Effects of Substitution on Cytotoxicity of Diphenyl Ditelluride in Cultured Vascular Endothelial Cells
Abstract
:1. Introduction
2. Results
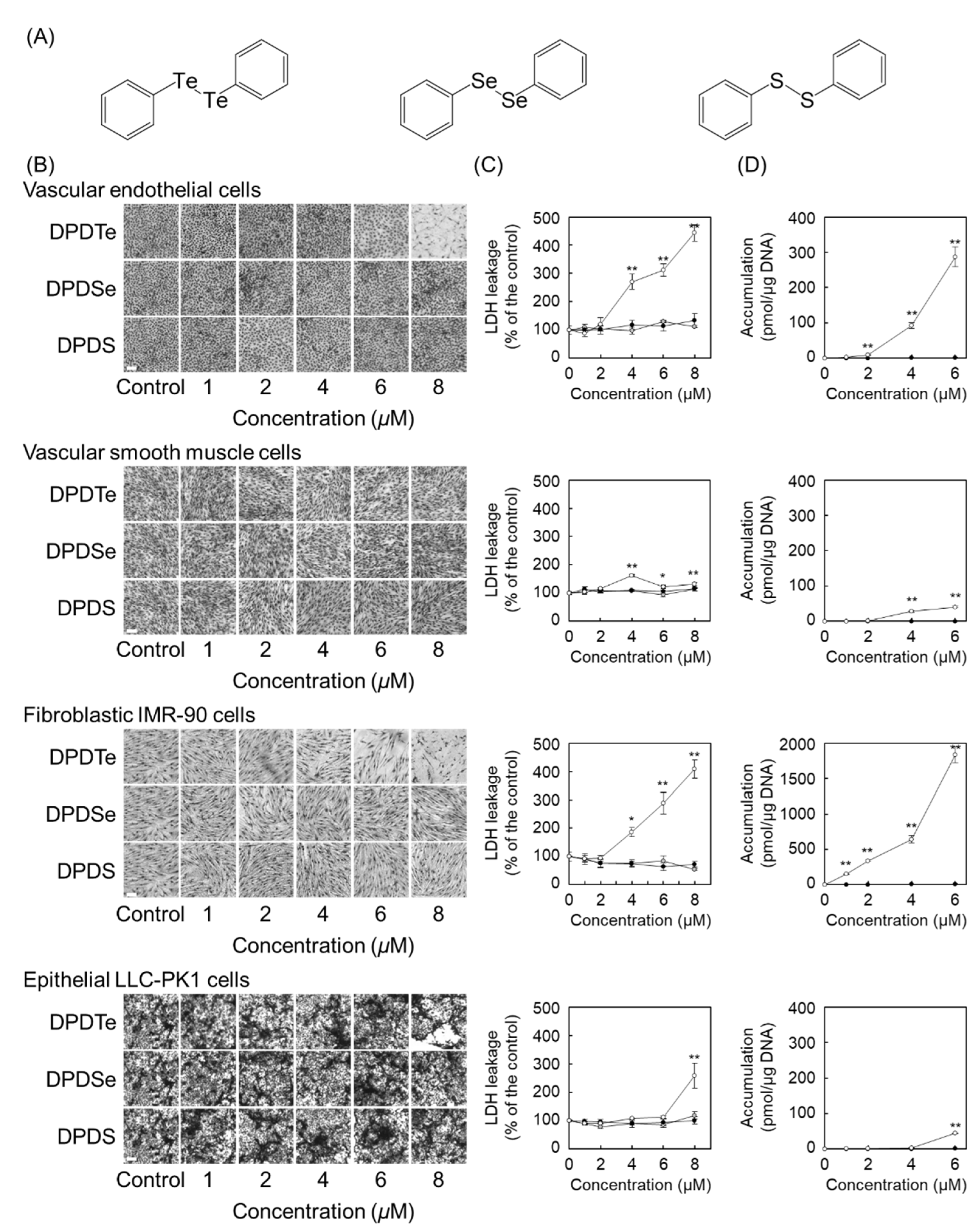
2.1. Diphenyl Ditelluride (DPDTe) Accumulates and Exhibits Cytotoxicity in Vascular Endothelial Cells and Fibroblastic IMR-90 Cells
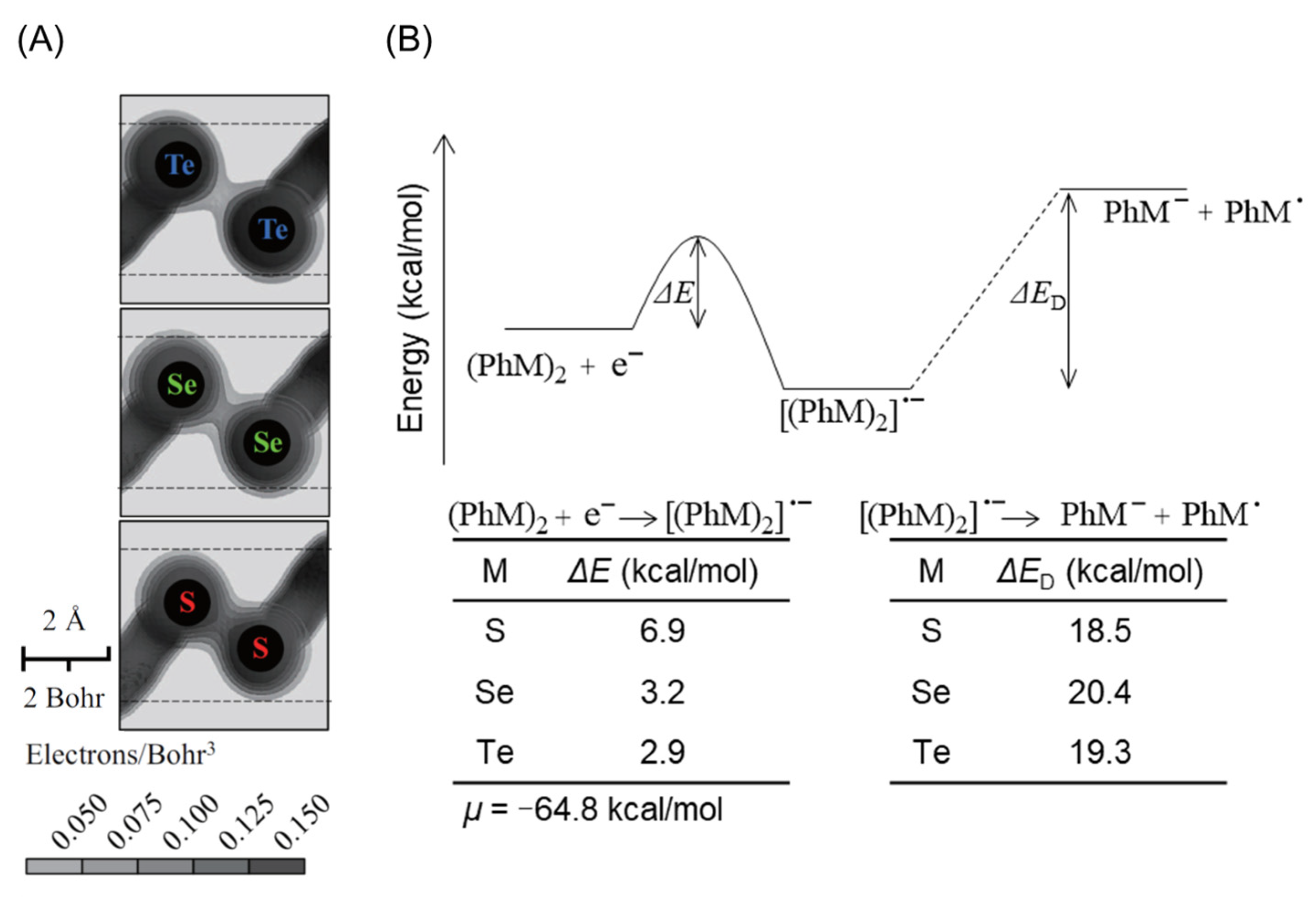
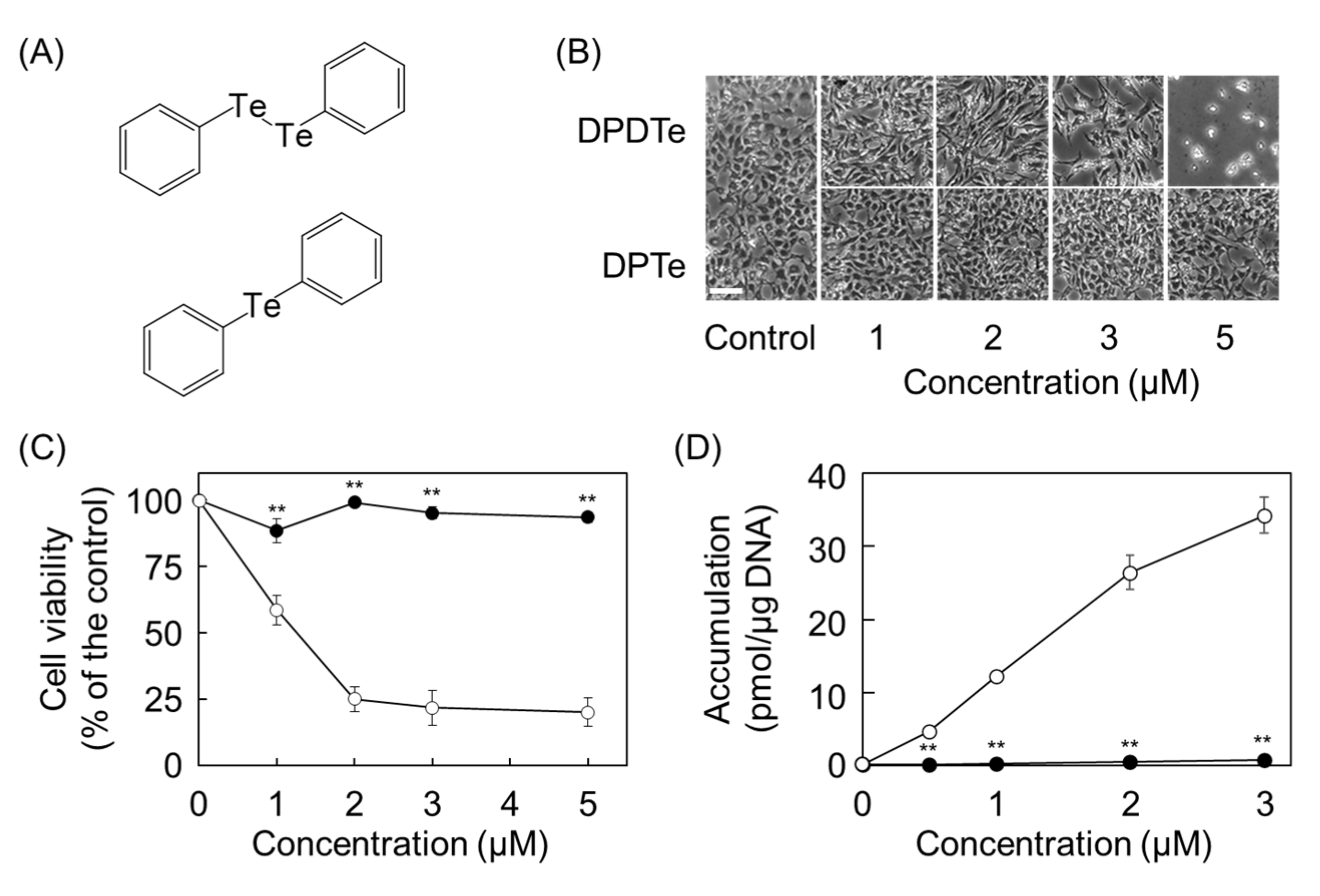
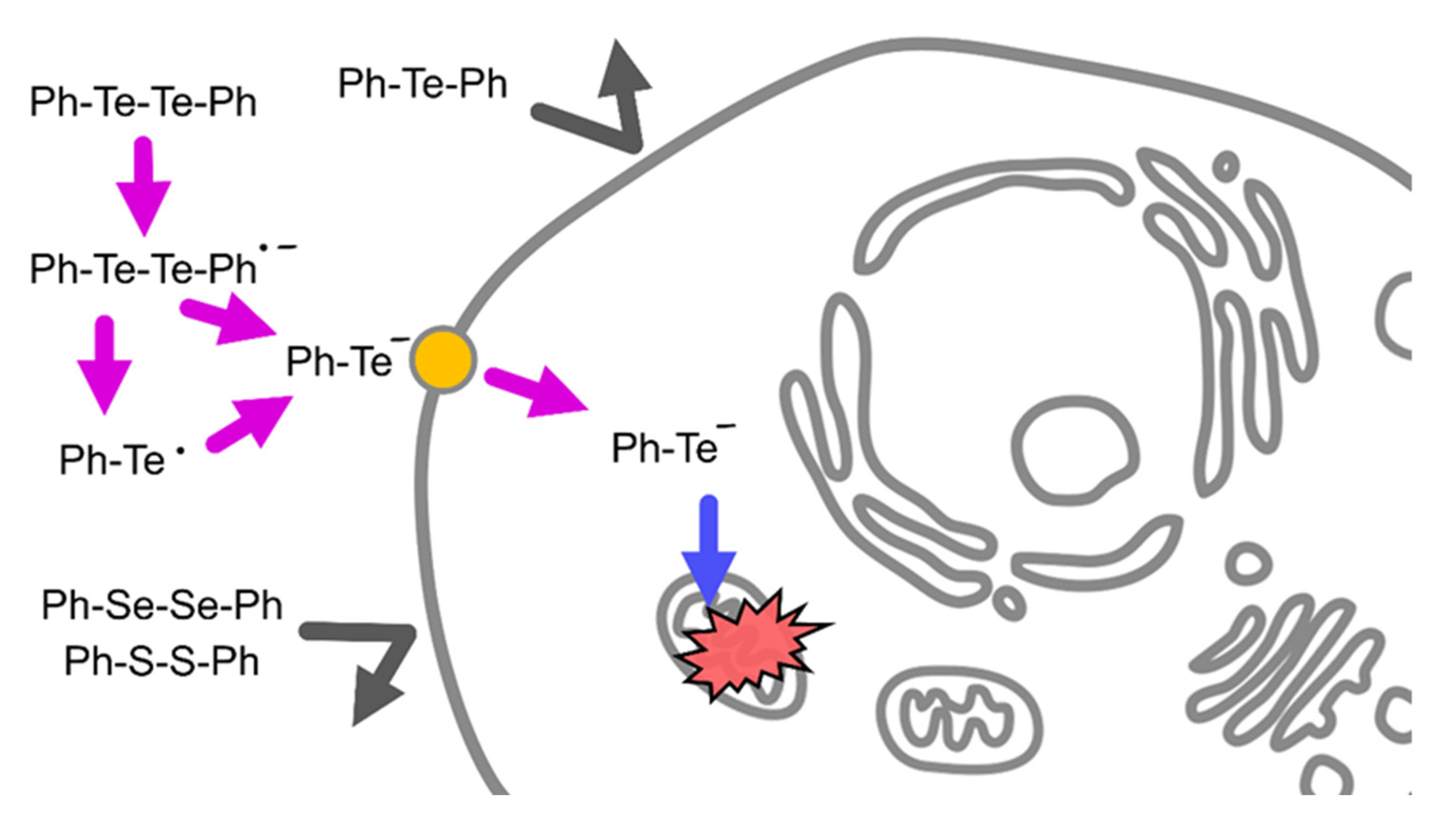
2.2. Cleavage of Te–Te Bond Is Important for Accumulation and Cytotoxicity of DPDTe
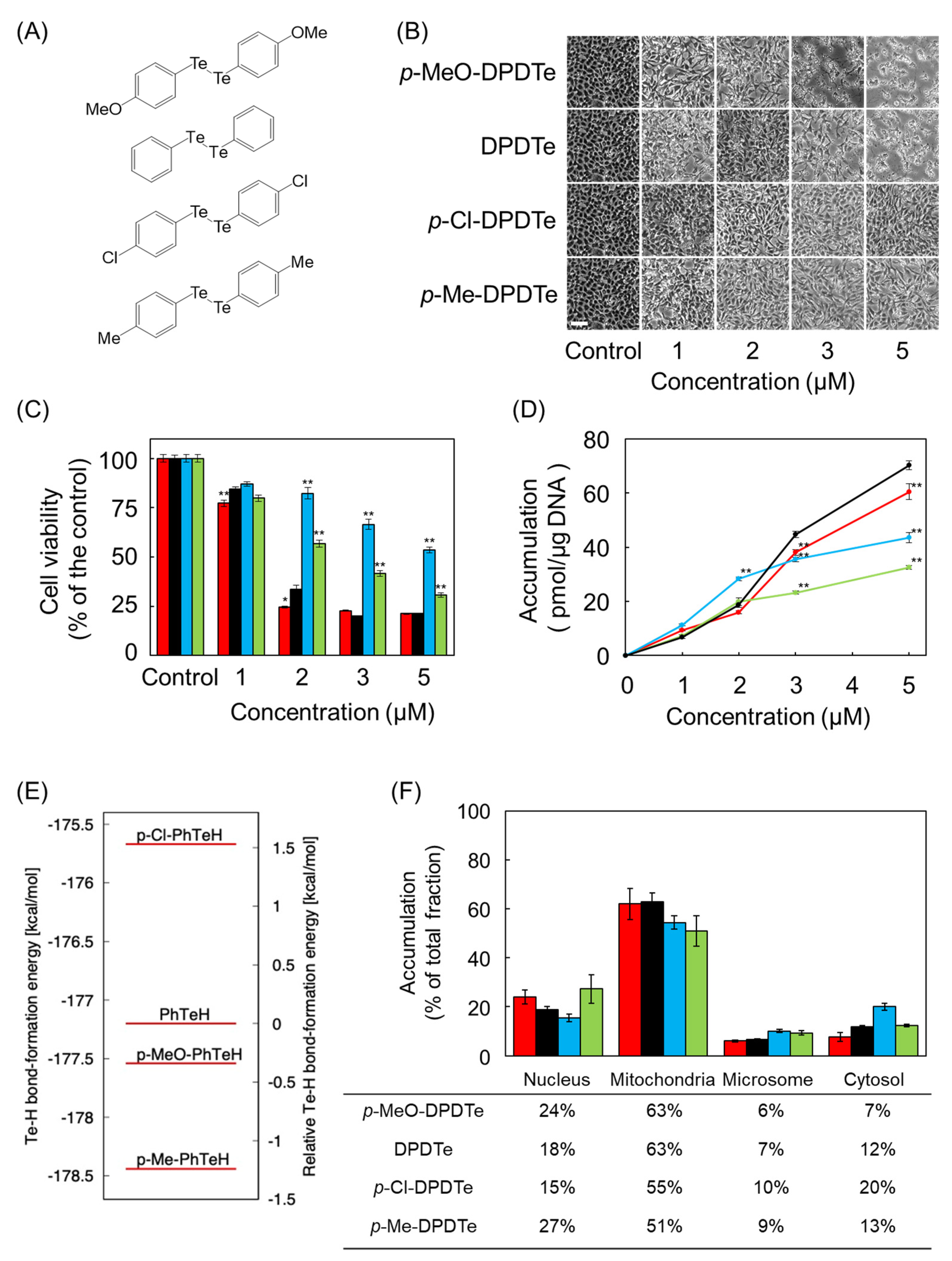
2.3. Substitution Modulate Cytotoxicity of DPDTe
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatments
4.3. Cytotoxicity Assay and Cell Viability Assay
4.4. Intracellular Accumulation of Organotellurium and Organoselenium Compounds
4.5. Ab Initio Quantum Chemical Calculation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grignard, V. Regarding some new organometallic combinations of magnesium and their application to alcohol synthesis and hydrocarbides. CR Acad. Sci. 1900, 130, 1322–1324. [Google Scholar]
- Fujie, T.; Hara, T.; Kaji, T. Toxicology of organic-inorganic hybrid molecules: Bio-organometallics and its toxicology. J. Toxicol. Sci. 2016, 41, SP81–SP88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, T.; Matsuzaki, H.; Nakamura, T.; Yoshida, E.; Ohkubo, T.; Maruyama, H.; Yamamoto, C.; Saito, S.; Kaji, T. Cytotoxicity of zinc, copper and rhodium complexes with 1, 10-phenanthroline or 2, 9-dimethyl-1, 10-phenanthroline in cultured vascular endothelial cells. Fundam. Toxicol. Sci. 2016, 3, 109–113. [Google Scholar] [CrossRef]
- Hara, T.; Nakano, S.; Kitamura, Y.; Yamamoto, C.; Yasuike, S.; Kaji, T. Intracellular accumulation-independent cytotoxicity of pentavalent organoantimony compounds in cultured vascular endothelial cells. J. Toxicol. Sci. 2019, 44, 845–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, T.; Nonaka, Y.; Yasuike, S.; Kaji, T.; Yamamoto, C. Structure-activity relationship of [1,5]azastibocines in cytotoxicity to vascular endothelial cells. J. Toxicol. Sci. 2018, 43, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Fujie, T.; Okino, S.; Yoshida, E.; Yamamoto, C.; Naka, H.; Kaji, T. Copper diethyldithiocarbamate as an inhibitor of tissue plasminogen activator synthesis in cultured human coronary endothelial cells. J. Toxicol. Sci. 2017, 42, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Fujie, T.; Segawa, Y.; Uehara, A.; Nakamura, T.; Kimura, T.; Yoshida, E.; Yamamoto, C.; Uchiyama, M.; Naka, H.; Kaji, T. Zinc diethyldithiocarbamate as an inducer of metallothionein in cultured vascular endothelial cells. J. Toxicol. Sci. 2016, 41, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Fujie, T.; Segawa, Y.; Yoshida, E.; Kimura, T.; Fujiwara, Y.; Yamamoto, C.; Satoh, M.; Naka, H.; Kaji, T. Induction of metallothionein isoforms by copper diethyldithiocarbamate in cultured vascular endothelial cells. J. Toxicol. Sci. 2016, 41, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Hara, T.; Tatsuishi, H.; Banno, T.; Fujie, T.; Yamamoto, C.; Naka, H.; Kaji, T. Copper(II) bis(diethyldithiocarbamate) induces the expression of syndecan-4, a transmembrane heparan sulfate proteoglycan, via p38 MAPK activation in vascular endothelial cells. Int. J. Mol. Sci. 2018, 19, 3302. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, E.; Kurita, M.; Eto, K.; Kumagai, Y.; Kaji, T. Methylmercury promotes prostacyclin release from cultured human brain microvascular endothelial cells via induction of cyclooxygenase-2 through activation of the EGFR-p38 MAPK pathway by inhibiting protein tyrosine phosphatase 1B activity. Toxicology 2017, 392, 40–46. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Antonello, S.; Benassi, R.; Gavioli, G.; Taddei, F.; Maran, F. Theoretical and electrochemical analysis of dissociative electron transfers proceeding through formation of loose radical anion species: reduction of symmetrical and unsymmetrical disulfides. J. Am. Chem. Soc. 2002, 124, 7529–7538. [Google Scholar] [CrossRef] [PubMed]
- Fujie, T.; Yamamoto, T.; Yamamoto, C.; Kaji, T. Bis(1,4-dihydro-2-methyl-1-phenyl-4-thioxo-3-pyridiolato)zinc(II) exhibits strong cytotoxicity and a high intracellular accumulation in cultured vascular endothelial cells. J. Toxicol. Sci. 2019, 44, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kohri, K.; Yoshida, E.; Yasuike, S.; Fujie, T.; Yamamoto, C.; Kaji, T. The cytotoxicity of organobismuth compounds with certain molecular structures can be diminished by replacing the bismuth atom with an antimony atom in the molecules. J. Toxicol. Sci. 2015, 40, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, Y.; Mitani, M.; Yasuike, S.; Kurita, J.; Kaji, T. An organobismuth compound that exhibits selective cytotoxicity to vascular endothelial cells in vitro. J. Health Sci. 2005, 51, 333–340. [Google Scholar] [CrossRef] [Green Version]
- de Freitas, A.S.; de Souza Prestes, A.; Wagner, C.; Sudati, J.H.; Alves, D.; Porciúncula, L.O.; Kade, I.J.; Rocha, J.B.T. Reduction of diphenyl diselenide and analogs by mammalian thioredoxin reductase is independent of their gluthathione peroxidase-like activity: A possible novel pathway for their antioxidant activity. Molecules 2010, 15, 7699–7714. [Google Scholar] [CrossRef]
- de Freitas, A.S.; Rocha, J.B.T. Diphenyl diselenide and analogs are substrates of cerebral rat thioredoxin reductase: A pathway for their neuroprotective effects. Neurosci. Lett. 2011, 503, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Puntel, R.L.; Roos, D.H.; Seeger, R.L.; Rocha, J.B.T. Mitochondrial electron transfer chain complexes inhibition by different organochalcogens. Toxicol. In Vitro 2013, 27, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Jorge, P.M.; de Oliveira, I.M.; Filippi Chiela, E.C.; Viau, C.M.; Saffi, J.; Horn, F.; Rosa, R.M.; Guecheva, T.N.; Pêgas Henriques, J.A. Diphenyl ditelluride-induced cell cycle arrest and apoptosis: A relation with topoisomerase I inhibition. Basic Clin. Pharmacol. Toxicol. 2015, 116, 273–280. [Google Scholar] [CrossRef]
- Singh, D.; Deobald, A.M.; Camargo, L.R.S.; Tabarelli, G.; Rodrigues, O.E.D.; Braga, A.L. An efficient one-pot synthesis of symmetrical diselenides or ditellurides from halides with CuO nanopowder/Se0 or Te0/Base. Org. Lett. 2010, 12, 3288–3291. [Google Scholar] [CrossRef]
- Zhang, S.; Karra, K.; Koe, A.; Jin, J. Catalyst free one-pot synthesis of symmetrical diaryl tellurides with Te0/KOH. Tetarahedron Lett. 2013, 54, 2452–2454. [Google Scholar] [CrossRef]
- Hara, T.; Saeki, M.; Negishi, Y.; Kaji, T.; Yamamoto, C. Cell density-dependent accumulation of low polarity gold nanocluster in cultured vascular endothelial cells. J. Toxicol. Sci. 2020, 45, 795–800. [Google Scholar] [CrossRef]
- Kissane, J.M.; Robins, E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J. Biol. Chem. 1958, 233, 184–188. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Huzinaga, S. 1994 Polanyi Award Lecture Concept of active electrons in chemistry. Can. J. Chem. 1995, 73, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Boys, S.F. Electronic wave functions—I. A general method of calculation for the stationary states of any molecular system. Proc. R. Soc. Lond. Ser. A. Math. Phys. Sci. 1950, 200, 542–554. [Google Scholar]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Sakai, Y.; Miyoshi, E.; Klobukowski, M.; Huzinaga, S. Model potentials for main group elements Li through Rn. J. Chem. Phys. 1997, 106, 8084–8092. [Google Scholar] [CrossRef]
- Noro, T.; Sekiya, M.; Koga, T. Contracted polarization functions for the atoms helium through neon. Theor. Chem. Acc. 1997, 98, 25–32. [Google Scholar] [CrossRef]
- Sekiya, M.; Noro, T.; Koga, T.; Matsuyama, H. Contracted polarization functions for the atoms magnesium through argon. J. Mol. Struct. 1998, 451, 51–60. [Google Scholar] [CrossRef]
- Miyoshi, E.; Sakai, Y.; Tanaka, K.; Masamura, M. Relativistic dsp-model core potentials for main group elements in the fourth, fifth and sixth row and their applications. J. Mol. Struct. 1998, 451, 73–79. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, T.; Okazaki, T.; Hashiya, T.; Nozawa, K.; Yasuike, S.; Kurita, J.; Yamamoto, C.; Hamada, N.; Kaji, T. Effects of Substitution on Cytotoxicity of Diphenyl Ditelluride in Cultured Vascular Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 10520. https://doi.org/10.3390/ijms221910520
Hara T, Okazaki T, Hashiya T, Nozawa K, Yasuike S, Kurita J, Yamamoto C, Hamada N, Kaji T. Effects of Substitution on Cytotoxicity of Diphenyl Ditelluride in Cultured Vascular Endothelial Cells. International Journal of Molecular Sciences. 2021; 22(19):10520. https://doi.org/10.3390/ijms221910520
Chicago/Turabian StyleHara, Takato, Takahiro Okazaki, Tamayo Hashiya, Kyohei Nozawa, Shuji Yasuike, Jyoji Kurita, Chika Yamamoto, Noriaki Hamada, and Toshiyuki Kaji. 2021. "Effects of Substitution on Cytotoxicity of Diphenyl Ditelluride in Cultured Vascular Endothelial Cells" International Journal of Molecular Sciences 22, no. 19: 10520. https://doi.org/10.3390/ijms221910520
APA StyleHara, T., Okazaki, T., Hashiya, T., Nozawa, K., Yasuike, S., Kurita, J., Yamamoto, C., Hamada, N., & Kaji, T. (2021). Effects of Substitution on Cytotoxicity of Diphenyl Ditelluride in Cultured Vascular Endothelial Cells. International Journal of Molecular Sciences, 22(19), 10520. https://doi.org/10.3390/ijms221910520






