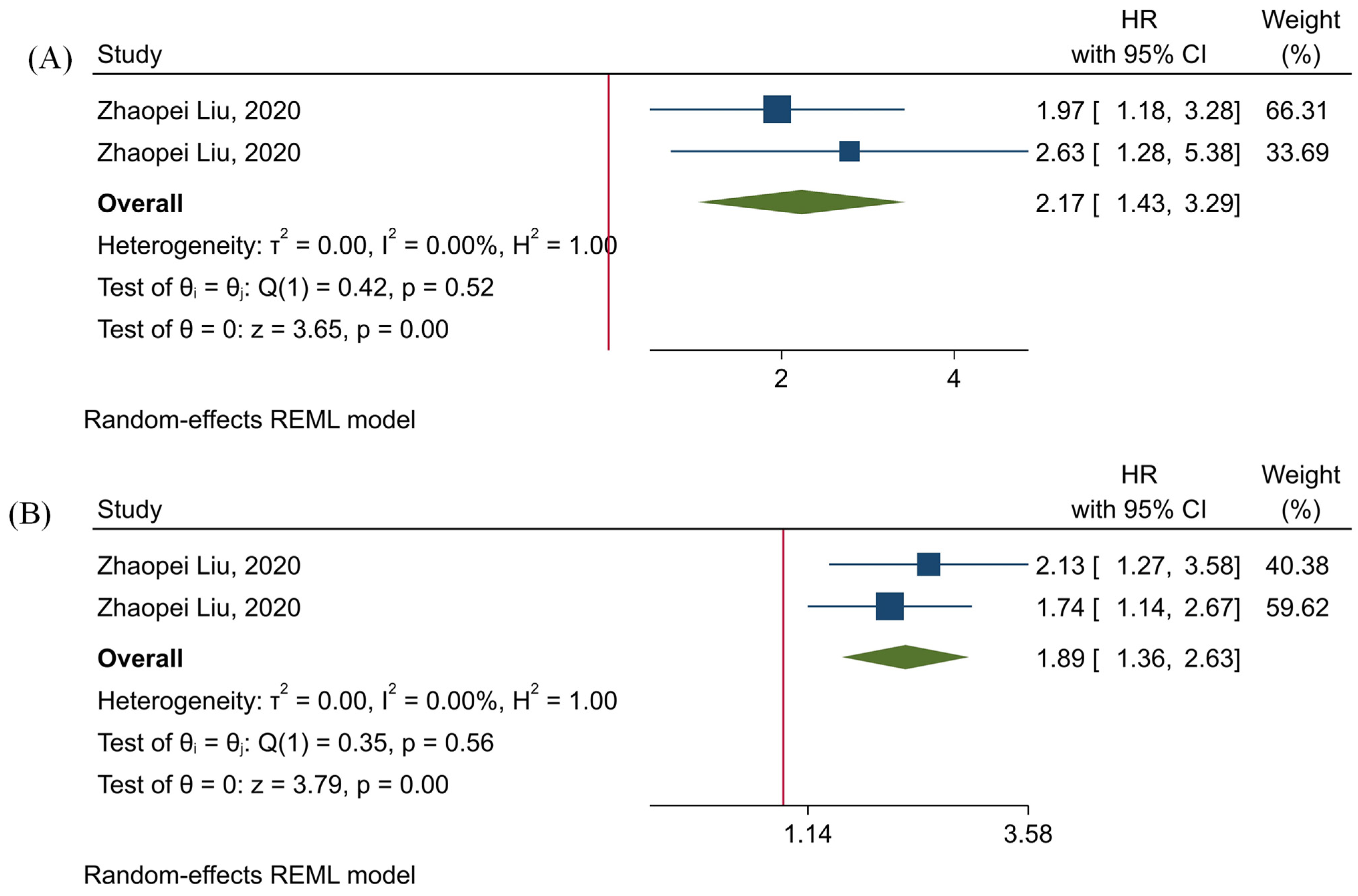
3.5. The Prognostic Value of TIGIT
3.6. The Prognostic Value of Tumor-Infiltrating TIGIT+CD8+ T-Cells
3.7. Assessing Potential Bias among the Included Studies
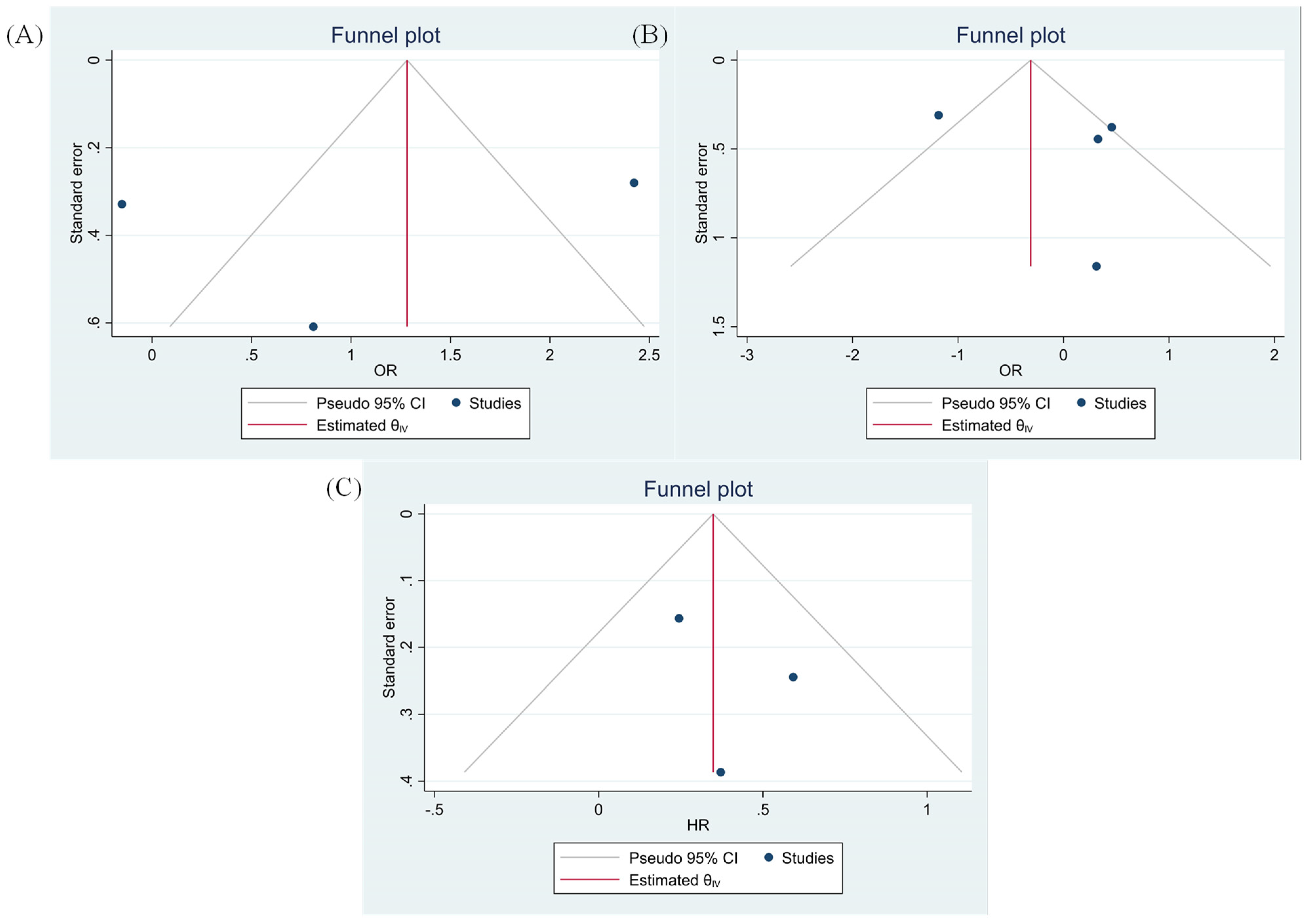
3.8. Evaluating Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sambi, M.; Bagheri, L.; Szewczuk, M.R. Current challenges in cancer immunotherapy: Multimodal approaches to improve efficacy and patient response rates. J. Oncol. 2019, 2019, 4508794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, J.; Wickman, E.; DeRenzo, C.; Gottschalk, S. CAR T-cell Therapy for Solid Tumors: Bright Future or Dark Reality? Mol. Ther. 2020, 28, 2320–2339. [Google Scholar] [CrossRef] [PubMed]
- Lotfinejad, P.; Asghari Jafarabadi, M.; Abdoli Shadbad, M.; Kazemi, T.; Pashazadeh, F.; Sandoghchian Shotorbani, S.; Jadidi Niaragh, F.; Baghbanzadeh, A.; Vahed, N.; Silvestris, N. Prognostic role and clinical significance of tumor-infiltrating lymphocyte (TIL) and programmed death ligand 1 (PD-L1) expression in triple-negative breast cancer (TNBC): A systematic review and meta-analysis study. Diagnostics 2020, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, A.; Rostami, Z.; Safarpour, H.; Shadbad, M.A.; Nourbakhsh, N.S.; Argentiero, A.; Taefehshokr, S.; Tabrizi, N.J.; Kooshkaki, O.; Astamal, R.V. From Oncogenic Signaling Pathways to Single-Cell Sequencing of Immune Cells: Changing the Landscape of Cancer Immunotherapy. Molecules 2021, 26, 2278. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, N.; Derakhshani, A.; Kooshkaki, O.; Abdoli Shadbad, M.; Hajiasgharzadeh, K.; Baghbanzadeh, A.; Safarpour, H.; Mokhtarzadeh, A.; Brunetti, O.; Yue, S.C. Immune Checkpoints and CAR-T Cells: The Pioneers in Future Cancer Therapies? Int. J. Mol. Sci. 2020, 21, 8305. [Google Scholar] [CrossRef]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1: PD-L1/2 pathway from discovery to clinical implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef] [Green Version]
- Shadbad, M.A.; Asadzadeh, Z.; Derakhshani, A.; Hosseinkhani, N.; Mokhtarzadeh, A.; Baghbanzadeh, A.; Hajiasgharzadeh, K.; Brunetti, O.; Argentiero, A.; Racanelli, V.; et al. A scoping review on the potentiality of PD-L1-inhibiting microRNAs in treating colorectal cancer: Toward single-cell sequencing-guided biocompatible-based delivery. Biomed. Pharmacother. 2021, 143, 112213. [Google Scholar] [CrossRef] [PubMed]
- Shadbad, M.A.; Asadzadeh, Z.; Hosseinkhani, N.; Derakhshani, A.; Alizadeh, N.; Brunetti, O.; Silvestris, N.; Baradaran, B. A Systematic Review of the Tumor-Infiltrating CD8+ T-Cells/PD-L1 Axis in High-Grade Glial Tumors: Toward Personalized Immuno-Oncology. Front. Immunol. 2021, 12, 634181. [Google Scholar] [CrossRef]
- Shadbad, M.A.; Safaei, S.; Brunetti, O.; Derakhshani, A.; Lotfinejad, P.; Mokhtarzadeh, A.; Hemmat, N.; Racanelli, V.; Solimando, A.G.; Argentiero, A. A Systematic Review on the Therapeutic Potentiality of PD-L1-Inhibiting MicroRNAs for Triple-Negative Breast Cancer: Toward Single-Cell Sequencing-Guided Biomimetic Delivery. Genes 2021, 12, 1206. [Google Scholar] [CrossRef]
- Deng, W.; Ma, Y.; Su, Z.; Liu, Y.; Liang, P.; Huang, C.; Liu, X.; Shao, J.; Zhang, Y.; Zhang, K.; et al. Single-cell RNA-sequencing analyses identify heterogeneity of CD8(+) T cell subpopulations and novel therapy targets in melanoma. Mol. Ther. Oncolytics 2020, 20, 105–118. [Google Scholar] [CrossRef]
- Kim, M.; Min, Y.K.; Jang, J.; Park, H.; Lee, S.; Lee, C.H. Single-cell RNA sequencing reveals distinct cellular factors for response to immunotherapy targeting CD73 and PD-1 in colorectal cancer. J. Immunother. Cancer 2021, 9, e002503. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Wu, L.; Mao, L.; Liu, J.-F.; Chen, L.; Yu, G.-T.; Yang, L.-L.; Wu, H.; Bu, L.-L.; Kulkarni, A.B.; Zhang, W.-F. Blockade of TIGIT/CD155 signaling reverses T-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol. Res. 2019, 7, 1700–1713. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.L.; Maxwell, R.; Theodros, D.; Belcaid, Z.; Mathios, D.; Luksik, A.S.; Kim, E.; Wu, A.; Xia, Y.; Garzon-Muvdi, T.; et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology 2018, 7, e1466769. [Google Scholar] [CrossRef] [PubMed]
- Sunseri, N.; Chen, X.; Wald, N.; Preillon, J.; Smith, S.M.; Driessens, G.; Kline, J. Beyond PD-1: Investigating the Therapeutic Potential of TIGIT Blockade in DLBCL; American Society of Hematology: Washington, DC, USA, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, J.A.; Côté, P.; Bombardier, C. Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 2006, 144, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, R.J.; Thompson, S.G. A likelihood approach to meta-analysis with random effects. Stat. Med. 1996, 15, 619–629. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-J.; Zhou, Z.-Q.; Wang, P.; Chen, C.-L.; Liu, Y.; Pan, Q.-Z.; Zhu, Q.; Tang, Y.; Weng, D.-S.; Xia, J.-C. Orchestration of immune checkpoints in tumor immune contexture and their prognostic significance in esophageal squamous cell carcinoma. Cancer Manag. Res. 2018, 10, 6457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Wang, X.; Wang, T.; Zhang, X. Correlation of T cell immunoglobulin and ITIM Domain (TIGIT) and programmed death 1 (PD-1) with clinicopathological characteristics of renal cell carcinoma may indicate potential targets for treatment. Med Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 6861. [Google Scholar] [CrossRef]
- Tang, W.; Pan, X.; Han, D.; Rong, D.; Zhang, M.; Yang, L.; Ying, J.; Guan, H.; Chen, Z.; Wang, X. Clinical significance of CD8+ T cell immunoreceptor with Ig and ITIM domains+ in locally advanced gastric cancer treated with SOX regimen after D2 gastrectomy. Oncoimmunology 2019, 8, e1593807. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Lee, Y.J.; Choi, M.E.; Yun, K.A.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E. Expression of lymphocyte-activating gene 3 and T-cell immunoreceptor with immunoglobulin and ITIM domains in cutaneous melanoma and their correlation with programmed cell death 1 expression in tumor-infiltrating lymphocytes. J. Am. Acad. Dermatol. 2019, 81, 219–227. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, J.; Chen, Y.; Cui, J.; Lei, Y.; Cui, Y.; Jiang, N.; Jiang, W.; Chen, L.; Chen, Y. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma). Int. Immunopharmacol. 2020, 80, 106198. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Q.; Wang, Z.; Zhang, H.; Zeng, H.; Huang, Q.; Chen, Y.; Jiang, W.; Lin, Z.; Qu, Y. Intratumoral TIGIT+ CD8+ T-cell infiltration determines poor prognosis and immune evasion in patients with muscle-invasive bladder cancer. J. Immunother. Cancer 2020, 8, e000978. [Google Scholar] [CrossRef]
- Shadbad, M.A.; Hajiasgharzadeh, K.; Derakhshani, A.; Silvestris, N.; Baghbanzadeh, A.; Racanelli, V.; Baradaran, B. From Melanoma Development to RNA-Modified Dendritic Cell Vaccines: Highlighting the Lessons From the Past. Front. Immunol. 2021, 12, 331. [Google Scholar] [CrossRef]
- Stanietsky, N.; Rovis, T.L.; Glasner, A.; Seidel, E.; Tsukerman, P.; Yamin, R.; Enk, J.; Jonjic, S.; Mandelboim, O. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur. J. Immunol. 2013, 43, 2138–2150. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Li, L.; Lu, F.; Yue, J.; Liu, Z.; Zhang, W.; Fu, R. Overexpression of TIGIT in NK and T Cells Contributes to Tumor Immune Escape in Myelodysplastic Syndromes. Front. Oncol. 2020, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, H.; Han, F.; Chen, X.; Lin, R.; Wang, W.; Qiu, H.; Zhuang, Z.; Liao, Q.; Zhang, W. CD155T/TIGIT signaling regulates CD8+ T-cell metabolism and promotes tumor progression in human gastric cancer. Cancer Res. 2017, 77, 6375–6388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.-M.; Li, W.-Q.; Wu, Y.-H.; Han, L.; Cao, X.-G.; Yang, X.-M.; Wang, H.-F.; Zhao, W.-S.; Zhai, W.-J.; Qi, Y.-M. Intrinsic expression of immune checkpoint molecule TIGIT could help tumor growth in vivo by suppressing the function of NK and CD8+ T cells. Front. Immunol. 2018, 9, 2821. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yu, Y.; Yang, S.; Zeng, B.; Zhang, Z.; Jiao, G.; Zhang, Y.; Cai, L.; Yang, R. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol. Immunother. 2009, 58, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J.; Ochoa, A.C. myeloid derived suppressor cells as disease modulators. Front. Immunol. 2020, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Dufait, I.; Schwarze, J.K.; Liechtenstein, T.; Leonard, W.; Jiang, H.; Escors, D.; De Ridder, M.; Breckpot, K. Ex vivo generation of myeloid-derived suppressor cells that model the tumor immunosuppressive environment in colorectal cancer. Oncotarget 2015, 6, 12369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuvers, M.E.; Muskens, F.; Bezemer, K.; Lambers, M.; Dingemans, A.-M.C.; Groen, H.J.; Smit, E.F.; Hoogsteden, H.C.; Hegmans, J.P.; Aerts, J.G. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer 2013, 81, 468–474. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, X.; Li, W.; Feng, Q.; Feng, H.; Tong, Y.; Rong, H.; Wang, W.; Zhang, D.; Zhang, Z. Circulating and tumor-infiltrating arginase 1-expressing cells in gastric adenocarcinoma patients were mainly immature and monocytic Myeloid-derived suppressor cells. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Pang, X.; Fan, H.-Y.; Tang, Y.-L.; Wang, S.-S.; Cao, M.-X.; Wang, H.-F.; Dai, L.-L.; Wang, K.; Yu, X.-H.; Wu, J.-B. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE 2020, 15, e0229089. [Google Scholar] [CrossRef] [Green Version]
- Han, H.S.; Jeong, S.; Kim, H.; Kim, H.-D.; Kim, A.R.; Kwon, M.; Park, S.-H.; Woo, C.G.; Kim, H.K.; Lee, K.H. TOX-expressing terminally exhausted tumor-infiltrating CD8+ T cells are reinvigorated by co-blockade of PD-1 and TIGIT in bladder cancer. Cancer Lett. 2020, 499, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Inozume, T.; Yaguchi, T.; Furuta, J.; Harada, K.; Kawakami, Y.; Shimada, S. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J. Investig. Dermatol. 2016, 136, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Abreu, D.; Johnson, M.L.; Hussein, M.A.; Cobo, M.; Patel, A.J.; Secen, N.M.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.-H. Primary Analysis of a Randomized, Double-Blind, Phase II Study of the Anti-TIGIT Antibody Tiragolumab (tira) Plus Atezolizumab (atezo) versus Placebo Plus Atezo as First-Line (1L) Treatment in Patients with PD-L1-Selected NSCLC (CITYSCAPE); American Society of Clinical Oncology: Alexandria, VA, USA, 2020. [Google Scholar]
| No. | The First Author and Publication Year | Country | Sample Size | Male/Female Ratio | Median Age | High Stage/Low Stage | Cancer Type | Endpoint | Cancer Therapy Record | TIGIT Evaluation Method | TIGIT Antibody ID |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zhao, 2018 | China | 154 | 4.13 | 55 | 0.57 | Esophageal squamous cell carcinoma | OS | No chemotherapy/immunotherapy before surgery | IHC | MBSA43 |
| 2 | Hong, 2018 | China | 60 | 1.72 | 55.6 | 0.07 | Renal cell carcinoma | Clinicopathological association | No radiotherapy, chemotherapy, and biological therapy before surgery | IHC | N/a |
| 3 | Tang, 2019 | China | 441 | 1.25 | N/a | 0.36 | Gastric adenocarcinoma | Clinicopathological association | No neoadjuvant chemotherapy/radiotherapy before surgery | IHC | ab233404 |
| 4 | Lee, 2019 | South Korea | 124 | 1.21 | N/a | 2.12 | Cutaneous melanoma | OS | N/a | IHC | TG1 |
| 5 | Sun, 2020 | China | 334 | 1.19 | 56 | 0.39 | Lung adenocarcinoma | OS | N/a | IHC | MBS20013451 |
| 6 | Liu, 2020 | China | 141 | 4.87 | 62 | 0.62 | Muscle-invasive bladder cancer | OS/RFS | One hundred nineteen patients of these cohorts received adjuvant cisplatin-based chemotherapy. | IHC | ab243903 |
| 7 | Liu, 2020 | China | 118 | 6.37 | 62 | 1.68 | Muscle-invasive bladder cancer | OS/RFS | IHC | ab243903 |
| No. | The First Author and Publication Year | Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Confounding Measurement and Account | Analysis |
|---|---|---|---|---|---|---|---|
| 1 | Zhao, 2018 | *** | *** | *** | *** | ** | *** |
| 2 | Lee, 2019 | ** | *** | *** | *** | * | *** |
| 3 | Sun, 2020 | *** | *** | *** | *** | ** | *** |
| 4 | Liu, 2020 | *** | *** | *** | *** | ** | *** |
| Major Components | Hong, 2018 | Tang, 2019 |
|---|---|---|
| 1. Were the criteria for inclusion in the sample clearly defined? | Yes | Yes |
| 2. Were the study subjects and the setting described in detail? | Yes | Yes |
| 3. Was the exposure measured in a valid and reliable way? | Yes | Yes |
| 4. Were objective, standard criteria used for measurement of the condition? | Yes | Yes |
| 5. Were confounding factors identified? | Unclear | Unclear |
| 6. Were strategies to deal with confounding factors stated? | Unclear | Unclear |
| 7. Were the outcomes measured in a valid and reliable way? | Yes | Yes |
| 8. Was appropriate statistical analysis used? | Yes | Yes |
| No. | Intervention | Mechanism of Action | Cancer Type | Clinical Trial Phase | Study Start Date | The Status | Country | Clinicaltrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|
| 1 | ASP8374, ASP8374 + Pembrolizumab | an anti-TIGIT mAb + an anti-PD-1 mAb | Advanced solid tumors | Phase 1 | 8-September-17 | Active, not recruiting | International | NCT03260322 |
| 2 | Tiragolumab, Tiragolumab + Atezolizumab | an anti-TIGIT mAb + an anti-PD-L1 mAb | Advanced/Metastatic tumors | Phase 1 | 23-May-16 | Recruiting | International | NCT02794571 |
| 3 | BGB-A1217+ Tislelizumab | an anti-TIGIT mAb | Advanced solid tumors | Phase 1 | 26-August-19 | Recruiting | International | NCT04047862 |
| 4 | Tiragolumab + Atezolizumab | an anti-TIGIT mAb + an anti-PD-L1 mAb | NSCLC | Phase 2 | 10-August-18 | Active, not recruiting | International | NCT03563716 |
| 5 | AB154 + Zimberelimab | an anti-TIGIT mAb + an anti-PD-1 mAb | Advanced solid tumors | Phase 1 | 12-September-18 | Recruiting | International | NCT03628677 |
| 6 | vibostolimab, vibostolimab + Pembrolizumab | an anti-TIGIT mAb + an anti-PD-1 mAb | Advanced solid tumors | Phase 1 | 13-December-16 | Recruiting | International | NCT02964013 |
| 7 | BMS-986207, BMS-986207 + Nivolumab | an anti-TIGIT mAb + an anti-PD-1 mAb | Broad solid tumors | Phase 1/2 | 29-November-16 | Active, not recruiting | International | NCT02913313 |
| 8 | Atezolizumab, Atezolizumab + Tiragolumab | an anti-PD-L1 mAb + an anti-TIGIT mAb | SCLC | Phase 3 | 4-February-20 | Recruiting | International | NCT04256421 |
| 9 | Tiragolumab + Atezolizumab | an anti-TIGIT mAb + an anti-PD-L1 mAb | NSCLC | Phase 3 | 4-March-20 | Recruiting | International | NCT04294810 |
| 10 | Tiragolumab+ Atezolizumab | an anti-TIGIT mAb + an anti-PD-L1 mAb | Gastric cancer | Phase 1/2 | 13-October-17 | Recruiting | International | NCT03281369 |
| 11 | AB154+ zimberelimab | an anti-TIGIT mAb + an anti-PD-1 mAb | NSCLC | Phase 2 | 10-February-20 | Recruiting | International | NCT04262856 |
| 13 | BMS-986207 + Nivolumab + COM701 | an anti-TIGIT mAb + an anti-PD-1 mAb + an anti-PVRIG mAb | Advanced solid tumors | Phase 1/2 | 31-August-20 | Recruiting | United States | NCT04570839 |
| 14 | Atezolizumab, Atezolizumab + Tiragolumab | an anti-PD-L1 mAb + an anti-TIGIT mAb | Esophageal squamous cell carcinoma | Phase 3 | 28-September-20 | Recruiting | International | NCT04543617 |
| 15 | Tislelizumab, Tislelizumab + BGB-A1217 | an anti-TIGIT mAb+ an anti-PD-1 mAb | Cervical cancer | Phase 2 | 25-January-21 | Not yet recruiting | China | NCT04693234 |
| 17 | M6223 | an anti-TIGIT mAb | Metastatic solid tumors | Phase 1 | 10-July-20 | Recruiting | International | NCT04457778 |
| 18 | IBI939 | an anti-TIGIT mAb | Advanced NSCLC | Phase 1 | 6-June-21 | Not yet recruiting | China | NCT04672369 |
| 19 | IBI939 + Sintilimab | an anti-TIGIT mAb + an anti-PD-1 mAb | Advanced lung cancer | Phase 1 | 28-January-21 | Not yet recruiting | China | NCT04672356 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseinkhani, N.; Shadbad, M.A.; Asghari Jafarabadi, M.; Karim Ahangar, N.; Asadzadeh, Z.; Mohammadi, S.M.; Lotfinejad, P.; Alizadeh, N.; Brunetti, O.; Fasano, R.; et al. A Systematic Review and Meta-Analysis on the Significance of TIGIT in Solid Cancers: Dual TIGIT/PD-1 Blockade to Overcome Immune-Resistance in Solid Cancers. Int. J. Mol. Sci. 2021, 22, 10389. https://doi.org/10.3390/ijms221910389
Hosseinkhani N, Shadbad MA, Asghari Jafarabadi M, Karim Ahangar N, Asadzadeh Z, Mohammadi SM, Lotfinejad P, Alizadeh N, Brunetti O, Fasano R, et al. A Systematic Review and Meta-Analysis on the Significance of TIGIT in Solid Cancers: Dual TIGIT/PD-1 Blockade to Overcome Immune-Resistance in Solid Cancers. International Journal of Molecular Sciences. 2021; 22(19):10389. https://doi.org/10.3390/ijms221910389
Chicago/Turabian StyleHosseinkhani, Negar, Mahdi Abdoli Shadbad, Mohammad Asghari Jafarabadi, Noora Karim Ahangar, Zahra Asadzadeh, Seyede Momeneh Mohammadi, Parisa Lotfinejad, Nazila Alizadeh, Oronzo Brunetti, Rossella Fasano, and et al. 2021. "A Systematic Review and Meta-Analysis on the Significance of TIGIT in Solid Cancers: Dual TIGIT/PD-1 Blockade to Overcome Immune-Resistance in Solid Cancers" International Journal of Molecular Sciences 22, no. 19: 10389. https://doi.org/10.3390/ijms221910389
APA StyleHosseinkhani, N., Shadbad, M. A., Asghari Jafarabadi, M., Karim Ahangar, N., Asadzadeh, Z., Mohammadi, S. M., Lotfinejad, P., Alizadeh, N., Brunetti, O., Fasano, R., Silvestris, N., & Baradaran, B. (2021). A Systematic Review and Meta-Analysis on the Significance of TIGIT in Solid Cancers: Dual TIGIT/PD-1 Blockade to Overcome Immune-Resistance in Solid Cancers. International Journal of Molecular Sciences, 22(19), 10389. https://doi.org/10.3390/ijms221910389










