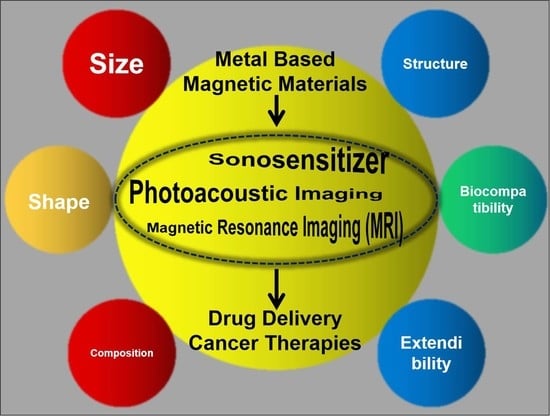
Recent Advances in Metal-Based Magnetic Composites as High-Efficiency Candidates for Ultrasound-Assisted Effects in Cancer Therapy
Abstract
:1. Introduction
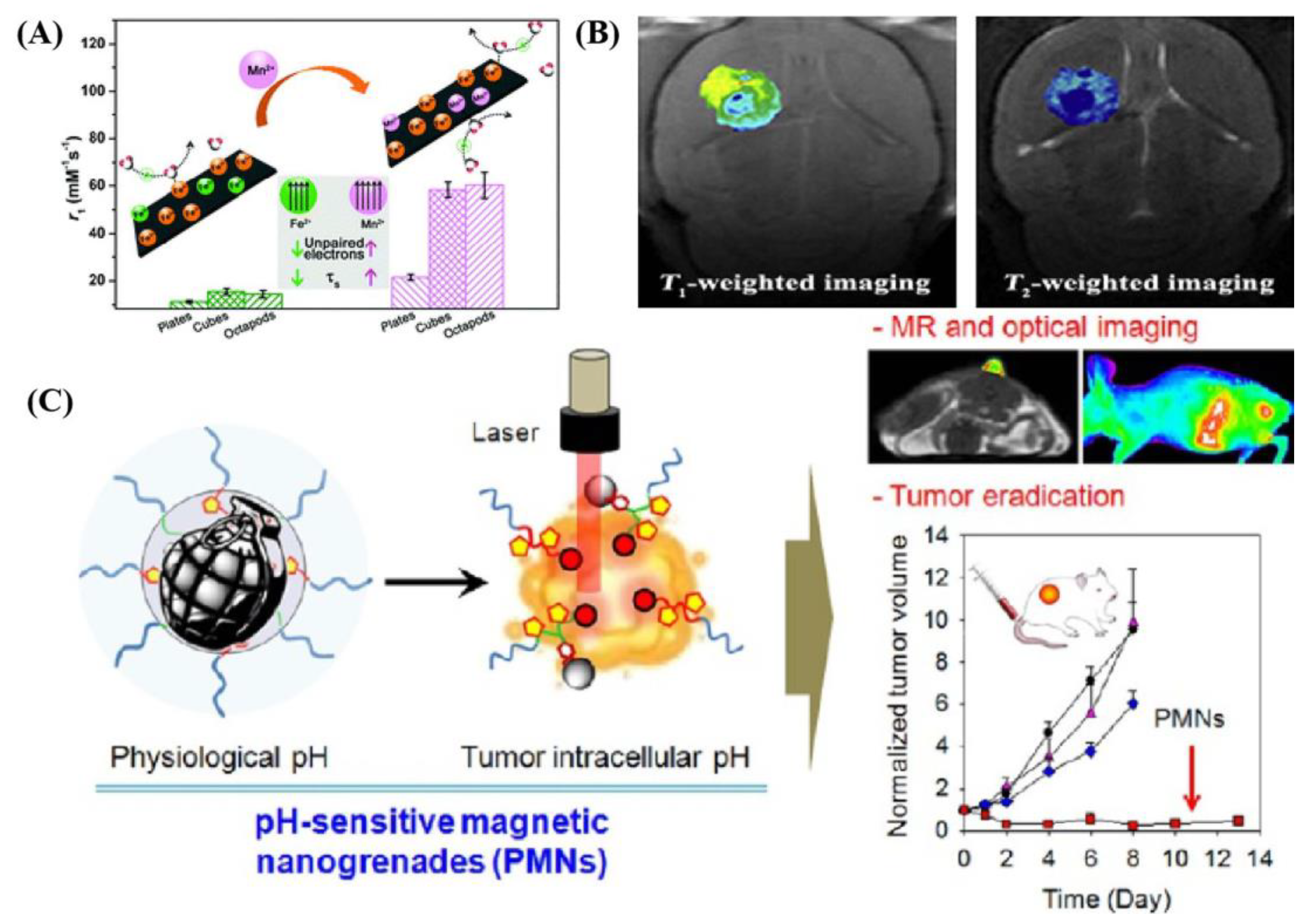
2. Superparamagnetic Iron Oxides Nanoparticles (SPIOs) as an Effective Agent in MRI Utilization
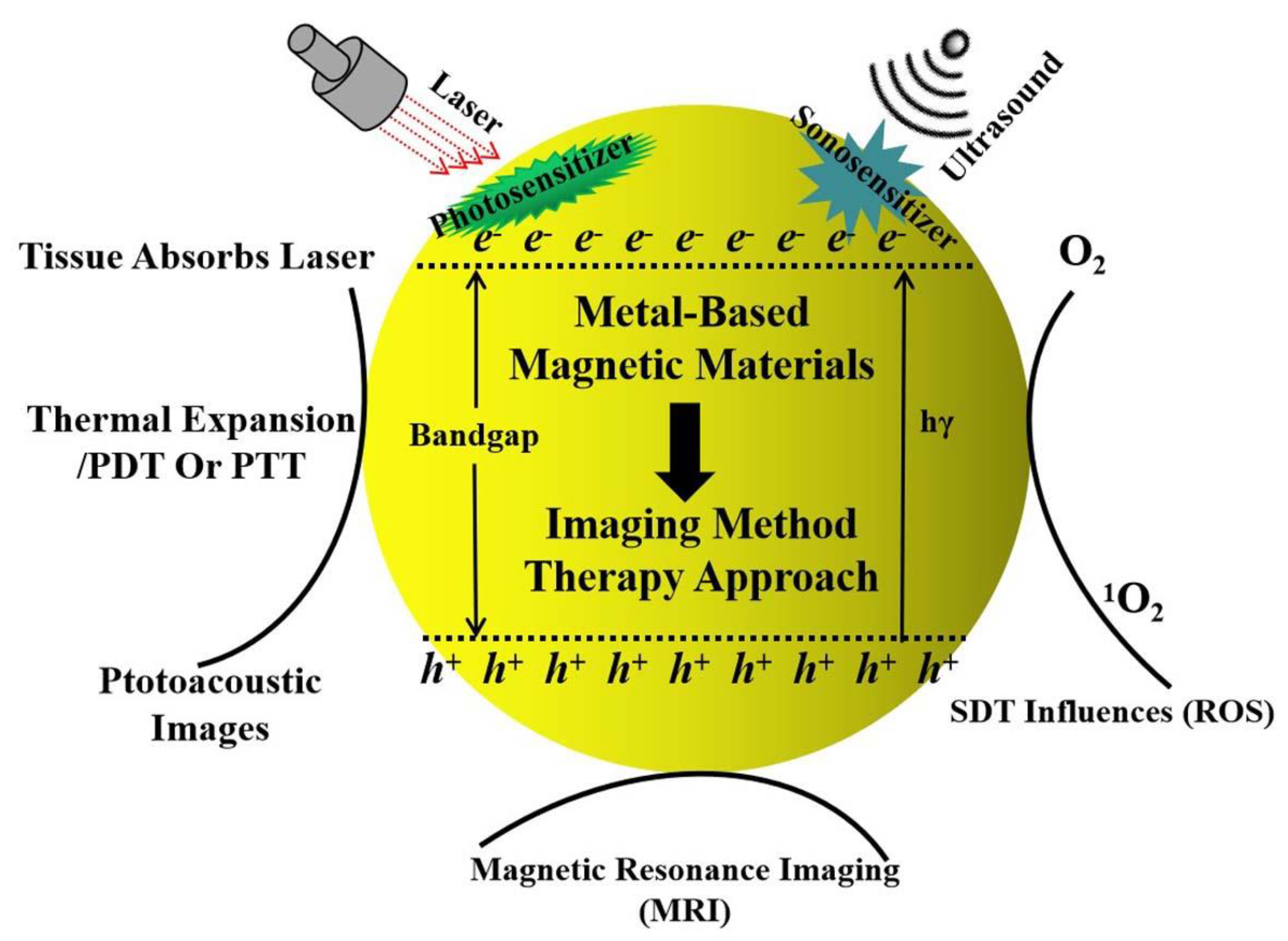
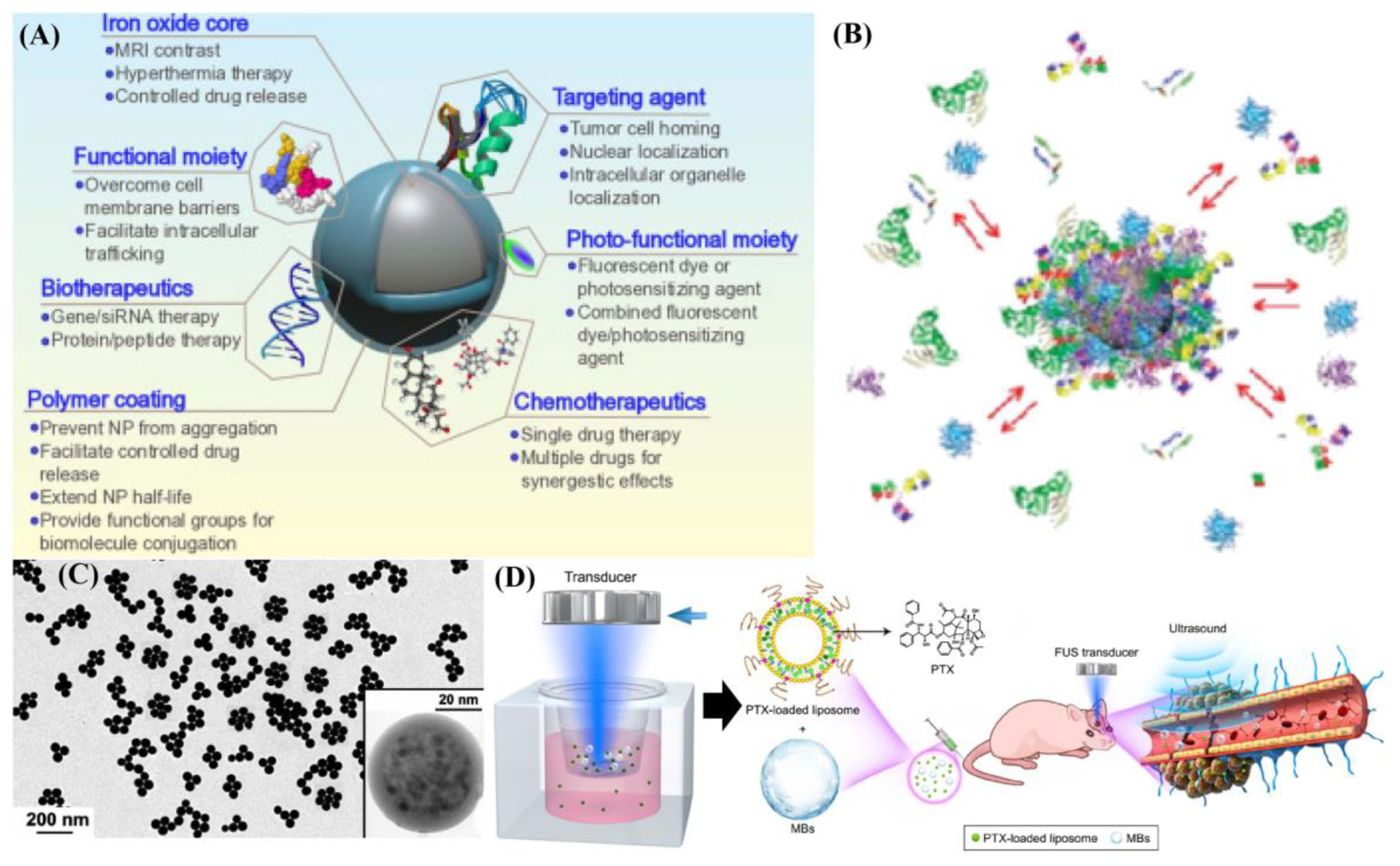
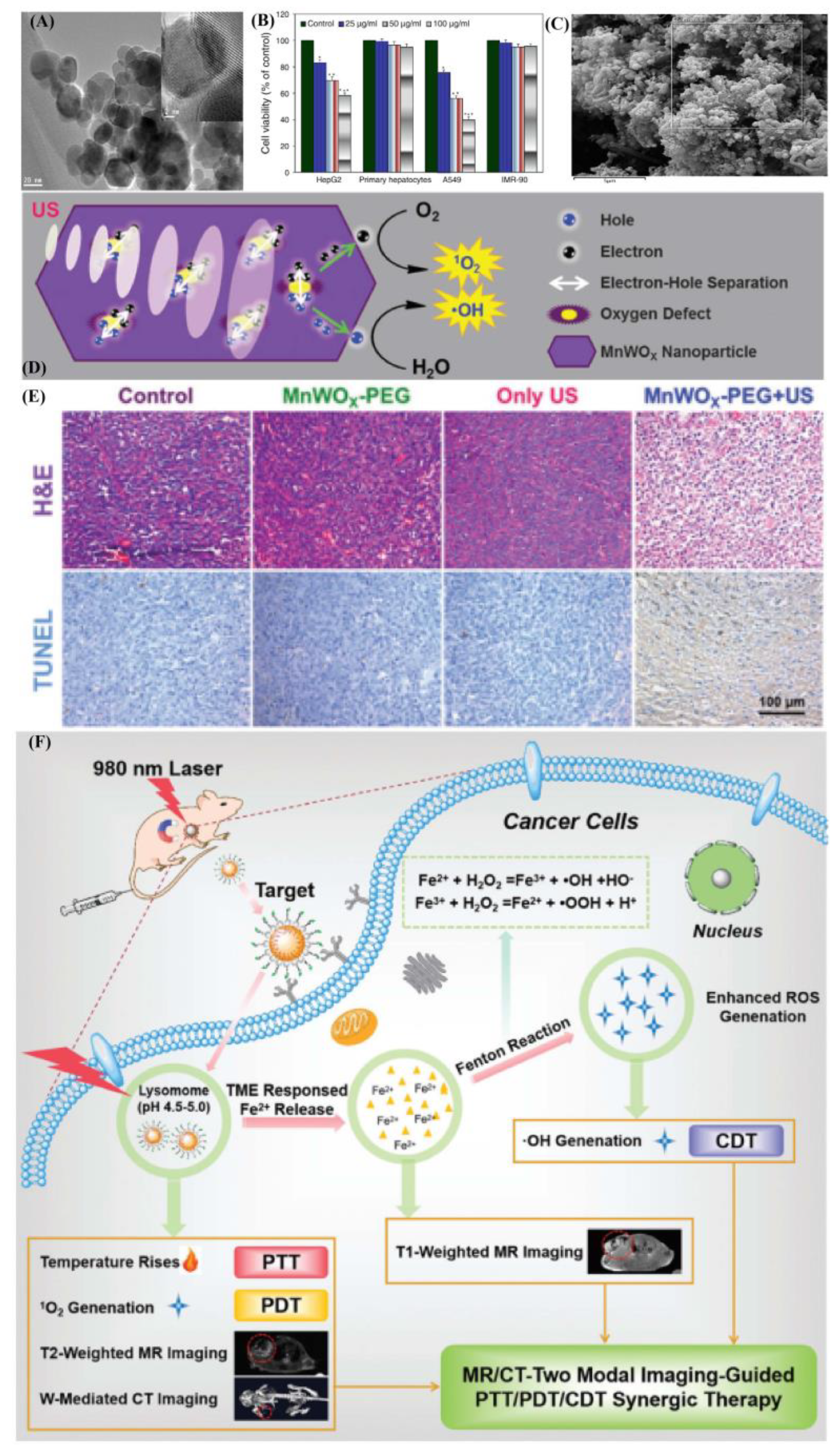
3. Magnetic Particles Based Heterogeneous Structure as One Effective Supporter toward Ultrasound (US) Integrated Cancer Therapies
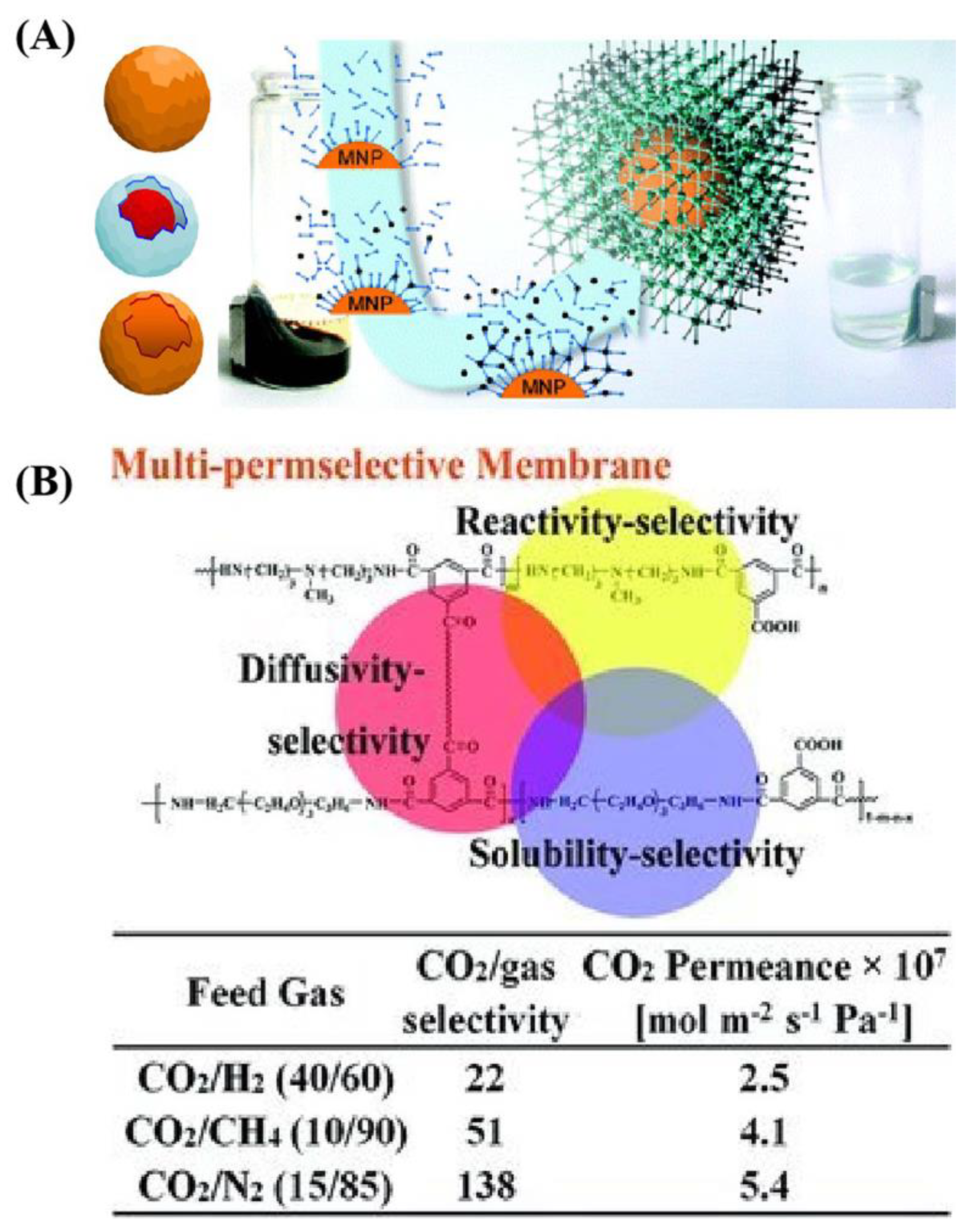
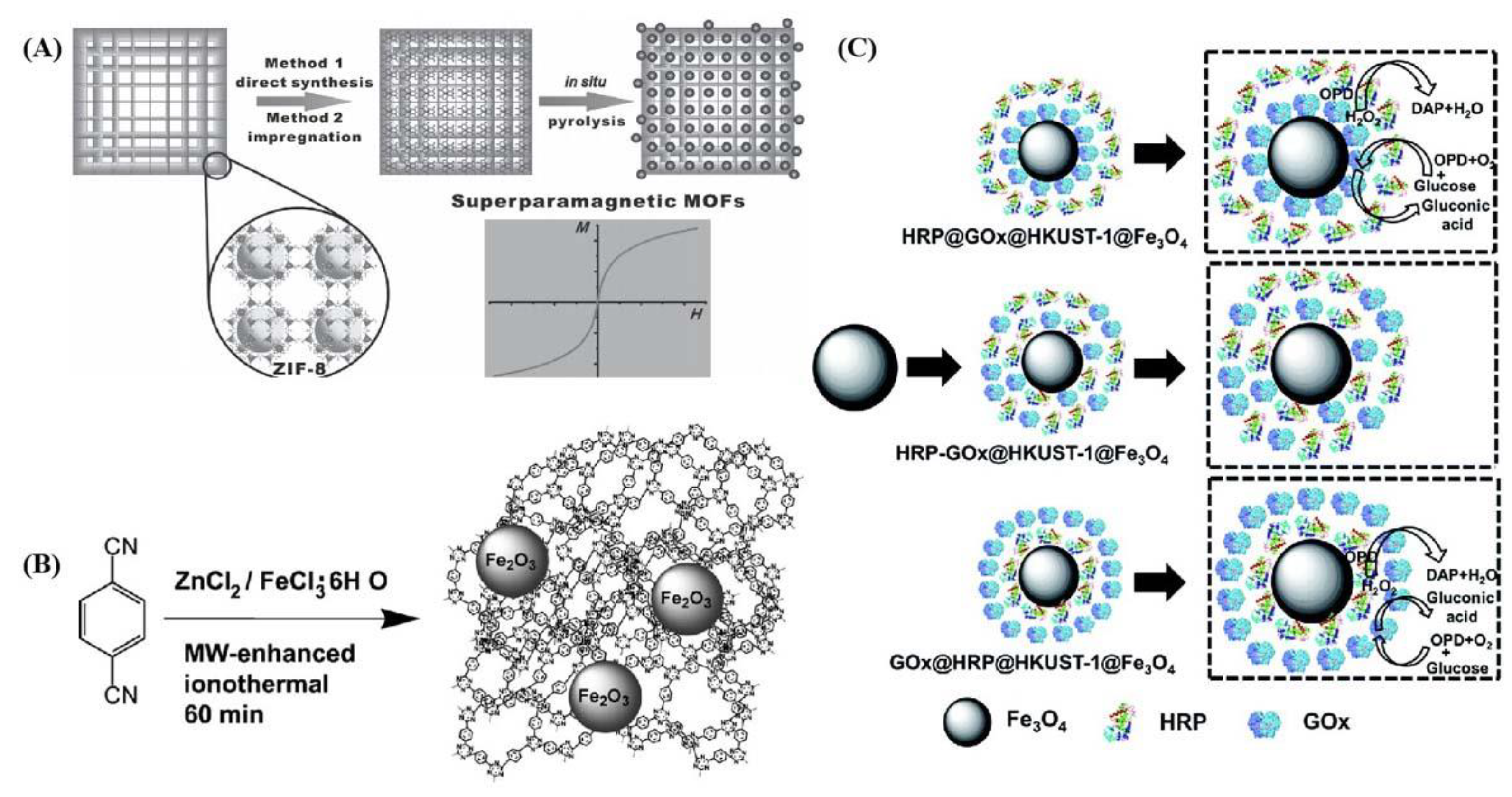
4. The Fabrication of Magnetic Metal–Organic-Frameworks (MMOF) and Related Biological Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Temperley, A.A.; Lefevre, H.W. The Mssbauer effect in marcasite structure iron compounds. J. Phys. Chem. Solids. 1965, 27, 85–92. [Google Scholar] [CrossRef]
- Singh, O.P.; Gupta, V.P. Electronic properties of iron chalcogenides FeS, FeS2, FeSe2, and FeTe2. Phys. Status Solidi C 2010, 133, 249–252. [Google Scholar] [CrossRef]
- Lionel, V.; Niclas, B.; Sten-Eric, L.; Anders, H. Controlled Aqueous Chemical Growth of Oriented Three-Dimensional Crystalline Nanorod Arrays: Application to Iron(III) Oxides. Chem. Mater. 2001, 13, 233–235. [Google Scholar]
- Mao, X.; Kim, J.G.; Han, J.; Jung, H.S.; Sang, G.L.; Kotov, N.A.; Lee, J. Phase-pure FeSe(x) (x = 1, 2) nanoparticles with one- and two-photon luminescence. J. Am. Chem. Soc. 2014, 136, 7189–7192. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, P.; Guetlich, P.; Mueller, E.W. Effect of metal dilution on the spin-crossover behavior in [FexM1−x(phen)2(NCS)2] (M = Mn, Co, Ni, Zn). Inorg. Chem. 1982, 21, 3429–3433. [Google Scholar] [CrossRef]
- Mao, X.; Kwon, J.; Koh, E.; Hwang, D.; Lee, J. Ligand Exchange Procedure for Bimetallic Magnetic Iron–Nickel Nanocrystals toward Biocompatible Activities. ACS Appl. Mater. Interfaces 2015, 7, 15522–15530. [Google Scholar] [CrossRef]
- Beveridge, J.S.; Buck, M.R.; Bondi, J.F.; Misra, R.; Schiffer, P.; Schaak, R.E.; Williams, M.E. Purification and Magnetic Interrogation of Hybrid Au-Fe3O4 and FePt-Fe3O4 Nanoparticles. Angew. Chem. Int. Ed. 2011, 23, 10049–10053. [Google Scholar] [CrossRef]
- Sun, S. Chemical synthesis of monodisperse magnetic nanoparticles for sensitive cancer detection. J. Inorg. Organomet. Polym. Mater. 2013, 24, 33–38. [Google Scholar] [CrossRef]
- Mao, X.; Lee, J. Facile synthesis of phase-pure FeCr2Se4 and FeCr2S4 nanocrystals via a wet chemistry method. J. Mater. Chem. C 2014, 2, 3744–3749. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Pasquale, N.J.; Garbuzenko, O.B.; Lee, K.B. Stem cell-based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer. Biomaterials 2016, 81, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Jan, E.; Byrne, S.J.; Cuddihy, M.; Davies, A.M.; Volkov, Y.; Gun’Ko, Y.; Kotov, N.A. High-Content Screening as a Universal Tool for Fingerprinting of Cytotoxicity of Nanoparticles. ACS Nano 2008, 2, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, J.; Guan, S.; Du, M.; Wu, K.; Xu, K.; Zou, L.; Tian, H.; Fang, Y. Magnetic actuation of flexible microelectrode arrays for neural activity recordings. Nano Lett. 2019, 19, 8032–8039. [Google Scholar] [CrossRef]
- Peng, Z.; Fang, S.; Hung, H.C.; Jain, P.; Leger, K.J.; Jiang, S. Sensitive and Quantitative Detection of Anti-Poly(ethylene glycol) (PEG) Antibodies by Methoxy-PEG-Coated Surface Plasmon Resonance Sensors. Anal. Chem. 2017, 89, 8217–8222. [Google Scholar]
- Stefan, M.; Leostean, C.; Pan, O.; Suciu, M.; Barbu-Tudoran, L. Synthesis and characterization of Fe3O4-ZnS:Mn nanocomposites for biomedical applications. Mater. Chem. Phys. 2021, 264, 124474–124482. [Google Scholar] [CrossRef]
- Miyazaki, T. Organic modification of magnetite nanoparticles for biomedical applications. Bioceramics 2021, 2021, 77–82. [Google Scholar]
- Kahn, O. Chemistry and Physics of Supramolecular Magnetic Materials. Acc. Chem. Res. 2000, 33, 647–657. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment: A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Ma, J.; Waxman, D.J. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 2008, 7, 3670–3684. [Google Scholar] [CrossRef] [Green Version]
- Lee, F.Y. Combination of Src Kinase Inhibitors and Chemotherapeutic Agents for the Treatment of Proliferative Diseases. WO2005013983A1, 13 March 2008. [Google Scholar]
- Fukui, S.; Abe, R.; Ogawa, J.; Oka, T.; Yamaguchi, M.; Sato, T.; Imaizumi, H. Study on optimization design of superconducting magnet for magnetic force assisted drug delivery system. Physica C 2007, 463–465, 1315–1318. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Linemann, T.; Birkelund, S.; Tarp, G.A.; Moos, T. Evaluation of Targeted Delivery to the Brain Using Magnetic Immunoliposomes and Magnetic Force. Materials 2019, 12, 3576. [Google Scholar] [CrossRef] [Green Version]
- Schober, O.; Lottes, G.; Junker, D. Radiation exposure during positron emission tomography (PET) and possibilities of reducing the radiation exposure of patients and personnel. Strahlenschutz Forsch. Prax. 1990, 31, 133–145. [Google Scholar] [PubMed]
- Bulte, J. Superparamagnetic Iron Oxides as MPI Tracers: A Primer and Review of early applications. Adv. Drug Deliv. Rev. 2018, 138, 293–301. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, H.J.; Nam, G.E.; Cho, K.; Son, J.H. In Vivo Dual-Modality Terahertz/Magnetic Resonance Imaging Using Superparamagnetic Iron Oxide Nanoparticles as a Dual Contrast Agent. IEEE Trans. Terahertz Sci. 2012, 2, 93–98. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, C.; Bao, J.; Yang, L.; Wei, R.; Cheng, J.; Lin, H.; Gao, J. Surface manganese substitution in magnetite nanocrystals enhances T1 contrast ability by increasing electron spin relaxation. J. Mater. Chem. B 2018, 6, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Gu, W.; Wang, H.; Deng, Y.; Shi, X.; Ye, L. T1-T2 dual-modal MRI of brain gliomas using PEGylated Gd-doped iron oxide nanoparticles. J. Colloid Interface Sci. 2014, 417, 159–165. [Google Scholar] [CrossRef]
- Persson, H.; Berthold, C.H.; Rydmark, M.; Fabricius, C. Metabolic relationships between proteins of myelin and paranodally shedded, partially degraded myelin fragments in the rabbit CNS. J. Neurosci. 2010, 33, 310–318. [Google Scholar] [CrossRef]
- Waysbort, D.; Navon, G. Pseudocontact contribution to the isotropic chemical shifts of protons in octahedral paramagnetic complex ions. Chem. Phys. 1980, 49, 333–337. [Google Scholar] [CrossRef]
- Luz, Z.; Shulman, R.G. Proton Magnetic Resonance Shifts in Aqueous Solutions of Paramagnetic Metal Ions. J. Chem. Phys. 1965, 43, 3750–3756. [Google Scholar] [CrossRef]
- Eckert, H.; Yesinowski, J.P.; Silver, L.A.; Stolper, E.M. Water in silicate glasses: Quantitation and structural studies by proton solid echo and magic angle spinning NMR methods. J. Phys. Chem. A 1988, 92, 2055–2064. [Google Scholar] [CrossRef]
- Ling, D.; Park, W.; Park, S.J.; Lu, Y.; Hyeon, T. Multifunctional Tumor pH-Sensitive Self-Assembled Nanoparticles for Bimodal Imaging and Treatment of Resistant Heterogeneous Tumors. J. Am. Chem. Soc. 2014, 136, 5647–5655. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Sun, Y.; Tang, X.; Ren, Q.; Yang, W. Tumor-Targeting Multifunctional Rattle-Type Theranostic Nanoparticles for MRI/NIRF Bimodal Imaging and Delivery of Hydrophobic Drugs. Small 2014, 11, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Pselt, E.H.; Kloust, H.; Tromsdorf, U.; Janschel, M.; Weller, H. Relaxivity-Optimization of a PEGylated Iron Oxide-Based Negative Magnetic Resonance Contrast Agent for T2-Weighted Spin Echo Imaging. ACS Nano 2012, 6, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Puppi, J.; Mitry, R.R.; Modo, M.; Dhawan, A.; Raja, K.; Hughes, R.D. Use of a clinically approved iron oxide MRI contrast agent to label human hepatocytes. Cell Transplant. 2011, 20, 963–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruggen, B.; Schaep, J.; Wilms, D. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 1999, 156, 29–41. [Google Scholar] [CrossRef]
- Verma, V.K.; Beevi, S.S.; Debnath, T.; Potlapuvu, U.S. Signal regulatory protein alpha (SIRPA) and kinase domain receptor (KDR) are key expression markers in cardiac specific precursor selection from hADSCs. New Horiz. Transl. Med. 2015, 2, 93–101. [Google Scholar]
- Fang, Y.; Li, Y.; Chen, Z.; Yu, Z.; Wu, J.; Ning, G. Superparamagnetic iron oxide nanoparticle-embedded encapsulated microbubbles as dual contrast agents of magnetic resonance and ultrasound imaging. Biomaterials 2009, 30, 3882–3890. [Google Scholar]
- Sheng, S.; Heze, G.; Zequan, J.; Yuqing, J.; Ying, W. Self-assembled microbubbles as contrast agents for ultrasound/magnetic resonance dual-modality imaging. Acta Biomater. 2015, 24, 266–278. [Google Scholar] [CrossRef]
- Chin, C.T.; Burns, P.N. Predicting the acoustic response of a microbubble population for contrast imaging in medical ultrasound. Ultrasound Med. Biol. 2000, 26, 1293–1300. [Google Scholar] [CrossRef]
- Kiessling, F.; Huppert, J.; Palmowski, M. Functional and Molecular Ultrasound Imaging: Concepts and Contrast Agents. Curr. Med. Chem. 2009, 16, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Eisenbrey, J.R.; Forsberg, F.; Lorenz, N.; Ntoulia, A. Ultrasound contrast agents: Microbubbles made simple for the pediatric radiologist. Pediatr. Radiol. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Leongpoi, H.; Christiansen, J.; Heppner, P.; Lewis, C.W.; Klibanov, A.L.; Kaul, S.; Lindner, J.R. Assessment of Endogenous and Therapeutic Arteriogenesis by Contrast Ultrasound Molecular Imaging of Integrin Expression. Circulation 2005, 111, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- Huynh, E.; Lovell, J.F.; Helfield, B.L.; Jeon, M.; Kim, C.; Goertz, D.E.; Wilson, B.C.; Gang, Z. Porphyrin Shell Microbubbles with Intrinsic Ultrasound and Photoacoustic Properties. J. Am. Chem. Soc. 2012, 134, 16464–16467. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Wang, J.; Dai, Z.; Jin, Y.; Qu, E.; Xing, Z.; Guo, C.; Liu, J.; Yue, X. Bifunctional gold nanorod-loaded polymeric microcapsules for both contrast-enhanced ultrasound imaging and photothermal therapy. J. Mater. Chem. 2011, 21, 5561–5564. [Google Scholar] [CrossRef]
- Malvindi, M.A.; Greco, A.; Conversano, F.; Figuerola, A.; Corti, M.; Bonora, M.; Lascialfari, A.; Doumari, H.A.; Moscardini, M.; Cingolani, R. Magnetic/silica nanocomposites as dual-mode contrast agents for combined magnetic resonance imaging and ultrasonography. Adv. Funct. Mater. 2011, 21, 2548–2555. [Google Scholar] [CrossRef]
- Liu, Z.; Lammers, T.; Ehling, J.; Fokong, S.; Bornemann, J.; Kiessling, F.; G’Tjens, J. Iron oxide nanoparticle-containing microbubble composites as contrast agents for MR and ultrasound dual-modality imaging. Biomaterials 2011, 32, 6155–6163. [Google Scholar] [CrossRef]
- Niu, C.; Wang, Z.; Lu, G.; Krupka, T.M.; Sun, Y.; You, Y.; Song, W.; Ran, H.; Li, P.; Zheng, Y. Doxorubicin loaded superparamagnetic PLGA-iron oxide multifunctional microbubbles for dual-mode US/MR imaging and therapy of metastasis in lymph nodes. Biomaterials 2013, 34, 2307–2317. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef]
- Ku, G.; Wang, X.; Xie, X.; Stoica, G.; Wang, L. Imaging of tumor angiogenesis in rat brains in vivo by photoacoustic tomography. Appl. Opt. 2005, 44, 770–775. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Pang, Y.; Geng, K.; Xie, X.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef]
- Hu, X.; Wei, C.W.; Xia, J.; Pelivanov, I.; O’Donnell, M.; Gao, X. Trapping and Photoacoustic Detection of CTCs at the Single Cell per Milliliter Level with Magneto-Optical Coupled Nanoparticles. Small 2013, 9, 2045. [Google Scholar] [CrossRef]
- Jin, Y.; Jia, C.; Huang, S.W.; O’Donnell, M.; Gao, X. Multifunctional nanoparticles as coupled contrast agents. Nat. Commun. 2010, 1, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.; Akhtar, M.J. Selective killing of cancer cells by iron oxide nanoparticles mediated through reactive oxygen species via p53 pathway. J. Nanopart. Res. 2013, 15, 1225. [Google Scholar] [CrossRef]
- Bakhshi, H.; Azari, M.M.; Shokuhfar, A. A facile approach for obtaining NiFe2O4@C core-shell nanoparticles and their magnetic properties assessment. Diam. Relat. Mater. 2020, 110, 108159. [Google Scholar] [CrossRef]
- Lee, J.H.; Huh, Y.M.; Jun, Y.W.; Seo, J.W.; Jang, J.T.; Song, H.T.; Kim, S.; Cho, E.J.; Yoon, H.G.; Suh, J.S.; et al. Artificially Engineered Magnetic Nanoparticles for Ultra-Sensitive Molecular Imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lu, H.; Yang, F.; Zhang, Y.; Dong, H. Biodegradable FeWOx Nanoparticles for CT/MR Imaging-Guided Synergistic Photothermal, Photodynamic and Chemodynamic Therapy. Nanoscale 2021, 13, 3049–3060. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, J.; Jing, X.; Yang, D.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. Oxygen-Deficient Black Titania for Synergistic/Enhanced Sonodynamic and Photoinduced Cancer Therapy at Near Infrared-II Biowindow. ACS Nano 2018, 12, 4545–4555. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Zhou, W.; Zhao, J.; Li, R. Hypoxia modulation by dual-drug nanoparticles for enhanced synergistic sonodynamic and starvation therapy. J. Nanobiotechnol. 2021, 19, 87. [Google Scholar] [CrossRef]
- Gong, F.; Cheng, L.; Yang, N.; Betzer, O.; Feng, L. Ultrasmall Oxygen-Deficient Bimetallic Oxide MnWOX Nanoparticles for Depletion of Endogenous GSH and Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 2019, 31, 1900730. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Rahi, A. Synthesis and Applications of Nanoflowers. Recent Pat. Nanotechnol. 2016, 10, 86–115. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, J.; Lin, L.; Zhang, C.; Shi, X. Multifunctional PVCL nanogels with redox-responsiveness enable enhanced MR imaging and ultrasound-promoted tumor chemotherapy. Theranostics 2020, 10, 4349–4358. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Bleyer, W.A. The clinical pharmacology of methotrexate: New applications of an old drug. Cancer 1978, 41, 36–51. [Google Scholar] [CrossRef]
- Xuan, Z.; Harris, J.M. Novel degradable poly(ethylene glycol) hydrogels for controlled release of protein. J. Pharm. Sci. 2010, 87, 1450–1458. [Google Scholar]
- Tartaj, P.; Serna, C.J. Synthesis of monodisperse superparamagnetic Fe/silica nanospherical composites. J. Am. Chem. Soc. 2003, 125, 15754–15755. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy—ScienceDirect. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Pi, Z.; Fei, Y.; Chih-Kuang, Y.; Zeng, X.; Diao, X.; Hu, Y.; Chen, S.; Xin, C.; Zheng, H. Enhanced delivery of paclitaxel liposomes using focused ultrasound with microbubbles for treating nude mice bearing intracranial glioblastoma xenografts. Int. J. Nanomed. 2017, 12, 5613–5629. [Google Scholar] [CrossRef] [Green Version]
- Moir, S.; Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009, 9, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Hammes, G.G.; Wu, C.W. Regulation of Enzyme Activity. Science 1971, 172, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Blundell, S.; Thouless, D. Magnetism in Condensed Matter. Am. J. Phys. 2003, 71, 94–95. [Google Scholar] [CrossRef] [Green Version]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Duyne, R.V.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Imaz, I.; Rubio-Martinez, R.; Garcia-Fernandez, R.; Garcia, R.; Ruiz-Molina, D.; Hernando, R.; Puntes, R.; Maspoch, R. Coordination polymer particles as potential drug delivery systems. Chem. Commun. 2010, 46, 4737–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figuerola, A.; Corato, R.D.; Manna, L.; Pellegrino, T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol. Res. 2010, 62, 126–143. [Google Scholar] [CrossRef]
- Li, S.; Zhi, W.; Yu, X.; Wang, J.; Wang, S. High-Performance Membranes with Multi-permselectivity for CO2 Separation. Adv. Mater. 2012, 24, 3196–3200. [Google Scholar] [CrossRef]
- Lohe, M.; Gedrich, K.; Freudenberg, T.; Kockrick, E.; Dellmann, K. Heating and separation using nanomagnet-functionalized metal-organic frameworks. Chem. Commun. 2011, 47, 3075–3077. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liang, F.; Li, C.; Qiu, L.G.; Yuan, Y.P.; Peng, F.M.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Microwave-enhanced synthesis of magnetic porous covalent triazine-based framework composites for fast separation of organic dye from aqueous solution. J. Hazard. Mater. 2011, 186, 984–990. [Google Scholar] [CrossRef]
- Chen, S.; Wen, L.; Svec, F.; Tan, T.; Lv, Y. Magnetic metal–organic frameworks as scaffolds for spatial co-location and positional assembly of multi-enzyme systems enabling enhanced cascade biocatalysis. RSC Adv. 2017, 7, 21205–21213. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.N.; Zhou, M.; Shu, L.; Li, Z.; Guan, X. Magnetic metal-organic frameworks: γ-Fe2O3@MOFs via confined in situ pyrolysis method for drug delivery. Small 2014, 10, 2927–2936. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; He, X.; Chen, S.; He, C.; Wang, T.; Mao, X. Recent Advances in Metal-Based Magnetic Composites as High-Efficiency Candidates for Ultrasound-Assisted Effects in Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 10461. https://doi.org/10.3390/ijms221910461
Wang Z, He X, Chen S, He C, Wang T, Mao X. Recent Advances in Metal-Based Magnetic Composites as High-Efficiency Candidates for Ultrasound-Assisted Effects in Cancer Therapy. International Journal of Molecular Sciences. 2021; 22(19):10461. https://doi.org/10.3390/ijms221910461
Chicago/Turabian StyleWang, Zhenyu, Xiaoxiao He, Shiyue Chen, Chengdian He, Teng Wang, and Xiang Mao. 2021. "Recent Advances in Metal-Based Magnetic Composites as High-Efficiency Candidates for Ultrasound-Assisted Effects in Cancer Therapy" International Journal of Molecular Sciences 22, no. 19: 10461. https://doi.org/10.3390/ijms221910461