The Function of SUMOylation and Its Critical Roles in Cardiovascular Diseases and Potential Clinical Implications
Abstract
1. Introduction
1.1. CVD
1.2. SUMOylation
2. SUMO Machinery and CVD
2.1. UBC9
2.2. SUMO1
2.3. SUMO2
2.4. SENP2
2.5. SENP3
2.6. SENP5
3. SUMOylation of Proteins and CVD
3.1. Atherosclerosis
3.1.1. ERK5 (Extracellular Signal-Regulated Kinase 5)
3.1.2. NF-κB
3.1.3. p53
3.1.4. PKC (Protein Kinase C)
3.2. CHD (Congenital Heart Disease)
3.2.1. GATA4
3.2.2. GATA5
3.2.3. GATA6
3.2.4. MEF2 (Myocyte Enhancer Factor 2)
3.2.5. NKX2-5
3.2.6. SRF (Serum Responsive Factor)
3.2.7. TBX2
3.2.8. TBX5
3.3. HF (Heart Failure)
3.3.1. HSF2 (Heat Shock Transcription Factor 2)
3.3.2. Myocardin
3.3.3. PARIS (Parkin-Interacting Substrate)
3.3.4. PPARγ1 (Peroxisome Proliferation-Activated Receptor γ1)
3.3.5. SERCA2a (Sarcoplasmic/Endoplasmic Reticulum Ca2+-ATPase 2a)
3.4. Ischemic Cardiomyopathy
3.4.1. HDAC4 (Histone Deacetylase 4)
3.4.2. HIF1α (Hypoxia-Inducible Factor 1α)
3.4.3. PML (Promyelocytic Leukemia)
3.4.4. Target Protein Network
4. Concluding Remarks and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abe, J.I.; Sandhu, U.G.; Hoang, N.M.; Thangam, M.; Quintana-Quezada, R.A.; Fujiwara, K.; Le, N.T. Coordination of Cellular Localization-Dependent Effects of Sumoylation in Regulating Cardiovascular and Neurological Diseases. Adv. Exp. Med. Biol. 2017, 963, 337–358. [Google Scholar] [PubMed]
- Dehnavi, S.; Sadeghi, M.; Penson, P.E.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. The Role of Protein SUMOylation in the Pathogenesis of Atherosclerosis. J. Clin. Med. 2019, 8, 1856. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.S.; Chang, E.; Le, N.T.; Cushman, H.; Yeh, E.T.; Fujiwara, K.; Abe, J. De-SUMOylation enzyme of sentrin/SUMO-specific protease 2 regulates disturbed flow-induced SUMOylation of ERK5 and p53 that leads to endothelial dysfunction and atherosclerosis. Circ. Res. 2013, 112, 911–923. [Google Scholar] [CrossRef]
- Ahuja, P.; Sdek, P.; MacLellan, W.R. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol. Rev. 2007, 87, 521–544. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J. Diagnosis and treatment of adults with congenital heart disease. Future Cardiol. 2020, 16, 317–342. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.L.; Horwich, T.B.; Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011, 8, 30–41. [Google Scholar] [CrossRef]
- Mendler, L.; Braun, T.; Muller, S. The Ubiquitin-Like SUMO System and Heart Function: From Development to Disease. Circ. Res. 2016, 118, 132–144. [Google Scholar] [CrossRef]
- Chan, J.Y.; Tsai, C.Y.; Wu, C.H.; Li, F.C.; Dai, K.Y.; Sun, E.Y.; Chan, S.H.; Chang, A.Y. Sumoylation of hypoxia-inducible factor-1alpha ameliorates failure of brain stem cardiovascular regulation in experimental brain death. PLoS ONE 2011, 6, e17375. [Google Scholar] [CrossRef]
- Ulrich, H.D. Regulating post-translational modifications of the eukaryotic replication clamp PCNA. DNA Repair. 2009, 8, 461–469. [Google Scholar] [CrossRef]
- Yeh, E.T. SUMOylation and De-SUMOylation: Wrestling with life’s processes. J. Biol. Chem. 2009, 284, 8223–8227. [Google Scholar] [CrossRef]
- Matunis, M.J.; Coutavas, E.; Blobel, G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 1996, 135 Pt 1, 1457–1470. [Google Scholar] [CrossRef]
- Mahajan, R.; Delphin, C.; Guan, T.; Gerace, L.; Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 1997, 88, 97–107. [Google Scholar] [CrossRef]
- Kim, E.Y.; Chen, L.; Ma, Y.; Yu, W.; Chang, J.; Moskowitz, I.P.; Wang, J. Enhanced desumoylation in murine hearts by overexpressed SENP2 leads to congenital heart defects and cardiac dysfunction. J. Mol. Cell. Cardiol. 2012, 52, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.; Sheng, H.; Liu, S.; Li, Y.; Zhao, J.Q.; Warner, D.S.; Paschen, W.; Yang, W. Neuron-specific SUMO knockdown suppresses global gene expression response and worsens functional outcome after transient forebrain ischemia in mice. Neuroscience 2017, 343, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Treuter, E.; Gustafsson, J.A. Wrestling rules in transrepression: As easy as SUMO-1, -2, -3? Mol. Cell 2007, 25, 178–180. [Google Scholar] [CrossRef]
- Bohren, K.M.; Nadkarni, V.; Song, J.H.; Gabbay, K.H.; Owerbach, D. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J. Biol. Chem. 2004, 279, 27233–27238. [Google Scholar] [CrossRef]
- Boulanger, M.; Paolillo, R.; Piechaczyk, M.; Bossis, G. The SUMO Pathway in Hematomalignancies and Their Response to Therapies. Int. J. Mol. Sci. 2019, 20, 3895. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Dasso, M. Modification in reverse: The SUMO proteases. Trends Biochem. Sci. 2007, 32, 286–295. [Google Scholar] [CrossRef]
- Cheng, J.; Kang, X.L.; Zhang, S.; Yeh, E.T.H. SUMO-Specific protease 1 is essential for stabilization of HIF1 alpha during hypoxia. Cell 2007, 131, 584–595. [Google Scholar] [CrossRef]
- Kang, X.L.; Qi, Y.T.; Zuo, Y.; Wang, Q.; Zou, Y.Q.; Schwartz, R.J.; Cheng, J.K.; Yeh, E.T.H. SUMO-Specific Protease 2 Is Essential for Suppression of Polycomb Group Protein-Mediated Gene Silencing during Embryonic Development. Mol. Cell 2010, 38, 191–201. [Google Scholar] [CrossRef]
- Qi, Y.T.; Zuo, Y.; Yeh, E.T.H.; Cheng, J.K. An Essential Role of Small Ubiquitin-like Modifier (SUMO)-specific Protease 2 in Myostatin Expression and Myogenesis. J. Biol. Chem. 2014, 289, 3288–3293. [Google Scholar] [CrossRef]
- Qi, Y.T.; Wang, J.X.; Bomben, V.C.; Li, D.P.; Chen, S.R.; Sun, H.; Xi, Y.T.; Reed, J.G.; Cheng, J.K.; Pan, H.L.; et al. Hyper-SUMOylation of the Kv7 Potassium Channel Diminishes the M-Current Leading to Seizures and Sudden Death. Neuron 2014, 83, 1159–1171. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Huang, J.; Dong, W.; Xiao, H.; Shao, H.; Cheng, J.; Wu, H.; Qi, Y. Hyper-SUMOylation of K(+) Channels in Sudden Unexplained Death in Epilepsy: Isolation and Primary Culture of Dissociated Hippocampal Neurons from Newborn Mice for Subcellular Localization. Methods Mol. Biol. 2018, 1684, 63–71. [Google Scholar]
- Wu, H.; Chen, X.; Cheng, J.; Qi, Y. SUMOylation and Potassium Channels: Links to Epilepsy and Sudden Death. Adv. Protein Chem. Struct. Biol. 2016, 103, 295–321. [Google Scholar]
- Smits, V.A.; Freire, R. USP7/HAUSP: A SUMO deubiquitinase at the heart of DNA replication. Bioessays 2016, 38, 863–868. [Google Scholar] [CrossRef]
- Bertke, M.M.; Dubiak, K.M.; Cronin, L.; Zeng, E.; Huber, P.W. A deficiency in SUMOylation activity disrupts multiple pathways leading to neural tube and heart defects in Xenopus embryos. BMC Genom. 2019, 20, 386. [Google Scholar] [CrossRef]
- Hay, R.T. SUMO: A history of modification. Mol. Cell 2005, 18, 1–12. [Google Scholar] [CrossRef]
- Johnson, E.S. Protein modification by SUMO. Annu. Rev. Biochem. 2004, 73, 355–382. [Google Scholar] [CrossRef]
- Gao, J.; Shao, K.; Chen, X.; Li, Z.; Liu, Z.; Yu, Z.; Aung, L.H.H.; Wang, Y.; Li, P. The involvement of post-translational modifications in cardiovascular pathologies: Focus on SUMOylation, neddylation, succinylation, and prenylation. J. Mol. Cell. Cardiol. 2020, 138, 49–58. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, T.H.; Li, D.W. Roles of SUMOylation in Heart Development and Cardiovascular Diseases. Curr. Mol. Med. 2017, 16, 877–884. [Google Scholar] [CrossRef]
- McMurray, J.J.; Smith, G.L. Calcium handling in the failing heart and SUMO—Weighing the evidence. N. Engl. J. Med. 2011, 365, 1738–1739. [Google Scholar] [CrossRef]
- Abe, J.; Manabe, I.; Aikawa, M.; Aikawa, E. Cardiovascular Inflammation 2012: Reactive Oxygen Species, SUMOylation, and Biomarkers in Cardiovascular Inflammation. Int. J. Inflam. 2013, 2013, 953463. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; McLendon, P.M.; Gulick, J.; James, J.; Khalili, K.; Robbins, J. UBC9-Mediated Sumoylation Favorably Impacts Cardiac Function in Compromised Hearts. Circ. Res. 2016, 118, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Nacerddine, K.; Lehembre, F.; Bhaumik, M.; Artus, J.; Cohen-Tannoudji, M.; Babinet, C.; Pandolfi, P.P.; Dejean, A. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev. Cell 2005, 9, 769–779. [Google Scholar] [CrossRef]
- Lee, Y.J.; Mou, Y.; Maric, D.; Klimanis, D.; Auh, S.; Hallenbeck, J.M. Elevated global SUMOylation in Ubc9 transgenic mice protects their brains against focal cerebral ischemic damage. PLoS ONE 2011, 6, e25852. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Wen, S.; Zhu, H.; Yu, W.; Moskowitz, I.P.; Shaw, G.M.; Finnell, R.H.; Schwartz, R.J. Defective sumoylation pathway directs congenital heart disease. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 468–476. [Google Scholar] [CrossRef]
- Pai, P.; Shibu, M.A.; Chang, R.L.; Yang, J.J.; Su, C.C.; Lai, C.H.; Liao, H.E.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. ERbeta targets ZAK and attenuates cellular hypertrophy via SUMO-1 modification in H9c2 cells. J. Cell. Biochem. 2018, 119, 7855–7864. [Google Scholar] [CrossRef]
- Lee, A.; Jeong, D.; Mitsuyama, S.; Oh, J.G.; Liang, L.; Ikeda, Y.; Sadoshima, J.; Hajjar, R.J.; Kho, C. The role of SUMO-1 in cardiac oxidative stress and hypertrophy. Antioxid. Redox Signal. 2014, 21, 1986–2001. [Google Scholar] [CrossRef] [PubMed]
- Haindl, M.; Harasim, T.; Eick, D.; Muller, S. The nucleolar SUMO-specific protease SENP3 reverses SUMO modification of nucleophosmin and is required for rRNA processing. EMBO Rep. 2008, 9, 273–279. [Google Scholar] [CrossRef]
- Kim, Y.R.; Jacobs, J.S.; Li, Q.; Gaddam, R.R.; Vikram, A.; Liu, J.; Kassan, M.; Irani, K.; Kumar, S. SUMO2 regulates vascular endothelial function and oxidative stress in mice. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1292–H1300. [Google Scholar] [CrossRef]
- Kim, E.Y.; Zhang, Y.; Ye, B.; Segura, A.M.; Beketaev, I.; Xi, Y.; Yu, W.; Chang, J.; Li, F.; Wang, J. Involvement of activated SUMO-2 conjugation in cardiomyopathy. Biochim. Biophys. Acta 2015, 1852, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, C.; Sun, X.; Xiang, B.; Wang, M.; Yeh, E.T.; Chen, Y.; Li, H.; Shi, G.; Cang, H.; et al. SENP3-mediated de-conjugation of SUMO2/3 from promyelocytic leukemia is correlated with accelerated cell proliferation under mild oxidative stress. J. Biol. Chem. 2010, 285, 12906–12915. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Zhang, Y.; Beketaev, I.; Segura, A.M.; Yu, W.; Xi, Y.; Chang, J.; Wang, J. SENP5, a SUMO isopeptidase, induces apoptosis and cardiomyopathy. J. Mol. Cell. Cardiol. 2015, 78, 154–164. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Iyer, D.; Feng, X.H.; Schwartz, R.J. Regulation of cardiac specific nkx2.5 gene activity by small ubiquitin-like modifier. J. Biol. Chem. 2008, 283, 23235–23243. [Google Scholar] [CrossRef]
- Rawlings, N.; Lee, L.; Nakamura, Y.; Wilkinson, K.A.; Henley, J.M. Protective role of the deSUMOylating enzyme SENP3 in myocardial ischemia-reperfusion injury. PLoS ONE 2019, 14, e0213331. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Yeh, E.T. Characterization of a family of nucleolar SUMO-specific proteases with preference for SUMO-2 or SUMO-3. J. Biol. Chem. 2006, 281, 15869–15877. [Google Scholar] [CrossRef]
- Zunino, R.; Schauss, A.; Rippstein, P.; Andrade-Navarro, M.; McBride, H.M. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J. Cell Sci. 2007, 120 Pt 7, 1178–1188. [Google Scholar] [CrossRef]
- Zunino, R.; Braschi, E.; Xu, L.; McBride, H.M. Translocation of SenP5 from the nucleoli to the mitochondria modulates DRP1-dependent fission during mitosis. J. Biol. Chem. 2009, 284, 17783–17795. [Google Scholar] [CrossRef]
- Bjorkbacka, H.; Nilsson, J. Innate immunity in atherosclerosis. J. Innate Immun. 2010, 2, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.S.; Lee, H.; Nigro, P.; Thomas, T.; Le, N.T.; Chang, E.; McClain, C.; Reinhart-King, C.A.; King, M.R.; Berk, B.C.; et al. PKCzeta mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation. J. Cell Biol. 2011, 193, 867–884. [Google Scholar] [CrossRef]
- Nicorescu, I.; Dallinga, G.M.; de Winther, M.P.J.; Stroes, E.S.G.; Bahjat, M. Potential epigenetic therapeutics for atherosclerosis treatment. Atherosclerosis 2019, 281, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Woo, C.H.; Shishido, T.; McClain, C.; Lim, J.H.; Li, J.D.; Yang, J.; Yan, C.; Abe, J. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ. Res. 2008, 102, 538–545. [Google Scholar] [CrossRef]
- Sato, M.; Kawai-Kowase, K.; Sato, H.; Oyama, Y.; Kanai, H.; Ohyama, Y.; Suga, T.; Maeno, T.; Aoki, Y.; Tamura, J.; et al. c-Src and hydrogen peroxide mediate transforming growth factor-beta1-induced smooth muscle cell-gene expression in 10T1/2 cells. Arter. Thromb. Vasc. Biol. 2005, 25, 341–347. [Google Scholar] [CrossRef]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Gareus, R.; Kotsaki, E.; Xanthoulea, S.; van der Made, I.; Gijbels, M.J.; Kardakaris, R.; Polykratis, A.; Kollias, G.; de Winther, M.P.; Pasparakis, M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008, 8, 372–383. [Google Scholar] [CrossRef]
- Chang, E.; Abe, J.I. Kinase-SUMO networks in diabetes-mediated cardiovascular disease. Metabolism 2016, 65, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Desterro, J.M.; Rodriguez, M.S.; Hay, R.T. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol. Cell 1998, 2, 233–239. [Google Scholar] [CrossRef]
- Mihara, M.; Erster, S.; Zaika, A.; Petrenko, O.; Chittenden, T.; Pancoska, P.; Moll, U.M. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 2003, 11, 577–590. [Google Scholar] [CrossRef]
- Teodoro, J.G.; Parker, A.E.; Zhu, X.; Green, M.R. p53-mediated inhibition of angiogenesis through up-regulation of a collagen prolyl hydroxylase. Science 2006, 313, 968–971. [Google Scholar] [CrossRef]
- Heo, K.S.; Le, N.T.; Cushman, H.J.; Giancursio, C.J.; Chang, E.; Woo, C.H.; Sullivan, M.A.; Taunton, J.; Yeh, E.T.; Fujiwara, K.; et al. Disturbed flow-activated p90RSK kinase accelerates atherosclerosis by inhibiting SENP2 function. J. Clin. Investig. 2015, 125, 1299–1310. [Google Scholar] [CrossRef]
- Tabit, C.E.; Shenouda, S.M.; Holbrook, M.; Fetterman, J.L.; Kiani, S.; Frame, A.A.; Kluge, M.A.; Held, A.; Dohadwala, M.M.; Gokce, N.; et al. Protein kinase C-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation 2013, 127, 86–95. [Google Scholar] [CrossRef]
- Dentice, M.; Cordeddu, V.; Rosica, A.; Ferrara, A.M.; Santarpia, L.; Salvatore, D.; Chiovato, L.; Perri, A.; Moschini, L.; Fazzini, C.; et al. Missense mutation in the transcription factor NKX2-5: A novel molecular event in the pathogenesis of thyroid dysgenesis. J. Clin. Endocrinol. Metab. 2006, 91, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Yuan, H.; Liu, X.; Wang, H.; Chen, S.; Chen, Z.; de The, H.; Zhou, J.; Zhu, J. GATA5 SUMOylation is indispensable for zebrafish cardiac development. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, W.; Zhu, J.; Yuan, H.; Chu, M.; Wen, B. Modification of cardiac transcription factor Gata6 by SUMO. Biochimie 2020, 170, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, B.; You, S.; Chen, L.; Dongmei, Q.; Gu, M.; Lu, Y.; Chen, Y.; Zhang, F.; Yu, B. SENP2 regulates MEF2A de-SUMOylation in an activity dependent manner. Mol. Biol. Rep. 2013, 40, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Chen, Z.; Bartunkova, S.; Yamasaki, N.; Izumo, S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 1999, 126, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Hikichi, T.; Miura, H.; Shibata, H.; Mitsunaga, K.; Yamada, Y.; Woltjen, K.; Miyamoto, K.; Hiratani, I.; Yamada, Y.; et al. Srf destabilizes cellular identity by suppressing cell-type-specific gene expression programs. Nat. Commun. 2018, 9, 1387. [Google Scholar] [CrossRef]
- Iwanicka-Pronicka, K.; Socha, M.; Jedrzejowska, M.; Krajewska-Walasek, M.; Jamsheer, A. Life-threatening cardiac episode in a Polish patient carrying contiguous gene microdeletion of the TBX5 and the TBX3 genes. Springerplus 2016, 5, 1638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Z.; Zhang, W.; Nam, Y.J. Stoichiometric optimization of Gata4, Hand2, Mef2c, and Tbx5 expression for contractile cardiomyocyte reprogramming. Sci. Rep. 2019, 9, 14970. [Google Scholar] [CrossRef]
- Shalizi, A.; Bilimoria, P.M.; Stegmuller, J.; Gaudilliere, B.; Yang, Y.; Shuai, K.; Bonni, A. PIASx is a MEF2 SUMO E3 ligase that promotes postsynaptic dendritic morphogenesis. J. Neurosci. 2007, 27, 10037–10046. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Kuo, C.H.; Pai, P.Y.; Ho, T.J.; Lin, Y.M.; Chen, R.J.; Tsai, F.J.; Vijaya Padma, V.; Kuo, W.W.; Huang, C.Y. Inhibition of HSF2 SUMOylation via MEL18 upregulates IGF-IIR and leads to hypertension-induced cardiac hypertrophy. Int. J. Cardiol. 2018, 257, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jiang, Y.; Yin, Y.; Li, Q.; He, J.; Jing, Y.; Qi, Y.T.; Xu, Q.; Li, W.; Lu, B.; et al. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J. Mol. Cell Biol. 2013, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Zhang, T.C.; Cao, D.; Wang, Z.; Antos, C.L.; Li, S.; Wang, Y.; Olson, E.N.; Wang, D.Z. Myocardin induces cardiomyocyte hypertrophy. Circ. Res. 2006, 98, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V.; Rizzu, P.; Squitieri, F.; Krieger, E.; Vanacore, N.; van Swieten, J.C.; Brice, A.; van Duijn, C.M.; Oostra, B.; Meco, G.; et al. DJ-1 (PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol. Sci. 2003, 24, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef]
- Ohshima, T.; Koga, H.; Shimotohno, K. Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J. Biol. Chem. 2004, 279, 29551–29557. [Google Scholar] [CrossRef]
- Kho, C.; Lee, A.; Jeong, D.; Oh, J.G.; Chaanine, A.H.; Kizana, E.; Park, W.J.; Hajjar, R.J. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 2011, 477, 601–605. [Google Scholar] [CrossRef]
- Oh, J.G.; Watanabe, S.; Lee, A.; Gorski, P.A.; Lee, P.; Jeong, D.; Liang, L.; Liang, Y.; Baccarini, A.; Sahoo, S.; et al. miR-146a Suppresses SUMO1 Expression and Induces Cardiac Dysfunction in Maladaptive Hypertrophy. Circ. Res. 2018, 123, 673–685. [Google Scholar] [CrossRef]
- Du, J.; Zhang, L.; Zhuang, S.; Qin, G.J.; Zhao, T.C. HDAC4 degradation mediates HDAC inhibition-induced protective effects against hypoxia/reoxygenation injury. J. Cell. Physiol. 2015, 230, 1321–1331. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Aparicio-Sanchez, J.J.; Buxton, S.; Brook, J.D. HDAC4 and 5 repression of TBX5 is relieved by protein kinase D1. Sci. Rep. 2019, 9, 17992. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Fan, Y.; Liu, X.; Zhou, L.; Cheng, J.; Cai, R.; Xue, S. SENP1 protects against myocardial ischaemia/reperfusion injury via a HIF1alpha-dependent pathway. Cardiovasc. Res. 2014, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ishov, A.M.; Sotnikov, A.G.; Negorev, D.; Vladimirova, O.V.; Neff, N.; Kamitani, T.; Yeh, E.T.; Strauss, J.F., 3rd; Maul, G.G. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 1999, 147, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Han, Y.; Shao, X.; Paulo, P.; Li, W.; Zhu, M.; Tang, N.; Guo, S.; Chen, Y.; Wu, H.; et al. Knockdown of endogenous RNF4 exacerbates ischaemia-induced cardiomyocyte apoptosis in mice. J. Cell. Mol. Med. 2020, 24, 9545–9559. [Google Scholar] [CrossRef] [PubMed]
- Evans, T. Regulation of Cardiac Gene Expression by GATA-4/5/6. Trends Cardiovasc. Med. 1997, 7, 75–83. [Google Scholar] [CrossRef]
- Winick, J.; Abel, T.; Leonard, M.W.; Michelson, A.M.; Chardon-Loriaux, I.; Holmgren, R.A.; Maniatis, T.; Engel, J.D. A GATA family transcription factor is expressed along the embryonic dorsoventral axis in Drosophila melanogaster. Development 1993, 119, 1055–1065. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, Y.; Zhao, H.; Qin, L.; Shi, Y.; Zhu, X.; Song, L.; Zhou, X.; Chen, J.; Zhou, H.; et al. The critical role of SENP1-mediated GATA2 deSUMOylation in promoting endothelial activation in graft arteriosclerosis. Nat. Commun. 2017, 8, 15426. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yang, Y.Q.; Ma, J.; Lin, X.P.; Zheng, J.H.; Bai, K.; Chen, Y.H. Novel GATA4 mutations identified in patients with congenital atrial septal defects. Zhonghua Xin Xue Guan Bing Za Zhi 2010, 38, 724–727. [Google Scholar]
- Riquelme, C.; Barthel, K.K.; Liu, X. SUMO-1 modification of MEF2A regulates its transcriptional activity. J. Cell. Mol. Med. 2006, 10, 132–144. [Google Scholar] [CrossRef]
- Clark, C.D.; Lee, K.H. Second heart field-specific expression of Nkx2-5 requires promoter proximal interaction with Srf. Mech. Dev. 2020, 162, 103615. [Google Scholar] [CrossRef]
- Lyons, I.; Parsons, L.M.; Hartley, L.; Li, R.; Andrews, J.E.; Robb, L.; Harvey, R.P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995, 9, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Chen, L.; Ma, Y.; Yu, W.; Chang, J.; Moskowitz, I.P.; Wang, J. Expression of sumoylation deficient Nkx2.5 mutant in Nkx2.5 haploinsufficient mice leads to congenital heart defects. PLoS ONE 2011, 6, e20803. [Google Scholar] [CrossRef]
- Norman, C.; Runswick, M.; Pollock, R.; Treisman, R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell 1988, 55, 989–1003. [Google Scholar] [CrossRef]
- Johansen, F.E.; Prywes, R. Serum response factor: Transcriptional regulation of genes induced by growth factors and differentiation. Biochim. Biophys. Acta 1995, 1242, 1–10. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Minami, T.; Tojo, M.; Honda, Y.; Uchimura, Y.; Saitoh, H.; Yasuda, H.; Nagahiro, S.; Saya, H.; Nakao, M. Serum response factor is modulated by the SUMO-1 conjugation system. Biochem. Biophys. Res. Commun. 2003, 306, 32–38. [Google Scholar] [CrossRef]
- Niu, Z.; Yu, W.; Zhang, S.X.; Barron, M.; Belaguli, N.S.; Schneider, M.D.; Parmacek, M.; Nordheim, A.; Schwartz, R.J. Conditional mutagenesis of the murine serum response factor gene blocks cardiogenesis and the transcription of downstream gene targets. J. Biol. Chem. 2005, 280, 32531–32538. [Google Scholar] [CrossRef]
- Di Gregorio, A. T-Box Genes and Developmental Gene Regulatory Networks in Ascidians. Curr. Top. Dev. Biol. 2017, 122, 55–91. [Google Scholar] [PubMed]
- Pang, S.; Liu, Y.; Zhao, Z.; Huang, W.; Chen, D.; Yan, B. Novel and functional sequence variants within the TBX2 gene promoter in ventricular septal defects. Biochimie 2013, 95, 1807–1809. [Google Scholar] [CrossRef]
- Zhang, R.R.; Cai, K.; Liu, L.; Yang, Q.; Zhang, P.; Gui, Y.H.; Wang, F. A regulatory variant in TBX2 promoter is related to the decreased susceptibility of congenital heart disease in the Han Chinese population. Mol. Genet. Genom. Med. 2019, 7, e00530. [Google Scholar] [CrossRef] [PubMed]
- Huber, P.; Crum, T.; Clary, L.M.; Ronan, T.; Packard, A.V.; Okkema, P.G. Function of the C. elegans T-box factor TBX-2 depends on SUMOylation. Cell. Mol. Life Sci. 2013, 70, 4157–4168. [Google Scholar] [CrossRef]
- Steimle, J.D.; Moskowitz, I.P. TBX5: A Key Regulator of Heart Development. Curr. Top. Dev. Biol. 2017, 122, 195–221. [Google Scholar]
- Jia, Y.; Chang, Y.; Guo, Z.; Li, H. Transcription factor Tbx5 promotes cardiomyogenic differentiation of cardiac fibroblasts treated with 5-azacytidine. J. Cell. Biochem. 2019, 120, 16503–16515. [Google Scholar] [CrossRef] [PubMed]
- Kathiriya, I.S.; Rao, K.S.; Iacono, G.; Devine, W.P.; Blair, A.P.; Hota, S.K.; Lai, M.H.; Garay, B.I.; Thomas, R.; Gong, H.Z.; et al. Modeling Human TBX5 Haploinsufficiency Predicts Regulatory Networks for Congenital Heart Disease. Dev. Cell 2021, 56, 292–309.e9. [Google Scholar] [CrossRef]
- Beketaev, I.; Kim, E.Y.; Zhang, Y.; Yu, W.; Qian, L.; Wang, J. Potentiation of Tbx5-mediated transactivation by SUMO conjugation and protein inhibitor of activated STAT 1 (PIAS1). Int. J. Biochem. Cell Biol. 2014, 50, 82–92. [Google Scholar] [CrossRef]
- Frey, N.; Olson, E.N. Cardiac hypertrophy: The good, the bad, and the ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef]
- Booz, G.W. Putting the brakes on cardiac hypertrophy: Exploiting the NO-cGMP counter-regulatory system. Hypertension 2005, 45, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Miano, J.M. Myocardin in biology and disease. J. Biomed. Res. 2015, 29, 3–19. [Google Scholar]
- Trembley, M.A.; Quijada, P.; Agullo-Pascual, E.; Tylock, K.M.; Colpan, M.; Dirkx, R.A., Jr.; Myers, J.R.; Mickelsen, D.M.; de Mesy Bentley, K.; Rothenberg, E.; et al. Mechanosensitive Gene Regulation by Myocardin-Related Transcription Factors Is Required for Cardiomyocyte Integrity in Load-Induced Ventricular Hypertrophy. Circulation 2018, 138, 1864–1878. [Google Scholar] [CrossRef] [PubMed]
- Cen, B.; Selvaraj, A.; Prywes, R. Myocardin/MKL family of SRF coactivators: Key regulators of immediate early and muscle specific gene expression. J. Cell. Biochem. 2004, 93, 74–82. [Google Scholar] [CrossRef]
- Wang, D.Z.; Olson, E.N. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr. Opin. Genet. Dev. 2004, 14, 558–566. [Google Scholar] [CrossRef]
- Shin, J.H.; Ko, H.S.; Kang, H.; Lee, Y.; Lee, Y.I.; Pletinkova, O.; Troconso, J.C.; Dawson, V.L.; Dawson, T.M. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell 2011, 144, 689–702. [Google Scholar] [CrossRef]
- Nishida, T.; Yamada, Y. SUMOylation of the KRAB zinc-finger transcription factor PARIS/ZNF746 regulates its transcriptional activity. Biochem. Biophys. Res. Commun. 2016, 473, 1261–1267. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: Potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef]
- Mukherjee, D.; Chander, V.; Bandyopadhyay, A. PARIS-DJ-1 Interaction Regulates Mitochondrial Functions in Cardiomyocytes, Which Is Critically Important in Cardiac Hypertrophy. Mol. Cell. Biol. 2020, 41, e00106-20. [Google Scholar] [CrossRef] [PubMed]
- Wadosky, K.M.; Willis, M.S. The story so far: Post-translational regulation of peroxisome proliferator-activated receptors by ubiquitination and SUMOylation. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H515–H526. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Fong, A.L.; Ogawa, S.; Gamliel, A.; Li, A.C.; Perissi, V.; Rose, D.W.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 2005, 437, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, J.K.; Copelas, L.; MacKinnon, R.; Schoen, F.J.; Feldman, M.D.; Grossman, W.; Morgan, J.P. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ. Res. 1987, 61, 70–76. [Google Scholar] [CrossRef] [PubMed]
- del Monte, F.; O’Gara, P.; Poole-Wilson, P.A.; Yacoub, M.; Harding, S.E. Cell geometry and contractile abnormalities of myocytes from failing human left ventricle. Cardiovasc. Res. 1995, 30, 281–290. [Google Scholar] [CrossRef]
- Hasenfuss, G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc. Res. 1998, 37, 279–289. [Google Scholar] [CrossRef]
- Kho, C.; Lee, A.; Jeong, D.; Oh, J.G.; Gorski, P.A.; Fish, K.; Sanchez, R.; DeVita, R.J.; Christensen, G.; Dahl, R.; et al. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Xu, J.; Zhao, H.; Zheng, Q.; Xiao, X.; Ma, X.; Li, Y.; Du, X.; Liu, X. Zinc-Induced SUMOylation of Dynamin-Related Protein 1 Protects the Heart against Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2019, 2019, 1232146. [Google Scholar] [CrossRef]
- Tilemann, L.; Lee, A.; Ishikawa, K.; Aguero, J.; Rapti, K.; Santos-Gallego, C.; Kohlbrenner, E.; Fish, K.M.; Kho, C.; Hajjar, R.J. SUMO-1 gene transfer improves cardiac function in a large-animal model of heart failure. Sci. Transl. Med. 2013, 5, 211ra159. [Google Scholar] [CrossRef]
- Shah, S.J.; Wasserstrom, J.A. SERCA2a gene therapy for the prevention of sudden cardiac death: A future theranostic for heart failure? Circulation 2012, 126, 2047–2050. [Google Scholar] [CrossRef]
- Samuel, T.J.; Rosenberry, R.P.; Lee, S.; Pan, Z. Correcting Calcium Dysregulation in Chronic Heart Failure Using SERCA2a Gene Therapy. Int. J. Mol. Sci. 2018, 19, 1086. [Google Scholar] [CrossRef]
- Lowenstein, C.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial ischemia reperfusion injury: From basic science to clinical bedside. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; Wang, S.; Zhu, H.; Li, D. Roles and mechanisms of SUMOylation on key proteins in myocardial ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2019, 134, 154–164. [Google Scholar] [CrossRef]
- Kuo, M.H.; Allis, C.D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 1998, 20, 615–626. [Google Scholar] [CrossRef]
- Fischle, W.; Emiliani, S.; Hendzel, M.J.; Nagase, T.; Nomura, N.; Voelter, W.; Verdin, E. A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1p. J. Biol. Chem. 1999, 274, 11713–11720. [Google Scholar] [CrossRef] [PubMed]
- Grozinger, C.M.; Hassig, C.A.; Schreiber, S.L. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. USA 1999, 96, 4868–4873. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.H.; Bertos, N.R.; Vezmar, M.; Pelletier, N.; Crosato, M.; Heng, H.H.; Th’ng, J.; Han, J.; Yang, X.J. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol. Cell. Biol. 1999, 19, 7816–7827. [Google Scholar] [CrossRef]
- Zhang, L.X.; DeNicola, M.; Qin, X.; Du, J.; Ma, J.; Tina Zhao, Y.; Zhuang, S.; Liu, P.Y.; Wei, L.; Qin, G.; et al. Specific inhibition of HDAC4 in cardiac progenitor cells enhances myocardial repairs. Am. J. Physiol. Cell Physiol. 2014, 307, C358–C372. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Han, Y.; Wang, Y.; Sun, X.; Yan, S.; Yeh, E.T.; Chen, Y.; Cang, H.; Li, H.; Shi, G.; et al. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009, 28, 2748–2762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Yang, K.; Cang, H.; Huang, X.Z.; Li, H.; Yi, J. The biphasic redox sensing of SENP3 accounts for the HIF-1 transcriptional activity shift by oxidative stress. Acta Pharmacol. Sin. 2012, 33, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Maroui, M.A.; Kheddache-Atmane, S.; El Asmi, F.; Dianoux, L.; Aubry, M.; Chelbi-Alix, M.K. Requirement of PML SUMO interacting motif for RNF4- or arsenic trioxide-induced degradation of nuclear PML isoforms. PLoS ONE 2012, 7, e44949. [Google Scholar] [CrossRef]
- Niwa-Kawakita, M.; Ferhi, O.; Soilihi, H.; Le Bras, M.; Lallemand-Breitenbach, V.; de The, H. PML is a ROS sensor activating p53 upon oxidative stress. J. Exp. Med. 2017, 214, 3197–3206. [Google Scholar] [CrossRef]
- Plechanovova, A.; Jaffray, E.G.; McMahon, S.A.; Johnson, K.A.; Navratilova, I.; Naismith, J.H.; Hay, R.T. Mechanism of ubiquitylation by dimeric RING ligase RNF4. Nat. Struct. Mol. Biol. 2011, 18, 1052–1059. [Google Scholar] [CrossRef]
- Guo, B.; Sharrocks, A.D. Extracellular signal-regulated kinase mitogen-activated protein kinase signaling initiates a dynamic interplay between sumoylation and ubiquitination to regulate the activity of the transcriptional activator PEA3. Mol. Cell. Biol. 2009, 29, 3204–3218. [Google Scholar] [CrossRef]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1alpha and HIF2alpha: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2011, 12, 9–22. [Google Scholar] [CrossRef]
- Yang, W.; Sheng, H.; Thompson, J.W.; Zhao, S.; Wang, L.; Miao, P.; Liu, X.; Moseley, M.A.; Paschen, W. Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: Putative protective proteins/pathways. Stroke 2014, 45, 1115–1122. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, Y.; He, J.; Yan, Y.; Xu, L.; Lin, N.; Ji, Q.; Tong, R.; Fu, Y.; Gao, Y.; et al. The desumoylating enzyme sentrin-specific protease 3 contributes to myocardial ischemia reperfusion injury. J. Genet. Genom. 2018, 45, 125–135. [Google Scholar] [CrossRef]
- Gartner, A.; Muller, S. PML, SUMO, and RNF4: Guardians of nuclear protein quality. Mol. Cell 2014, 55, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Keiten-Schmitz, J.; Wagner, K.; Piller, T.; Kaulich, M.; Alberti, S.; Muller, S. The Nuclear SUMO-Targeted Ubiquitin Quality Control Network Regulates the Dynamics of Cytoplasmic Stress Granules. Mol. Cell 2020, 79, 54–67.e7. [Google Scholar] [CrossRef] [PubMed]
- Hotz, P.W.; Wiesnet, M.; Tascher, G.; Braun, T.; Muller, S.; Mendler, L. Profiling the Murine SUMO Proteome in Response to Cardiac Ischemia and Reperfusion Injury. Molecules 2020, 25, 5571. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.J. Cardiac development: New concepts. Clin. Perinatol. 2005, 32, 845–855, vii. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, P.; Boltryk, R.J.; Hill, M.; Zhang, F.; Dong, L.; Wilkinson, J.S.; Melvin, T.; Harris, N.R.; Brown, T. Flexible acoustic particle manipulation device with integrated optical waveguide for enhanced microbead assays. Anal. Sci. 2009, 25, 285–291. [Google Scholar] [CrossRef]
- Plant, L.D.; Xiong, D.; Romero, J.; Dai, H.; Goldstein, S.A.N. Hypoxia Produces Pro-arrhythmic Late Sodium Current in Cardiac Myocytes by SUMOylation of NaV1.5 Channels. Cell Rep. 2020, 30, 2225–2236.e4. [Google Scholar] [CrossRef]
- Lumpkin, R.J.; Gu, H.; Zhu, Y.; Leonard, M.; Ahmad, A.S.; Clauser, K.R.; Meyer, J.G.; Bennett, E.J.; Komives, E.A. Site-specific identification and quantitation of endogenous SUMO modifications under native conditions. Nat. Commun. 2017, 8, 1171. [Google Scholar] [CrossRef]
- Hendriks, I.A.; Lyon, D.; Su, D.; Skotte, N.H.; Daniel, J.A.; Jensen, L.J.; Nielsen, M.L. Site-specific characterization of endogenous SUMOylation across species and organs. Nat. Commun. 2018, 9, 2456. [Google Scholar] [CrossRef]
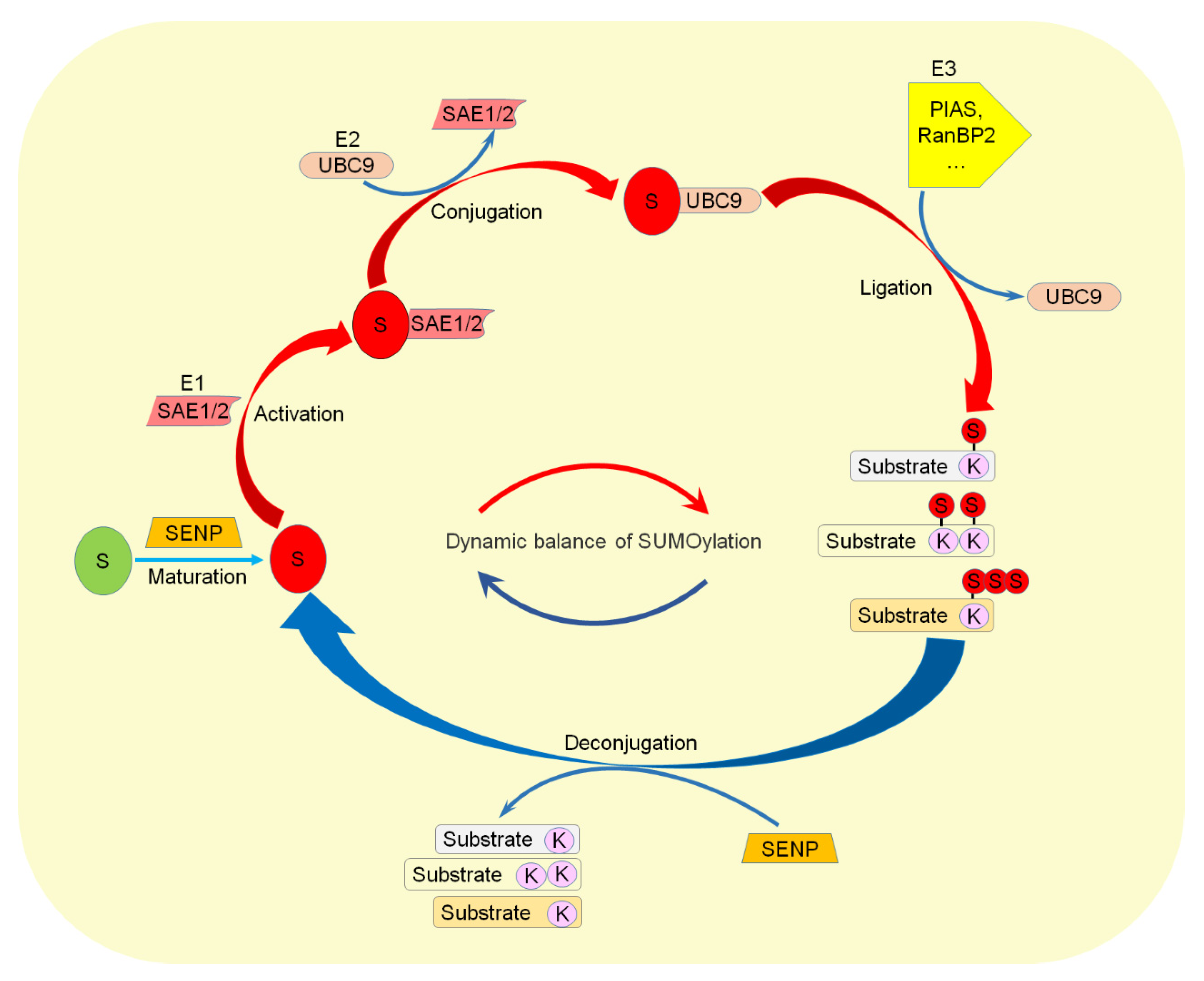





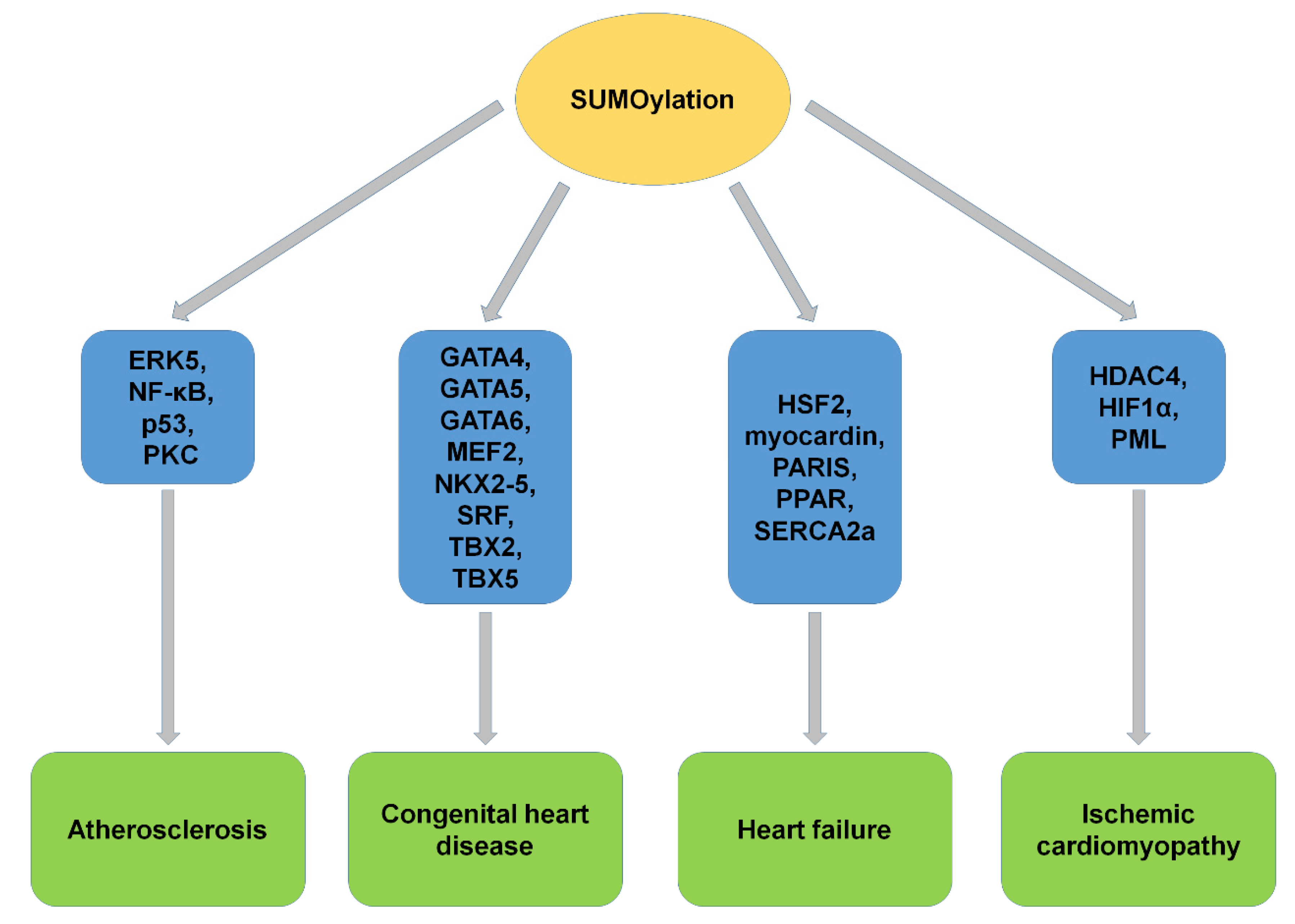
| SUMO Machinery | Disease | Biophysical and Biological Effects of SUMOylation |
|---|---|---|
| UBC9 | CHD | UBC9-mediated SUMOylation induced a higher level of autophagy and increased autophagy flux and the incidence of cardiac development [35,39]. |
| SUMO1 | CHD and HF | SUMO1 plays a key role in normal cardiac development and decreased SUMOylation activity induces cardiac pathology [36]. |
| SUMO2 | HF | Endogenous SUMO2 is important in maintaining normal endothelium-dependent vascular function [40], and SUMO2 upregulation leads to endothelial dysfunction due to cholesterolemia [41]. |
| SENP2 | CHD | Overexpression of SENP2 led to ASD and VSD, reduced proliferation of cardiomyocytes, and induced cardiomyopathy with aging [13]. |
| SENP3 | Ischemic cardiomyopathy | SENP3-mediated deSUMOylation plays an important role in the regulation of myocardial cell survival after ischemic injury [42]. |
| SENP5 | HF | SENP5 level increased in the failing heart, and the increase of mitochondrial division mediated by Drp1 leads to changes in the morphology and structure of cardiac mitochondria, resulting in mitochondrial dysfunction [43]. |
| Disease | Protein Substrate | Biophysical Function | Biophysical and Biological Effects of SUMOylation |
|---|---|---|---|
| Atherosclerosis | ERK5 | ERK5 plays anti-inflammatory role in regulating PPARγ and KLF2 and inhibiting TNFα mediated adhesion gene expression. | SUMOylation of ERK5 significantly inhibits the transcriptional activity of MEF2, causing inflammation in the onset and promoting the progression of atherosclerosis [32]. |
| NF-κB | NF-κB is a nuclear transcription factor and is one of the earliest known cardiac progenitor cell markers. | SUMO1-mediated SUMOylation of NF-κB downregulates its activation, whereas SUMO2/3 leads to the detachment of NF-κB from IκBα and activation [57,58]. | |
| p53 | p53 is an important tumor suppressor gene that regulates cell apoptosis and cell cycle. | SUMOylated p53 promotes cell apoptosis and causes the occurrence of atherosclerosis, whereas deSUMOylated p53 inhibits atherosclerosis [59]. | |
| PKC | PKC plays an important role in cell growth, apoptosis, inflammation, and contractility of cardiomyocytes. | SUMOylation of PKC inhibits the kinase activity of PKC and accelerates the occurrence of atherosclerosis [4]. | |
| CHD | GATA4 | GATA4 is one of the most important members of the GATA superfamily and is known to be the primary cause of CHD. | SUMOylation of GATA4 promotes cardiac specific gene expression in pluripotent cells and regulates the development of CHD [63]. |
| GATA5 | GATA5 is an important transcription factor in cardiac development. | SUMOylation of GATA5 restores cardiac insufficiency caused by cardiac dysplasia and plays an important role in cardiac development [64]. | |
| GATA6 | GATA6 is one of the most important members of the GATA superfamily and is an important factor in the regulation of cardiac development. | SUMOylation of GATA6 inhibits its transcriptional activity in cardiac development [65]. | |
| MEF2 | The MEF2 family contains transcription factors that regulate the activity of muscle-specific genes and involved in cardiac development. | MEF2A, MEF2C, and MEF2D were modified by SUMO, and SUMOylation affects their transcriptional activity [66]. | |
| NKX2-5 | NKX2-5 is involved in the differentiation of cardiac progenitor cells and the formation of atrioventricular septum and atrioventricular outflow tract. | SUMOylation promotes the transcriptional activity of NKX2-5 and is associated with the progression of CHD [67]. | |
| SRF | SRF is a member of the DNA binding protein family MADS Box and is involved in cardiac development. | SUMOylation of SRF enhanced activation of c-fos promoter, but inhibited cardiac α-actin promoter, playing critical roles in cardiac development [68]. | |
| TBX2 | TBX2 is a T-Box transcription factor and plays vital roles in cardiac cushion development. | TBX2 SUMOylation plays an important role in cardiac development, especially non-atrioventricular myocardium in the atrioventricular and outflow canals [69]. | |
| TBX5 | TBX5 is a T-Box transcription factor and plays a critical role in cardiac development. | SUMOylation of TBX5 enhanced the transcriptional activity of TBX5 and enhanced the coordinate regulation with NKX2-5 and GATA4, regulating the progression of cardiac development [70,71]. | |
| HF | HSF2 | HSF2 plays a key role in cardiomyocyte apoptosis and hypertrophy induced by hypertensive angiotensin II. | HSF2 is modified by SUMO1 during HF, and PGCF2 significantly reduced the SUMOylation of HSF2 in spontaneously hypertensive rat hearts [72,73]. |
| Myocardin | Myocardin regulates the growth of cardiomyocytes by regulating the metabolic activity and plays a key role in the process of cardiomyocyte hypertrophy. | SUMOylation enhanced the activity of myocardin and leads to an increase in cardiac-specific gene expression in cardiomyocytes and promoted myocardin-mediated cardiac hypertrophy [74]. | |
| PARIS | PARIS regulates DNA binding in the promoter region of target genes and plays transcriptional repressor activity. | In cardiac hypertrophy, SUMOylated PARIS inhibited PGC1α transcription to regulate mitochondrial function [75,76]. | |
| PPARγ1 | PPARγ1 regulates the utilization of cardiac energy substrates. | SUMOylation of PPARγ1 inhibits the transcriptional activity and plays important roles in cardiac hypertrophy [77]. | |
| SERCA2a | SERCA2a regulates the calcium cycle in cardiomyocytes and is responsible for the reuptake of calcium during excitation-contraction coupling. | SUMOylation is essential to maintain the stability and activity of SERCA2a [78,79]. | |
| Ischemic cardiomyopathy | HDAC4 | HDAC4 is a histone deacetylase and is highly expressed in heart, brain, and skeletal muscle. | SUMOylation of HDAC4 mediates ubiquitination and promotes the degradation of HDAC4 in I/R and decreases the production of ROS [80,81]. |
| HIF1α | HIF1α is an important transcription factor that plays critical roles under hypoxia conditions. | HIF1α SUMOylation regulates the expression of heme oxygenase-1 in rostral ventrolateral medulla (RVLM), whereas its deSUMOylation leads to cell necrosis and apoptosis [82]. | |
| PML | PML is the important organizer of NBs. | PML SUMOylation is critical in PML NBs formation [83] and aggravates ischemia-induced cardiomyocyte apoptosis in vivo [84]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Chen, X.; Su, Q.; Lu, W.; Wang, Q.; Yuan, H.; Zhang, Z.; Wang, X.; Wu, H.; Qi, Y. The Function of SUMOylation and Its Critical Roles in Cardiovascular Diseases and Potential Clinical Implications. Int. J. Mol. Sci. 2021, 22, 10618. https://doi.org/10.3390/ijms221910618
Du C, Chen X, Su Q, Lu W, Wang Q, Yuan H, Zhang Z, Wang X, Wu H, Qi Y. The Function of SUMOylation and Its Critical Roles in Cardiovascular Diseases and Potential Clinical Implications. International Journal of Molecular Sciences. 2021; 22(19):10618. https://doi.org/10.3390/ijms221910618
Chicago/Turabian StyleDu, Congcong, Xu Chen, Qi Su, Wenbin Lu, Qiqi Wang, Hong Yuan, Zhenzhen Zhang, Xiaotong Wang, Hongmei Wu, and Yitao Qi. 2021. "The Function of SUMOylation and Its Critical Roles in Cardiovascular Diseases and Potential Clinical Implications" International Journal of Molecular Sciences 22, no. 19: 10618. https://doi.org/10.3390/ijms221910618
APA StyleDu, C., Chen, X., Su, Q., Lu, W., Wang, Q., Yuan, H., Zhang, Z., Wang, X., Wu, H., & Qi, Y. (2021). The Function of SUMOylation and Its Critical Roles in Cardiovascular Diseases and Potential Clinical Implications. International Journal of Molecular Sciences, 22(19), 10618. https://doi.org/10.3390/ijms221910618





