A Lower Irradiation Dose of 308 nm Monochromatic Excimer Light Might Be Sufficient for Vitiligo Treatment: A Novel Insight Gained from In Vitro and In Vivo Analyses
Abstract
:1. Introduction
2. Results
2.1. The Nontoxic Dose of MEL Irradiation Induced Expressions of Stem Cell Factor (SCF), Endothelin-1 (ET-1), and Glycoprotein Nonmetastatic Melanoma Protein B (GPNMB) in Cultured Human Skin Epidermal Keratinocytes
2.2. MEL Irradiation Using a Toxic Dose Induced Expressions of IL-1β, IL-6, and TNF-α in Cultured Human Skin Epidermal Keratinocytes
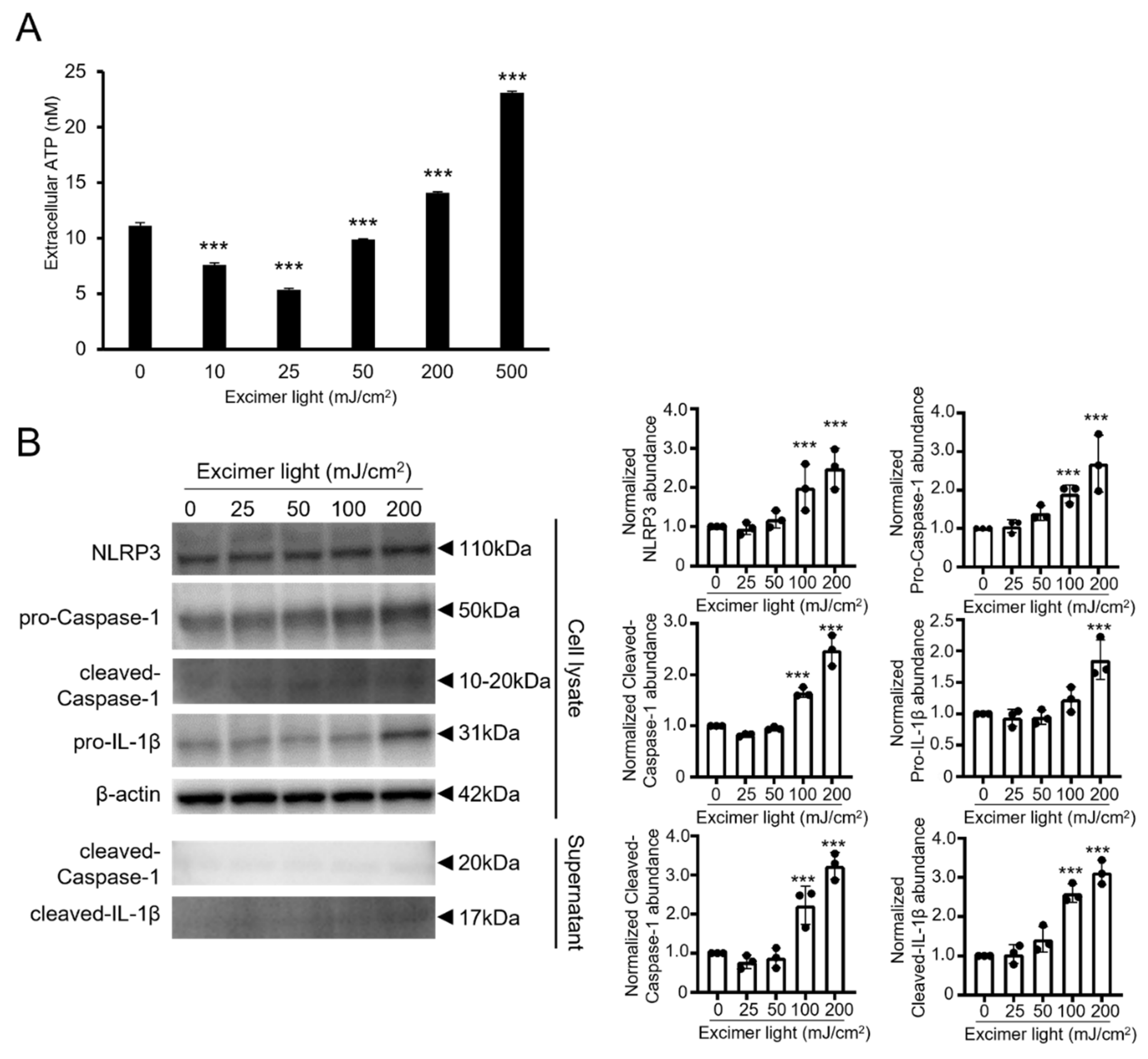
2.3. A Toxic Dose of MEL Irradiation Promoted ATP Release and Inflammasome Activation
2.4. A Lower Than Minimal Erythemal Dose Was Sufficient for Pigmentation in Guinea Pig Skin
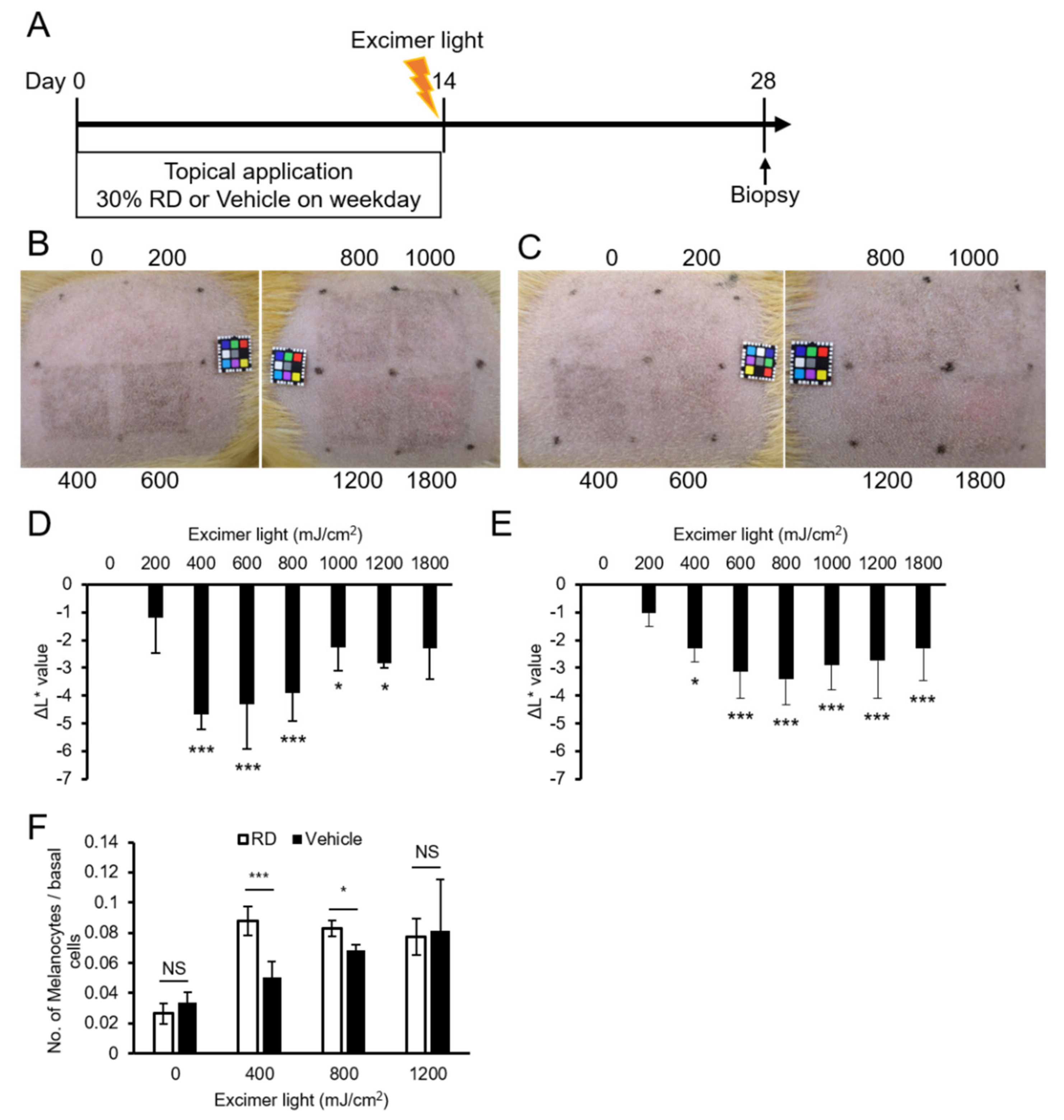
2.5. A Lower Dose Was Effective for Pigmentation in the Rhododendrol-Treated Guinea Pig Skin
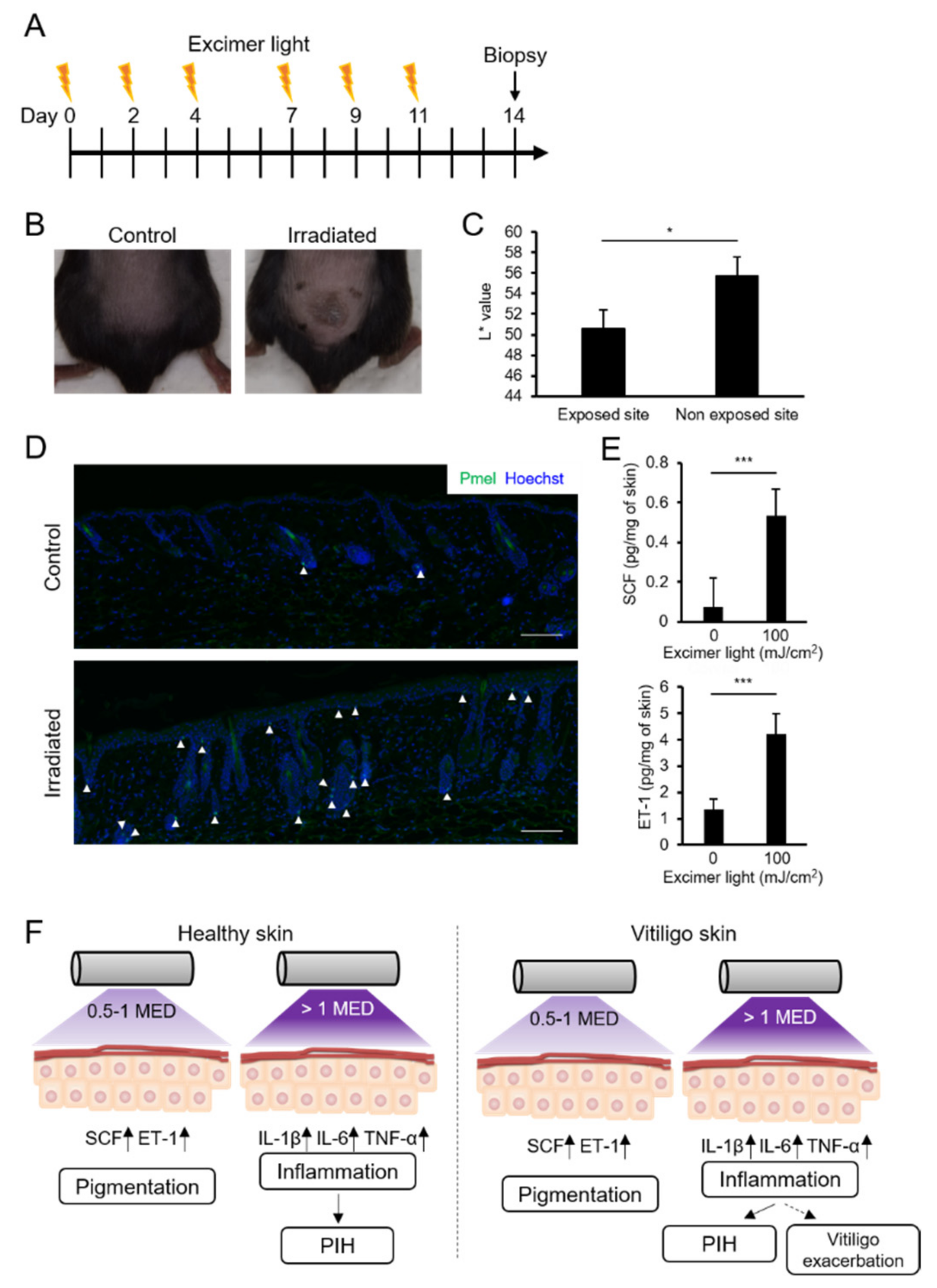
2.6. MEL Irradiation with a Suberythemal Dose Induced Skin Pigmentation and Melanocyte Recruitment in Mice
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Cell Treatment
4.3. Cytotoxicity Assay
4.4. RNA Isolation and qRT-PCR Analysis
4.5. ELISA Assay
4.6. Western Blotting Analysis
4.7. Quantification of Extracellular ATP
4.8. Animals and In Vivo Experimental Procedures
4.9. Histology
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Yamaguchi, Y.; Batzer, J.; Coelho, S.G.; Zmudzka, B.Z.; Miller, S.A.; Wolber, R.; Beer, J.Z.; Hearing, V.J. Mechanisms of Skin Tanning in Different Racial/Ethnic Groups in Response to Ultraviolet Radiation. J. Investig. Dermatol. 2005, 124, 1326–1332. [Google Scholar] [CrossRef] [Green Version]
- Hönigsmann, H. Erythema and pigmentation. Photodermatol. Photoimmunol. Photomed. 2002, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef] [Green Version]
- Imokawa, G. Autocrine and Paracrine Regulation of Melanocytes in Human Skin and in Pigmentary Disorders. Pigment. Cell Res. 2004, 17, 96–110. [Google Scholar] [CrossRef]
- Hachiya, A.; Kobayashi, A.; Yoshida, Y.; Kitahara, T.; Takema, Y.; Imokawa, G. Biphasic Expression of Two Paracrine Melanogenic Cytokines, Stem Cell Factor and Endothelin-1, in Ultraviolet B-Induced Human Melanogenesis. Am. J. Pathol. 2004, 165, 2099–2109. [Google Scholar] [CrossRef] [Green Version]
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Primers 2015, 1, 15011. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Eleftheriadou, V.; Whitton, M.; van Geel, N. Vitiligo. Lancet 2015, 386, 74–84. [Google Scholar] [CrossRef]
- Rodrigues, M.; Ezzedine, K.; Hamzavi, I.; Pandya, A.G.; Harris, J.E.; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J. Am. Acad. Dermatol. 2017, 77, 1–13. [Google Scholar] [CrossRef]
- Taieb, A.; Alomar, A.; Bohm, M.; Dell’anna, M.L.; De Pase, A.; Eleftheriadou, V.; Ezzedine, K.; Gauthier, Y.; Gawkrodger, D.J.; Jouary, T.; et al. Guidelines for the management of vitiligo: The European Dermatology Forum consensus. Br. J. Dermatol. 2013, 168, 5–19. [Google Scholar] [CrossRef]
- Grimes, P.E. New insights and new therapies in vitiligo. JAMA 2005, 293, 730–735. [Google Scholar] [CrossRef]
- Park, K.K.; Liao, W.; Murase, J.E. A review of monochromatic excimer light in vitiligo. Br. J. Dermatol. 2012, 167, 468–478. [Google Scholar] [CrossRef]
- Pacifico, A.; Leone, G. Photo(chemo)therapy for vitiligo. Photodermatol. Photoimmunol. Photomed. 2011, 27, 261–277. [Google Scholar] [CrossRef]
- Bae, J.M.; Jung, H.M.; Hong, B.Y.; Lee, J.H.; Choi, W.J.; Lee, J.H.; Kim, G.M. Phototherapy for Vitiligo: A Systematic Review and Meta-analysis. JAMA Dermatol. 2017, 153, 666–674. [Google Scholar] [CrossRef]
- Spencer, J.M.; Nossa, R.; Ajmeri, J. Treatment of vitiligo with the 308-nm excimer laser: A pilot study. J. Am. Acad. Dermatol. 2002, 46, 727–731. [Google Scholar] [CrossRef]
- Baltas, E.; Nagy, P.; Bonis, B.; Novak, Z.; Ignacz, F.; Szabo, G.; Bor, Z.; Dobozy, A.; Kemeny, L. Repigmentation of localized vitiligo with the xenon chloride laser. Br. J. Dermatol. 2001, 144, 1266–1267. [Google Scholar] [CrossRef]
- Taneja, A.; Trehan, M.; Taylor, C.R. 308-nm excimer laser for the treatment of localized vitiligo. Int. J. Dermatol. 2003, 42, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Valejo Coelho, M.M.; Apetato, M. The dark side of the light: Phototherapy adverse effects. Clin. Dermatol. 2016, 34, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhowaish, A.K.; Dietrich, N.; Onder, M.; Fritz, K. Effectiveness of a 308-nm excimer laser in treatment of vitiligo: A review. Lasers Med. Sci. 2013, 28, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Kunisada, T.; Lu, S.-Z.; Yoshida, H.; Nishikawa, S.; Nishikawa, S.-i.; Mizoguchi, M.; Hayashi, S.-i.; Tyrrell, L.; Williams, D.A.; Wang, X.; et al. Murine Cutaneous Mastocytosis and Epidermal Melanocytosis Induced by Keratinocyte Expression of Transgenic Stem Cell Factor. J. Exp. Med. 1998, 187, 1565–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imokawa, G.; Yada, Y.; Miyagishi, M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J. Biol. Chem. 1992, 267, 24675–24680. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Rabbani, P.; Sun, Q.; Hu, H.; Lim, C.H.; Manga, P.; Ito, M. EdnrB Governs Regenerative Response of Melanocyte Stem Cells by Crosstalk with Wnt Signaling. Cell Rep. 2016, 15, 1291–1302. [Google Scholar] [CrossRef] [Green Version]
- Swope, V.B.; Abdel-Malek, Z.; Kassem, L.M.; Nordlund, J.J. Interleukins 1α and 6 and Tumor Necrosis Factor-α Are Paracrine Inhibitors of Human Melanocyte Proliferation and Melanogenesis. J. Investig. Dermatol. 1991, 96, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Swope, V.B.; Krug, K.A.; Nordlund, J.J.; Abdel-Malek, Z.A.; Sramkoski, R.M.; Babcock, G.F. Synthesis of Interleukin-1α and β by Normal Human Melanocytes. J. Investig. Dermatol. 1994, 102, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Levandowski, C.B.; Mailloux, C.M.; Ferrara, T.M.; Gowan, K.; Ben, S.; Jin, Y.; McFann, K.K.; Holland, P.J.; Fain, P.R.; Dinarello, C.A.; et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1β processing via the NLRP1 inflammasome. Proc. Natl. Acad. Sci. USA 2013, 110, 2952–2956. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, S.; Rani, S.; Srivastava, N.; Kumar, R.; Parsad, D. Increased systemic and epidermal levels of IL-17A and IL-1β promotes progression of non-segmental vitiligo. Cytokine 2017, 91, 153–161. [Google Scholar] [CrossRef]
- Moretti, S.; Spallanzani, A.; Amato, L.; Hautmann, G.; Gallerani, I.; Fabiani, M.; Fabbri, P. New Insights into the Pathogenesis of Vitiligo: Imbalance of Epidermal Cytokines at Sites of Lesions. Pigment. Cell Res. 2002, 15, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, L.; Ha, D.; Qu, L.; Liu, L.; Tao, Y. Changes in sICAM-1 and GM-CSF levels in skin tissue fluid and expression of IL-6, IL-17 and TNF-alpha in blood of patients with vitiligo. Exp. Ther. Med. 2019, 17, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Ahn, S.; Lee, B.G.; Chang, I.; Hwang, J.S. Inhibition of skin pigmentation by an extract of Lepidium apetalum and its possible implication in IL-6 mediated signaling. Pigment. Cell Res. 2005, 18, 439–446. [Google Scholar] [CrossRef]
- Tu, C.-X.; Gu, J.-S.; Lin, X.-R. Increased interleukin-6 and granulocyte–macrophage clony stimulating factor levels in the sera of patients with non-segmental vitiligo. J. Dermatol. Sci. 2003, 31, 73–78. [Google Scholar] [CrossRef]
- Li, S.; Kang, P.; Zhang, W.; Jian, Z.; Zhang, Q.; Yi, X.; Guo, S.; Guo, W.; Shi, Q.; Li, B.; et al. Activated NLR family pyrin domain containing 3 (NLRP3) inflammasome in keratinocytes promotes cutaneous T-cell response in patients with vitiligo. J. Allergy Clin. Immunol. 2020, 145, 632–645. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.; Seo, J.; Lee, E.J.; Kim, J.Y.; Park, M.-Y.; Hwang, S.; Almurayshid, A.; Lim, B.J.; Yu, J.-W.; Oh, S.H. ATP-P2X7–Induced Inflammasome Activation Contributes to Melanocyte Death and CD8+ T-Cell Trafficking to the Skin in Vitiligo. J. Investig. Dermatol. 2020, 140, 1794–1804. [Google Scholar] [CrossRef]
- Kuroda, Y.; Takahashi, Y.; Sakaguchi, H.; Matsunaga, K.; Suzuki, T. Depigmentation of the skin induced by 4-(4-hydroxyphenyl)-2-butanol is spontaneously re-pigmented in brown and black guinea pigs. J. Toxicol. Sci. 2014, 39, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, A.; Yoshihisa, Y.; Yamakoshi, T.; Ur Rehman, M.; Norisugi, O.; Hara, H.; Matsunaga, K.; Makino, T.; Nishihira, J.; Shimizu, T. UV-B Radiation Induces Macrophage Migration Inhibitory Factor–Mediated Melanogenesis through Activation of Protease-Activated Receptor-2 and Stem Cell Factor in Keratinocytes. Am. J. Pathol. 2011, 178, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Takeo, M.; Rabbani, P.; Hu, H.; Lee, W.; Chung, Y.R.; Carucci, J.; Overbeek, P.; Ito, M. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat. Med. 2013, 19, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, A.; Kobayashi, A.; Ohuchi, A.; Takema, Y.; Imokawa, G. The Paracrine Role of Stem Cell Factor/c-kit Signaling in the Activation of Human Melanocytes in Ultraviolet-B-Induced Pigmentation. J. Investig. Dermatol. 2001, 116, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Biswas, K.B.; Takahashi, A.; Mizutani, Y.; Takayama, S.; Ishitsuka, A.; Yang, L.; Yang, F.; Iddamalgoda, A.; Katayama, I.; Inoue, S. GPNMB is expressed in human epidermal keratinocytes but disappears in the vitiligo lesional skin. Sci. Rep. 2020, 10, 4930. [Google Scholar] [CrossRef]
- Silpa-archa, N.; Kohli, I.; Chaowattanapanit, S.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. J. Am. Acad. Dermatol. 2017, 77, 591–605. [Google Scholar] [CrossRef]
- Nasti, T.H.; Timares, L. Inflammasome Activation of IL-1 Family Mediators in Response to Cutaneous Photodamage†. Photochem. Photobiol. 2012, 88, 1111–1125. [Google Scholar] [CrossRef] [Green Version]
- Abe, Y.; Hozumi, Y.; Okamura, K.; Suzuki, T. Expression of discoidin domain receptor 1 and E-cadherin in epidermis affects melanocyte behavior in rhododendrol-induced leukoderma mouse model. J. Dermatol. 2020, 47, 1330–1334. [Google Scholar] [CrossRef]
- Abe, Y.; Okamura, K.; Kawaguchi, M.; Hozumi, Y.; Aoki, H.; Kunisada, T.; Ito, S.; Wakamatsu, K.; Matsunaga, K.; Suzuki, T. Rhododenol-induced leukoderma in a mouse model mimicking Japanese skin. J. Dermatol. Sci. 2016, 81, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Kwon, S.H.; Ju, H.J.; Kang, H.Y. Suberythemic and erythemic doses of a 308-nm excimer laser treatment of stable vitiligo in combination with topical tacrolimus: A randomized controlled trial. J. Am. Acad. Dermatol. 2020, 83, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Yang, L.; Kuroda, Y.; Guo, J.; Teng, L.; Tsuruta, D.; Katayama, I. Local Epidermal Endocrine Estrogen Protects Human Melanocytes against Oxidative Stress, a Novel Insight into Vitiligo Pathology. Int. J. Mol. Sci. 2021, 22, 269. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroda, Y.; Yang, L.; Lai, S.; Guo, J.; Sayo, T.; Takahashi, Y.; Tsuruta, D.; Katayama, I. A Lower Irradiation Dose of 308 nm Monochromatic Excimer Light Might Be Sufficient for Vitiligo Treatment: A Novel Insight Gained from In Vitro and In Vivo Analyses. Int. J. Mol. Sci. 2021, 22, 10409. https://doi.org/10.3390/ijms221910409
Kuroda Y, Yang L, Lai S, Guo J, Sayo T, Takahashi Y, Tsuruta D, Katayama I. A Lower Irradiation Dose of 308 nm Monochromatic Excimer Light Might Be Sufficient for Vitiligo Treatment: A Novel Insight Gained from In Vitro and In Vivo Analyses. International Journal of Molecular Sciences. 2021; 22(19):10409. https://doi.org/10.3390/ijms221910409
Chicago/Turabian StyleKuroda, Yasutaka, Lingli Yang, Sylvia Lai, Jiao Guo, Tetsuya Sayo, Yoshito Takahashi, Daisuke Tsuruta, and Ichiro Katayama. 2021. "A Lower Irradiation Dose of 308 nm Monochromatic Excimer Light Might Be Sufficient for Vitiligo Treatment: A Novel Insight Gained from In Vitro and In Vivo Analyses" International Journal of Molecular Sciences 22, no. 19: 10409. https://doi.org/10.3390/ijms221910409
APA StyleKuroda, Y., Yang, L., Lai, S., Guo, J., Sayo, T., Takahashi, Y., Tsuruta, D., & Katayama, I. (2021). A Lower Irradiation Dose of 308 nm Monochromatic Excimer Light Might Be Sufficient for Vitiligo Treatment: A Novel Insight Gained from In Vitro and In Vivo Analyses. International Journal of Molecular Sciences, 22(19), 10409. https://doi.org/10.3390/ijms221910409







