Metabolic Analysis of the Development of the Plant-Parasitic Cyst Nematodes Heterodera schachtii and Heterodera trifolii by Capillary Electrophoresis Time-of-Flight Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Data Collection
2.2. Major Metabolic Biosynthetic Pathways in Nematode Species
2.3. Relative Peak Areas of Metabolites and Patterns of Variation across the Stages
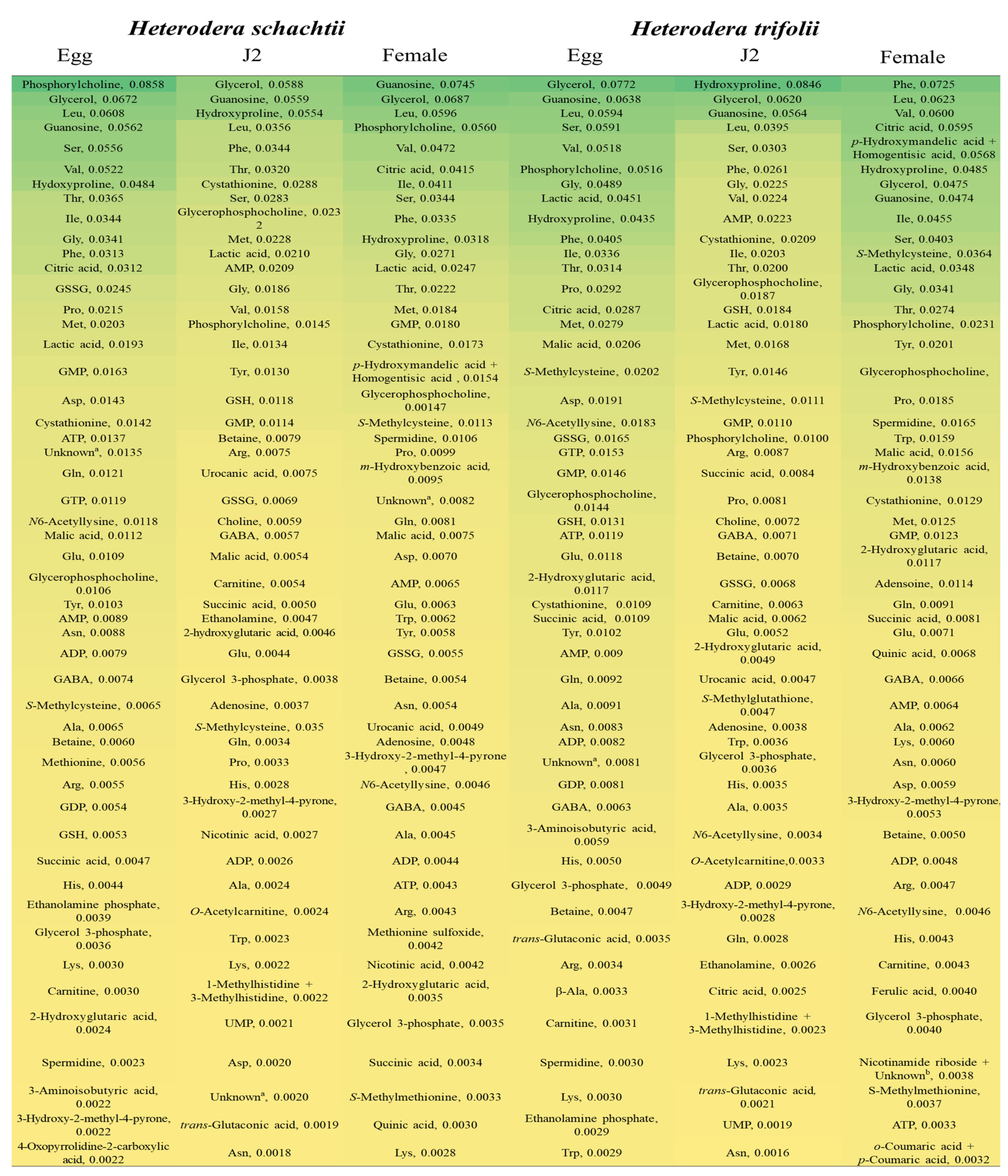
2.4. Highly Detected Metabolites in Nematode Species
2.5. Pearson’s Correlation and Multivariate Analysis of Metabolic Profiles
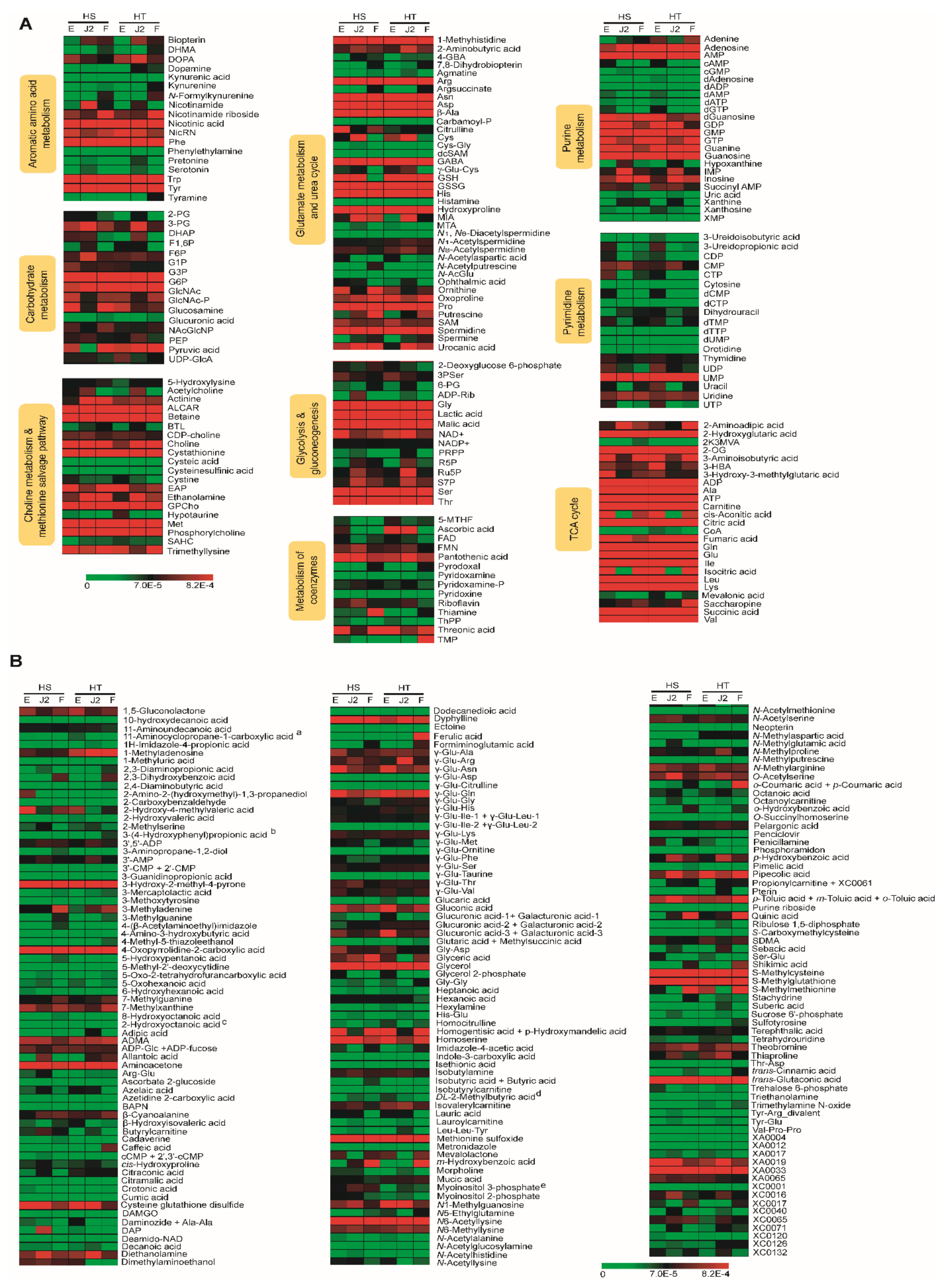
2.6. Heat Map and Biological Properties of Stage-Specific Metabolites
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. CE-TOF/MS Analysis of Metabolites
4.3. Data Processing and Analysis
4.4. Statistical Analysis
4.5. Plotting on the Pathway Map
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Reuss, S.H.; Schroeder, F.C. Combinatorial chemistry in nematodes: Modular assembly of primary metabolism-derived building blocks. Nat. Prod. Rep. 2015, 32, 994–1006. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, P.; Oh, B.-J.; Mani, V.; Lee, J.K.; Lee, C.-M.; Sim, J.-S.; Koo, J.C.; Hahn, B.-S. Differential Metabolic Profiles during the Developmental Stages of Plant-Parasitic Nematode Meloidogyne incognita. Int. J. Mol. Sci. 2017, 18, 1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madani, M.; Kyndt, T.; Colpaert, N.; Subbotin, S.A.; Gheysen, G.; Moens, M. Polymorphism among sugar beet cyst nematode Heterodera schachtii populations as inferred from AFLP and ITS rDNA gene analyses. Russ. J. Nematol. 2007, 15, 117–128. [Google Scholar]
- Dispersal of sugar beet cyst nematode (Heterodera schachtii) by water and soil in highland Chinese cabbage fields. Korean J. Hortic. Sci. 2016, 34, 195–205. [CrossRef]
- Kwon, S.B.; Park, D.K.; Won, H.S.; Moon, Y.G.; Lee, J.H.; Kim, Y.B.; Choi, B.G.; Seo, H.T.; Ko, H.R.; Lee, J.K.; et al. Spread of cyst nematodes in highland Chinese cabbage field in Gangwon-do. Korean J. Appl. Entomol. 2018, 57, 339–345. [Google Scholar] [CrossRef]
- Kaushal, K.K.; Chawala, G.; Pankaj; Sirohi, A.; Singh, K. A Report on Heterodera trifolii from Kangra, Himachal Pradesh. Indian J. Nematol. 2008, 28, 261–262. [Google Scholar]
- Mwamula, A.O.; Ko, H.-R.; Kim, Y.; Kim, Y.H.; Lee, J.-K.; Lee, D.W. Morphological and Molecular Characterization of Heterodera schachtii and the Newly Recorded Cyst Nematode, H. trifolii Associated with Chinese Cabbage in Korea. Plant Pathol. J. 2018, 34, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.E.; Whitehand, L. Comparative Morphometrics of Eggs and Second-Stage Juveniles of Heterodera schachtii and a Race of H. trifolii Parasitic on Sugarbeet in The Netherlands. J. Nematol. 1984, 16, 171–177. [Google Scholar]
- Gillet, F.-X.; Bournaud, C.; Júnior, J.D.A.D.S.; Grossi-De-Sa, M.F. Plant-parasitic nematodes: Towards understanding molecular players in stress responses. Ann. Bot. 2017, 119, 775–789. [Google Scholar] [CrossRef] [Green Version]
- Perrine-Walker, F. Interactions of endoparasitic and ectoparasitic nematodes within the plant root system. Funct. Plant Biol. 2019, 46, 295–303. [Google Scholar] [CrossRef]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an Emerging Tool for the Study of Plant–Pathogen Interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Szakasits, D.; Heinen, P.; Wieczorek, K.; Hofmann, J.; Wagner, F.; Kreil, D.P.; Sykacek, P.; Grundler, F.M.W.; Bohlmann, H. The transcriptome of syncytia induced by the cyst nematode Heterodera schachtiiin Arabidopsis roots. Plant J. 2009, 57, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Williamson, V.M.; Hussey, R.S. Nematode pathogenesis and resistance in plants. Plant Cell 1996, 8, 1735–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammerhofer, N.; Radakovic, Z.; Regis, J.M.A.; Dobrev, P.; Vankova, R.; Grundler, F.M.W.; Siddique, S.; Hofmann, J.; Wieczorek, K. Role of stress-related hormones in plant defence during early infection of the cyst nematode Heterodera schachtii in Arabidopsis. New Phytol. 2015, 207, 778–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariyar, S.R.; Nakarmi, J.; Anwer, M.A.; Siddique, S.; Ilyas, M.; Elashry, A.; Dababat, A.A.; Leon, J.; Grundler, F.M. Amino acid permease 6 modulates host response to cyst nematodes in wheat and Arabidopsis. Nematology 2018, 20, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Anwar, S.; Wieczorek, K.; Inselsbacher, E. Analysis of Arabidopsis amino acid metabolism in response to Heterodera schachtii infection. Pak. J. Nematol. 2018, 36, 131–150. [Google Scholar] [CrossRef]
- Hofmann, J.; Ashry, A.E.N.E.; Anwar, S.; Erban, A.; Kopka, J.; Grundler, F. Metabolic profiling reveals local and systemic responses of host plants to nematode parasitism. Plant J. 2010, 62, 1058–1071. [Google Scholar] [CrossRef] [Green Version]
- Anwar, S.; Inselsbacher, E.; Grundler, F.M.; Hofmann, J. Arginine metabolism of Arabidopsis thaliana is modulated by Heterodera schachtii infection. Nematology 2015, 17, 1027–1043. [Google Scholar] [CrossRef]
- Schroeder, F.C. Modular Assembly of Primary Metabolic Building Blocks: A Chemical Language. Chem. Biol. 2015, 22, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Wewer, V.; Makepeace, B.L.; Tanya, V.N.; Peisker, H.; Pfarr, K.; Hoerauf, A.; Dörmann, P. Lipid profiling of the filarial nematodes Onchocerca volvulus, Onchocerca ochengi and Litomosoides sigmodontis reveals the accumulation of nematode-specific ether phospholipids in the host. Int. J. Parasitol. 2017, 47, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Pang, Q.; Zhang, T.; Wang, Y.; Kong, W.; Guan, Q.; Yan, X.; Chen, S. Metabolomics of Early Stage Plant Cell–Microbe Interaction Using Stable Isotope Labeling. Front. Plant Sci. 2018, 9, 760. [Google Scholar] [CrossRef] [Green Version]
- Allwood, J.W.; Clarke, A.; Goodacre, R.; Mur, L.A. Dual metabolomics: A novel approach to understanding plant–pathogen interactions. Phytochemistry 2010, 71, 590–597. [Google Scholar] [CrossRef]
- Myers, R.F.; Krusberg, L.R. Organic Substances Discharged by Plant-Parasitic Nematodes. Phytopathology 1965, 55, 429–437. [Google Scholar]
- Wang, E.L.H.; Bergeson, G.B. Amino Acids and Carbohydrates Secreted by Meloidogyne incognita. J. Nematol. 1978, 10, 367–368. [Google Scholar]
- Huijbregts, A.W.M.; Gijssel, P.D.; Munning, R.G.; Heijbroek, W. Estimation of the viability of Heterodera schachtii field populations by measuring ATP, ADP and AMP contents of eggs and juveniles using HPLC. Eur. J. Plant Pathol. 1996, 102, 277–282. [Google Scholar] [CrossRef]
- Huijbregts, A.W.M.; Gijssel, P.D.; Munning, R.G.; Heijbroek, W. Estimation of the field population and the vitality of Heterodera schachtii, measuring ATP, ADP and amp contents of eggs and juveniles by HPLC. Commun. Soil Sci. Plant Anal. 1996, 27, 1153–1168. [Google Scholar] [CrossRef]
- Sudama, G.; Zhang, J.; Isbister, J.; Willett, J.D. Metabolic profiling in Caenorhabditis elegans provides an unbiased approach to investigations of dosage dependent lead toxicity. Metabolomics 2012, 9, 189–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Reuss, S.H. Exploring Modular Glycolipids Involved in Nematode Chemical Communication. Chim. Int. J. Chem. 2018, 72, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Heiger, D.N. Amino Acid Analysis by Capillary Electrophoresis Electrospray Ionization Mass Spectrometry. Anal. Chem. 2000, 72, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ueno, Y.; Naraoka, H.; Ohashi, Y.; Tomita, M.; Nishioka, T. Simultaneous Determination of Anionic Intermediates for Bacillus subtilis Metabolic Pathways by Capillary Electrophoresis Electrospray Ionization Mass Spectrometry. Anal. Chem. 2002, 74, 2233–2239. [Google Scholar] [CrossRef]
- Lamitina, S.T.; Morrison, R.; Moeckel, G.W.; Strange, K. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am. J. Physiol. Physiol. 2004, 286, C785–C791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Womersley, C.; Smith, L. Anhydrobiosis in nematodes—I. The role of glycerol myo-inositol and trehalose during desiccation. Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 70, 579–586. [Google Scholar] [CrossRef]
- Lanznaster, D.; Dal-Cim, T.; Piermartiri, T.C.B.; Tasca, C.I. Guanosine: A Neuromodulator with Therapeutic Potential in Brain Disorders. Aging Dis. 2016, 7, 657–679. [Google Scholar] [CrossRef] [Green Version]
- Bettio, L.; Gil-Mohapel, J.; Rodrigues, A.L.S. Guanosine and its role in neuropathologies. Purinergic Signal. 2016, 12, 411–426. [Google Scholar] [CrossRef] [Green Version]
- Da Silveira, T.L.; Machado, M.L.; Arantes, L.P.; Zamberlan, D.C.; Cordeiro, L.M.; Obetine, F.B.B.; da Silva, A.F.; Tassi, C.L.; Soares, F.A.A. Guanosine Prevents against Glutamatergic Excitotoxicity in C. elegans. Neuroscience 2019, 414, 265–272. [Google Scholar] [CrossRef]
- Lopez-vera, E.; Walewska, A.; Skalicky, J.J.; Olivera, B.M.; Bulaj, G. Role of Hydroxyprolines in the in Vitro Oxidative Folding and Biological Activity of Conotoxins. Biochemistry 2008, 47, 1741–1751. [Google Scholar] [CrossRef]
- Lochnit, G.; Dennis, R.D.; Geyer, R. Phosphorylcholine Substituents in Nematodes: Structures, Occurrence and Biological Implications. Biol. Chem. 2000, 381, 839–847. [Google Scholar] [CrossRef]
- Harnett, W.; Harnett, M.M. Phosphorylcholine: Friend or foe of the immune system? Immunol. Today 1999, 20, 125–129. [Google Scholar] [CrossRef]
- Cooper, D.; Eleftherianos, I. Parasitic Nematode Immunomodulatory Strategies: Recent Advances and Perspectives. Pathogens 2016, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Curtis, R.H.C.; Jones, J.T.; Davies, K.G.; Sharon, E.; Spiegel, Y. Plant Nematode Surfaces. In Biological Control of Plant-Parasitic Nematodes: Building Coherence between Microbial Ecology and Molecular Mechanisms, Progress in Biological Control 11; Davies, K., Spiegel, Y., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 115–144. [Google Scholar]
- Song, S.; Han, Y.; Zhang, Y.; Ma, H.; Zhang, L.; Huo, J.; Wang, P.; Liang, M.; Gao, M. Protective role of citric acid against oxidative stress induced by heavy metals in Caenorhabditis elegans. Environ. Sci. Pollut. Res. 2019, 26, 36820–36831. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Baldacci-Cresp, F.; Chang, C.; Maucourt, M.; Deborde, C.; Hopkins, J.; Lecomte, P.; Bernillon, S.; Brouquisse, R.; Moing, A.; Abad, P.; et al. (Homo)glutathione Deficiency Impairs Root-knot Nematode Development in Medicago truncatula. PLoS Pathog. 2012, 8, e1002471. [Google Scholar] [CrossRef]
- Brownlee, D.; Holden-Dye, L.; Walker, R. The range and biological activity of FMR Famide-related peptides and classical neurotransmitters in nematodes. Adv. Parasitol. 2000, 45, 109–180. [Google Scholar] [CrossRef]
- Perry, R.N.; Maule, A.G. Physiological and biochemical basis of behaviour. In Nematode Behaviour; Gaugler, R., Bilgrami, A.L., Eds.; CAB International: Wallingford, UK, 2004; pp. 197–238. [Google Scholar]
- Stewart, G.R.; Perry, R.N.; Wright, D.J. Immunocytochemical studies on the occurrence of gamma-aminobutyric acid in the nervous system of the nematodes Panagrellus redivivus, Meloidogyne incognita and Globodera rostochiensis. Fundam. Appl. Nematol. 1994, 17, 433–439. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/fan/40781.pdf (accessed on 8 April 2021).
- Stewart, G.; Perry, R.; Wright, D. Occurrence of dopamine in Panagrellus redivivus and Meloidogyne incognita. Nematology 2001, 3, 843–848. [Google Scholar] [CrossRef]
- Fu, X.; Chin, R.M.; Vergnes, L.; Hwang, H.; Deng, G.; Xing, Y.; Pai, M.Y.; Li, S.; Ta, L.; Fazlollahi, F.; et al. 2-Hydroxyglutarate Inhibits ATP Synthase and mTOR Signaling. Cell Metab. 2015, 22, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Soga, T.; Ohashi, Y.; Ueno, Y.; Naraoka, H.; Tomita, M.; Nishioka, T. Quantitative Metabolome Analysis Using Capillary Electrophoresis Mass Spectrometry. J. Proteome Res. 2003, 2, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Bolstad, B.; Irizarry, R.; Astrand, M.; Speed, T. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Saeed, A.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A Free, Open-Source System for Microarray Data Management and Analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junker, B.H.; Klukas, C.; Schreiber, F. VANTED: A system for advanced data analysis and visualization in the context of biological networks. BMC Bioinform. 2006, 7, 109. [Google Scholar] [CrossRef] [Green Version]



| HS-E | HS-J2 | HS-F | HT-E | HT-J2 | HT-F | |
|---|---|---|---|---|---|---|
| HS-E | 1 | |||||
| HS-J2 | 0.8203 * | 1 | ||||
| HS-F | 0.9417 * | 0.8450 * | 1 | |||
| HT-E | 0.9545 * | 0.8594 * | 0.9357 * | 1 | ||
| HT-J2 | 0.7975 * | 0.9651 * | 0.8103 * | 0.8394 * | 1 | |
| HT-F | 0.8008 * | 0.7545 * | 0.8814 * | 0.8509 * | 0.7481 * | 1 |
| Stage | Metabolites with No Significant Difference between HS and HT | Metabolites Significantly Higher in HS than HT | Metabolites Significantly Higher in HT than HS |
|---|---|---|---|
| Egg | XC0120, Leu-Leu-Tyr, Lauric acid, Spermine, dCMP, Val-Pro-Pro, Gly-Asp, N-Methylproline, γ-Glu-Ile + γ-Glu-Leu, Glycerol 2-phosphate, Dimethylaminoethanol, dATP, Decanoic acid, N-Acetylhistidine, N-Acetylalanine, Pyridoxamine-P, 2-Methylserine, dADP, ATP, Dyphylline, Octanoic acid, dTMP, XC0071, Hexanoic acid, Pantothenic acid, N-Acetylaspartic acid, N-Acetylmethionine, Citric acid, XC0126, γ-Glu-Ornitine, cis-Hydroxyproline, 2-Aminoisobutyric acid + 2-Aminobutyric acid, Thymidine, Asn, Pelargonic acid, S7P, XA0065, Lys, γ-Glu-Gln, Leu, XA0017, γ-Glu-Ala, Ile, Isocitric acid, γ-Glu, Asn, Val, Gly-Gly, 8-Hydroxyoctanoic acid + 2-Hydroxyoctanoic acid, Tyr, Aminoacetone, 1-Methylhistidine + 3-Methylhistidine, dAMP, γ-Glu-Lys, β-Hydroxyisovaleric acid, NADP+, γ-Glu-Asp, ADP, Carnitine, Triethanolamine, Terephthalic acid, p-Toluic acid + m-Toluic acid+ o-Toluic acid, 4-Oxopyrrolidine-2-carboxylic acid, Ser, dGuanosine, NAD+, Glu, γ-Glu-Ser, CDP-choline, γ-Glu-Val, AMP, MTA, Tetrahydrouridine, N-Acetyllysine, N-Acetylserine, ADMA, ADP-Glc + GDP-fucose, Isobutylamine, Glycerol, Heptanoic acid, O-Succinylhomoserine, cAMP, Theobromine, 11-Aminoundecanoic acid, N-Acetylglucosylamine, 7-Methylguanine, FAD, Xanthosine, Azelaic acid, Diethanolamine, UTP, Glucaric acid, and 5-MTHF | Citrulline, Methionine sulfoxide, Urocanic acid, XC0132, Penicillamine, XC0065, MIA, Choline, 3-PG, Thiamine, Myoinositol 1-phosphate + Myoinositol 3-phosphate, Imidazole-4-acetic acid, CTP, PEP, Ethanolamine, Ophthalmic acid, CDP, GlcNAc-P, Myoinositol 2-phosphate, G1P, Ser-Glu, Acetylcholine, 5-Hydroxylysine, TMP, Putrescine, XC0016, Arg-Glu, Octanoylcarnitine, 3-Methyladenine, CMP, NAcGlcNP, 3-Hydroxy-3-methylglutaric acid, 3′,5′-ADP, 2-Deoxyglucose 6-phosphate, XA0033, Phosphorylcholine, 6-PG, Arg, 6-Hydroxyhexanoic acid, 2-Hydroxy-4-methylvaleric acid, Riboflavin, Ru5P, γ-Glu-Arg, XA0019, GSSG, Cysteine glutathione disulfide, 4-GBA, Ornithine, Inosine, EAP, Gln, Threonic acid, Cystathionine, Betaine, Cystine, 7-Methylxanthine, ThPP, His-Glu, γ-Glu-Thr, N-Acetylgalactosamine + ManNA + GlcNAc, IMP, XC0089 + Nicotinamide riboside, GABA, 3-Hydroxy-2-methyl-4-pyrone, Thr, GMP, Hydroxyproline, and FMN | Adenine, S-Methylmethionine, Cys, Hypotaurine, Uracil, 2-Hydroxyglutaric acid, trans-Glutaconic acid, b β-Cyanoalanine, Thiaproline, Quinic acid, Actinine, 1-Methyladenosine, Pipecolic acid, Glucuronic acid-1 + Galacturonic acid-1, Dihydrouracil, S-Methylcysteine, UDP-GlcA, N1-Methylguanosine, Glucuronic acid-2 + Galacturonic acid-2, 3-Aminoisobutyric acid, Isovalerylcarnitine, γ-Glu-Cys, GSH, 3-HBA, Oxoproline, β-Ala, Lactic acid, Succinic acid, Mevalonic acid, Adenosine, S-Methylglutathione, Malic acid, dcSAM, Mucic acid, O-Acetylhomoserine + 2-Aminoadipic acid, Fumaric acid, 3-Ureidopropionic acid, SDMA, Daminozide + Ala-Ala, 1,5-Gluconolactone, Succinyl AMP, γ-Glu-Citrulline, Glucuronic acid-3 + Galacturonic acid-3, Guanine, CoA, γ-Glu-His, 1-Aminocyclopropane-1-carboxylic acid +, Homoserinelactone, UDP, SAHC, Saccharopine, N6-Acetyllysine, Mevalolactone, 2-OG, DOPA, NicRN, F6P, GDP, N8-Acetylspermidine, O-Acetylserine, Gluconic acid, N1-Acetylspermidine, Gly, Homogentisic acid + p-Hydroxymandelic acid, F1,6P, ALCAR, p-Hydroxybenzoic acid, G6P, SAM, 3PSer, N6, Methyllysine, Ala, Nω-Methylarginine, Met, Trp, G3P, GPCho, Pro, γ-Glu-Phe, Asp, γ-Glu-Gly, Spermidine, Phe, 3′-AMP, GTP, dGTP, UMP, cis-Aconitic acid, Uridine, Nicotinic acid, Homoserine, Trimethyllysine, His, Guanosine, and γ-Glu-Met |
| J2 | p-Toluic acid + m-Toluic acid + o-Toluic acid, Myoinositol 2-phosphate, XA0017, XC0071, Dihydrouracil, XC0065, ThPP, Xanthine, TMP, dTMP, GPCho, Gluconic acid, 3-PG, γ-Glu-Asn, Penciclovir, Spermidine, Formiminoglutamic acid, XA0004, Pantothenic acid, CMP, γ-Glu-Asp, Asn, Lactic acid, Uridine, Betaine, Tyr-Glu, Kynurenic acid, 2-Deoxyglucose 6-phosphate, Terephthalic acid, Cystine, 3′-CMP + 2′-CMP, Nω-Methylarginine, Guanine, Heptanoic acid, Isobutylamine, o-Coumaric acid + p-Coumaric acid, G3P, Kynurenine, UMP, 1,5-Gluconolactone, GMP, SAHC, 5-Hydroxylysine, Serotonin, 3′,5′-ADP, 2-PG, Spermine, Cysteine glutathione disulfide, GSSG, His-Glu, Aminoacetone, Octanoic acid, γ-Glu-Met, Oxoproline, ATP, SAM, γ-Glu-Gly, GTP, Fumaric acid, Guanosine, Inosine, Quinic acid, 3PSer, Isethionic acid, Adenosine, Hexanoic acid, Lys, 3-Hydroxy-2-methyl-4-pyrone, Ophthalmic acid, Glycerol, ADMA, S7P, AMP, UDP, Ser, dcSAM, N-Acetylgalactosamine + ManNAc + GlcNAc, 3-Mercaptolactic acid, 2-Hydroxyglutaric acid, 1-Methylhistidine + 3-Methylhistidine, XA0019, 11-Aminoundecanoic acid, trans-Glutaconic acid, Citraconic acid, p-Hydroxybenzoic acid, Acetylcholine, N-Acetylmethionine, Pelargonic acid, ADP, Theobromine, XC0089 + Nicotinamide riboside, Leu, 3-Hydroxy-3-methylglutaric acid, N-Acetylglucosylamine, Tyr, γ-Glu-His, Lauric acid, N6-Methyllysine, NAcGlcNP, Lauroylcarnitine, Malic acid, Arg, Decanoic acid, γ-Glu-Ile + γ-Glu-Leu, Glu, ADP-Glc + GDP-fucose, BTL, FAD, γ-Glu-Lys, CoA, PEP, γ-Glu-Gln, Glucuronic acid-3 + Galacturonic acid-3, GABA, 1H-Imidazole-4-propionic acid, His, Orotidine, γ-Glu, Phe, 3′-AMP, Uric acid, 3-Aminoisobutyric acid, DHAP, Pretonine, γ-Glu-Ser, N-Acetylalanine, XC0126, Succinyl AMP, Ascorbate 2-glucoside, Mucic acid, Thiaproline, Diethanolamine, and Agmatine | O-Acetylhomoserine + 2-Aminoadipic acid, ADP-Rib, Nicotinamide, Myoinositol 1-phosphate + Myoinositol 3-phosphate, Asp, Butyrylcarnitine, Putrescine, Thiamine, Hypoxanthine, Tyr-Arg_divalent, Acetylserine, Allantoic acid, Homoserine, IMP, UDP-GlcA, 4-GBA, Pipecolic acid, XC0040, Cadaverine, Methionine sulfoxide, Arg-Glu, F1,6P, MTA, Riboflavin, N-Acetylhistidine, Gly-Asp, 2-Methylserine, Nicotinic acid, 2,3-Diaminopropionic acid, cGMP, Ethanolamine, GDP, Octanoylcarnitine, XA0065, 2-Hydroxy-4-methylvaleric acid, G6P, F6P, 3-HBA, γ-Glu-Thr, Propionylcarnitine + XC0061, Trimethyllysine, dGuanosine, N-Acetylserine, Thr, Urocanic acid, XA0033, Ser-Glu, G1P, Sucrose 6′-phosphate, MIA, XC0016, Actinine, Pyridoxamine-P, β-Cyanoalanine, Phosphorylcholine, FMN, Citrulline, Cystathionine, Met, CDP-choline, Phe, Thymidine, 7-Methylxanthine, EAP, Saccharopine, Gln, and Adenine | 1-Methyladenosine, N6-Acetyllysine, N-Methylproline, Octopamine + Dopamine, N1-Methylguanosine, S-Methylcysteine, S-Methylglutathione, Pterin, XC0132, DOPA, Xanthosine, Sebacic acid, Pro, 3-Methyladenine, γ-Glu-Cys, γ-Glu-Ala, N1-Acetylspermidine, Glucuronic acid-2 + Galacturonic acid-2, γ-Glu-Arg, Suberic acid, 2-OG, NAD+, 6-PG, SDMA, Cys, Homogentisic acid + p-Hydroxymandelic acid, Isovalerylcarnitine, Threonic acid, Succinic acid, Ru5P, N8-Acetylspermidine, 7-Methylguanine, PRPP, GSH, Hydroxyproline, Trp, cis-Hydroxyproline, Ile, Leu-Leu-Tyr, N-Formylkynurenine, GlcNAc-P, Biopterin, NicRN, Ala, Citric acid, Val, R5P, NADP+, γ-Glu-Val, ALCAR, Ornithine, 2-Aminoisobutyric acid + 2-Aminobutyric acid, Dyphylline, β-Ala, Choline, Gly, 4-Oxopyrrolidine-2-carboxylic acid, Azelaic acid, and Carnitine |
| Female | GDP, 6-PG, Stachydrine, BTL, XC0017, Sebacic acid, Isobutyric acid + Butyric acid, Glycerol, Azetidine 2-carboxylic acid, Ectoine, Myoinositol 2-phosphate, G1P, 11-Aminoundecanoic acid, β-Hydroxyisovaleric acid, Putrescine, FAD, Agmatine, Azelaic acid, Cystine, Deamido-NAD, Asp, γ-Glu-Citrulline, Dyphylline, R5P, UMP, MTA, 2-Hydroxyvaleric acid, dAdenosine + 5′-Deoxyadenosine, Hexanoic acid, CDP-choline, 4-GBA, Val, Pro-Pro, Cysteic acid, Biopterin, Isovaleric acid + DL-2-Methylbutyric acid + Valeric acid, GTP, Hypoxanthine, NADP+, XC0120, N-Acetylalanine, dcSAM, Tetrahydrouridine, Betaine, Diethanolamine, 3′,5′-ADP, 3-Methyladenine, Ru5P, Threonic acid, γ-Glu-Arg, NAD+, cGMP, UTP, NicRN, Xanthosine, Pimelic acid, ArgSuccinate, AMP, N6-Acetyllysine, Glycerol 2-phosphate, Pyruvic acid, Leu, Ophthalmic acid, N-Acetylserine, Galactosamine + Glucosamine, 3-Methoxytyrosine, Theobromine, Pyridoxal, Isobutylamine, Arg, 1-Aminocyclopropane-1-carboxylic acid + Homoserinelactone, ADP, SAHC, 7,8-Dihydrobiopterin, Ile, Asn, dTMP, Decanoic acid, 3-Ureidopropionic acid, Octanoylcarnitine, Choline, Gln, S-Methylmethionine, 3PSer, N-Acetyllysine, S7P, G3P, γ-Glu-Met, cis-Hydroxyproline, F1,6P, Leu-Leu-Tyr, cAMP, N-Acetylglucosylamine, 2-Methylserine, N-AcGlu, γ-Glu-Ornitine, Lauric acid, F6P, 3-Mercaptolactic acid, Terephthalic acid, Hexylamine, XA0017, Pterin, Glutaric acid + Methylsuccinic acid, p-Hydroxybenzoic acid, GPCho, 7-Methylguanine, Octanoic acid, XA0019, UDP, m-Hydroxybenzoic acid, Propionylcarnitine + XC0061, 5-Oxohexanoic acid, Pyridoxamine, 3′-CMP + 2′-CMP, N-Acetylaspartic acid, Pelargonic acid, Glucaric acid, p-Toluic acid + m-Toluic acid + o-Toluic acid, His-Glu, 3-Guanidinopropionic acid, Mevalolactone, and γ-Glu-Asp | Thiamine, MIA, Dimethylaminoethanol, Urocanic acid, N-Methylputrescine, Citrulline, 2-Deoxyglucose 6-phosphate, 5-Hydroxypentanoic acid, XC0065, Nicotinamide, Cadaverine, Myoinositol 1-phosphate + Myoinositol 3-phosphate, XA0033, 2-Hydroxy-4-methylvaleric acid, GSSG, Ethanolamine, TMP, Glyceric acid, Phosphorylcholine, Ornithine, GlcNAc-P, CTP, Penicillamine, CDP, 3-Methylguanine, 8-Hydroxyoctanoic acid, o-Hydroxybenzoic acid, 3-Hydroxy-3-methylglutaric acid, 3-PG, XC0132, CMP, NAcGlcNP, EAP, Inosine, ThPP, Riboflavin, Methionine sulfoxide, dCMP, XA0065, Nicotinic acid, Guanosine, ADP-Glc + GDP-fucose, PEP, Trehalose 6-phosphate, Met, GMP, Pyridoxamine-P, Gly-Asp, XC0040, FMN, IMP, 5-Hydroxylysine, Cysteine glutathione disulfide, Cystathionine, ATP, dGuanosine, Actinine, Uric acid, and Uridine | Ferulic acid, o-Coumaric acid + p-Coumaric acid, GSH, Allantoic acid, Adenine, 1-Methyladenosine, 2,3-Diaminopropionic acid, Shikimic acid, DOPA, Guanine, Homogentisic acid + p-Hydroxymandelic acid, Pipecolic acid, Mucic acid, Tyr, 2-Hydroxyglutaric acid, γ-Glu-Gly, SDMA, Indole-3-carboxylic acid, S-Methylcysteine, O-Acetylhomoserine + 2-Aminoadipic acid, Saccharopine, Succinyl AMP, γ-Glu-His, ADMA, Sulfotyrosine, Formiminoglutamic acid, γ-Glu-Asn, Isovalerylcarnitine, Citraconic acid, Trp, Glucuronic acid-1 + Galacturonic acid-1, N1-Methylguanosine, Ser-Glu, γ-Glu-Ser, XC0089 + Nicotinamide riboside, 3-Aminoisobutyric acid, Succinic acid, Adenosine, trans-Glutaconic acid, γ-Glu-Thr, trans-Cinnamic acid, Quinic acid, Isethionic acid, DHMA, γ-Glu-Phe, N-Formylkynurenine, O-Acetylserine, Phe, Lys, XC0126, Gly-Gly, S-Methylglutathione, Ascorbate 2-glucoside, Malic acid, S-Carboxymethylcysteine, Fumaric acid, XC0016, Carnitine, XC0071, ALCAR, Pyridoxine, Nω-Methylarginine, N1-Acetylspermidine, γ-Glu-Val, Pro, Aminoacetone, N6-Methyllysine, N5-Ethylglutamine, N8-Acetylspermidine, Oxoproline, Thiaproline, γ-Glu-Gln, Triethanolamine, γ-Glu-Ile + γ-Glu-Leu, Arg-Glu, γ-Glu-Lys, Carboxymethyllysine, γ-Glu-Ala, N-Methylproline, Mevalonic acid, 3′-AMP, Kynurenine, 3-HBA, UDP-GlcA, Homoserine, Imidazole-4-acetic acid, His, Spermidine, Hydroxyproline, β-Ala, Isocitric acid, β-Cyanoalanine, Heptanoic acid, GABA, 2-OG, Metronidazole Glucuronic acid-3 + Galacturonic acid-3, G6P, Trimethyllysine, Citric acid, cis-Aconitic acid, Glucuronic acid-2 + Galacturonic acid-2, BAPN, N-Acetylgalactosamine + ManNAc + GlcNAc, 7-Methylxanthine, Lactic acid, 2-Aminoisobutyric acid + 2-Aminobutyric acid, Spermine, 2,3-Dihydroxybenzoic acid, Ala, Dihydrouracil, Homocitrulline, Phosphoramidon, 1-Methylhistidine + 3-Methylhistidine, Val, Gly, Gluconic acid, Thr, XA0004, 1,5-Gluconolactone, Thymidine, SAM, Uracil, Pantothenic acid, Ser, 4-Oxopyrrolidine-2-carboxylic acid, Glu, and 3-Hydroxy-2-methyl-4-pyrone |
| Sample Name | Replicates | Dried Extract (mg) | Solvent (ACN:H2O;1:1) (µL) |
|---|---|---|---|
| H. schachtii Egg | HS-egg-1 | 24.6 | 1200 |
| HS-egg-2 | 27.9 | 1200 | |
| HS-egg-3 | 27.4 | 1200 | |
| H. schachtii Juvenile-2 | HS-J2-1 | 20.8 | 900 |
| HS-J2-2 | 28.3 | 1200 | |
| HS-J2-3 | 24.9 | 1200 | |
| H. schachtii Female | HS-F-1 | 25.1 | 1200 |
| HS-F-2 | 19.1 | 900 | |
| HS-F-3 | 27.5 | 1200 | |
| H. trifolii Egg | HT-egg-1 | 20.7 | 900 |
| HT-egg-2 | 26.1 | 1200 | |
| HT-egg-3 | 25.3 | 1200 | |
| H. trifolii Juvenile-2 | HT-J2-1 | 22.5 | 900 |
| HT-J2-2 | 29.7 | 1200 | |
| HT-J2-3 | 30.1 | 1500 | |
| H. trifolii Female | HT-F-1 | 24.7 | 1200 |
| HT-F-2 | 14.4 | 600 | |
| HT-F-3 | 12.3 | 600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assefa, A.D.; Kim, S.-H.; Mani, V.; Ko, H.-R.; Hahn, B.-S. Metabolic Analysis of the Development of the Plant-Parasitic Cyst Nematodes Heterodera schachtii and Heterodera trifolii by Capillary Electrophoresis Time-of-Flight Mass Spectrometry. Int. J. Mol. Sci. 2021, 22, 10488. https://doi.org/10.3390/ijms221910488
Assefa AD, Kim S-H, Mani V, Ko H-R, Hahn B-S. Metabolic Analysis of the Development of the Plant-Parasitic Cyst Nematodes Heterodera schachtii and Heterodera trifolii by Capillary Electrophoresis Time-of-Flight Mass Spectrometry. International Journal of Molecular Sciences. 2021; 22(19):10488. https://doi.org/10.3390/ijms221910488
Chicago/Turabian StyleAssefa, Awraris Derbie, Seong-Hoon Kim, Vimalraj Mani, Hyoung-Rai Ko, and Bum-Soo Hahn. 2021. "Metabolic Analysis of the Development of the Plant-Parasitic Cyst Nematodes Heterodera schachtii and Heterodera trifolii by Capillary Electrophoresis Time-of-Flight Mass Spectrometry" International Journal of Molecular Sciences 22, no. 19: 10488. https://doi.org/10.3390/ijms221910488







