Involvement of Microglia in Neurodegenerative Diseases: Beneficial Effects of Docosahexahenoic Acid (DHA) Supplied by Food or Combined with Nanoparticles
Abstract
1. Introduction
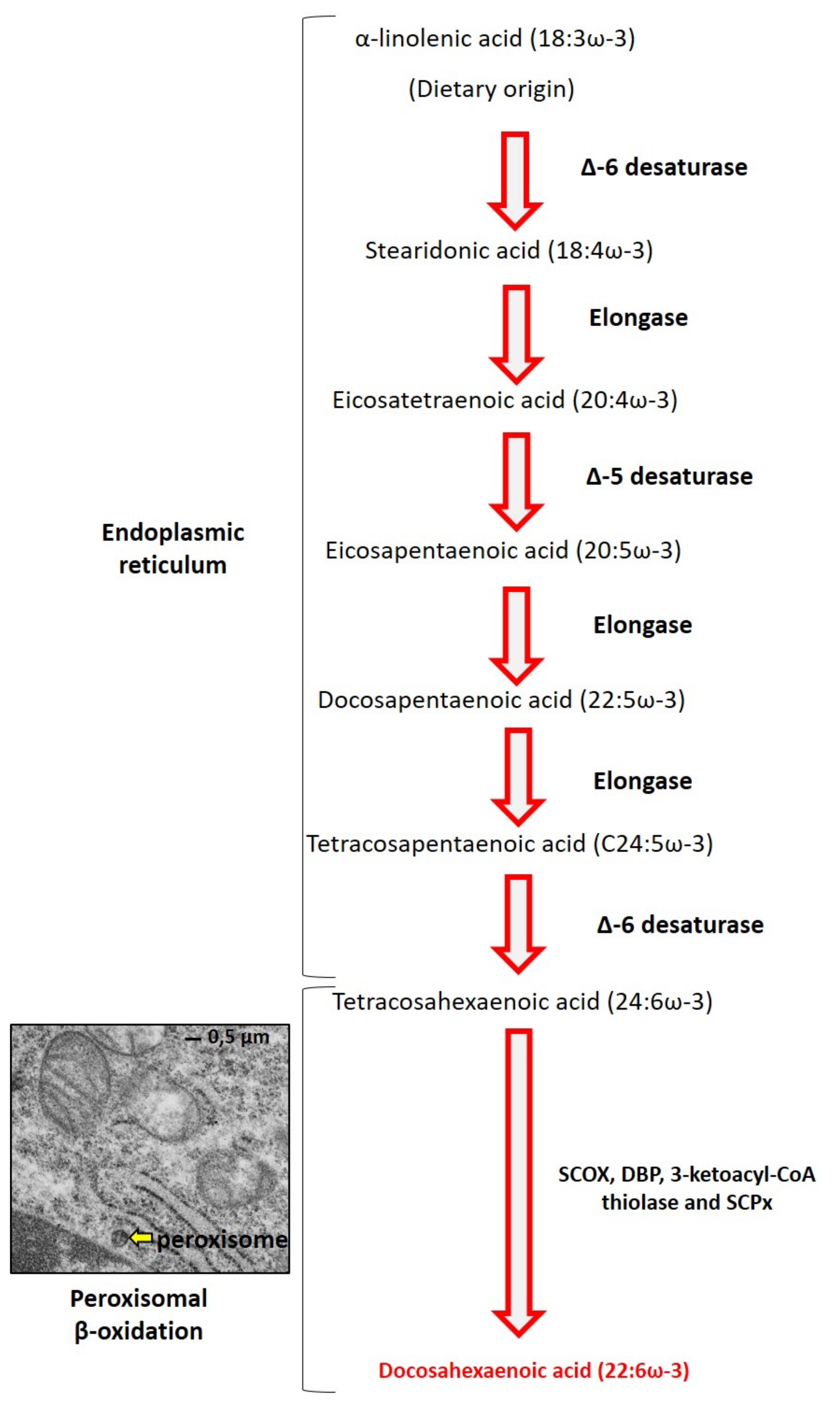
2. DHA Biochemistry
3. Impact of DHA on Cell Death and Oxidative Stress Induced in Microglial: In Vitro Studies
4. Impact of DHA on Inflammation Induced by Microglia in Neurodegenerative Diseases: In Vitro and Animal Studies
4.1. In Vitro Results
4.2. In Vivo Models
4.2.1. Neuroinflammation Mediated by LPS
4.2.2. Alzheimer Disease
4.2.3. Multiple Sclerosis
4.3. Some Other Neuropathologies
5. DHA, Clinical Trials, and Neurodegenerative Diseases
6. Brain Nanomedicine and Microglia
6.1. Nanoparticles
6.1.1. Lipid-Based Nanoparticles
6.1.2. Metal Nanoparticles
6.1.3. Polymer Nanoparticles and Mesoporous Silica Nanoparticles
6.1.4. Cell-Derived Nanoparticles
6.1.5. Antioxidant Nanoparticles
6.2. Dendrosomal Nanoparticles
6.3. Dendrimers and Other Dendritic Polymers
6.4. Quantum Dots
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and Neuroinflammation: A Systematic Review of the Effects of Stress on Microglia and the Implications for Mental Illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; Khoury, J.E. Microglia in Neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Lillo, A.; Rivas-Santisteban, R.; Reyes-Resina, I.; Navarro, G. Microglial Adenosine Receptors: From Preconditioning to Modulating the M1/M2 Balance in Activated Cells. Cells 2021, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Guesnet, P.; Alessandri, J.-M. Docosahexaenoic Acid (DHA) and the Developing Central Nervous System (CNS)—Implications for Dietary Recommendations. Biochimie 2011, 93, 7–12. [Google Scholar] [CrossRef]
- Champeil-Potokar, G.; Denis, I.; Goustard-Langelier, B.; Alessandri, J.-M.; Guesnet, P.; Lavialle, M. Astrocytes in Culture Require Docosahexaenoic Acid to Restore the N-3/n-6 Polyunsaturated Fatty Acid Balance in Their Membrane Phospholipids. J. Neurosci. Res. 2004, 75, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, J.-M.; Guesnet, P.; Vancassel, S.; Astorg, P.; Denis, I.; Langelier, B.; Aïd, S.; Poumès-Ballihaut, C.; Champeil-Potokar, G.; Lavialle, M. Polyunsaturated Fatty Acids in the Central Nervous System: Evolution of Concepts and Nutritional Implications throughout Life. Reprod. Nutr. Dev. 2004, 44, 509–538. [Google Scholar] [CrossRef]
- Alessandri, J.-M.; Extier, A.; Astorg, P.; Lavialle, M.; Simon, N.; Guesnet, P. Métabolisme des acides gras oméga-3: Différences entre hommes et femmes. Nutr. Clin. Métabolisme 2009, 23, 55–66. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; Mooijer, P.A.; Zhang, Z.; Reddy, J.K.; Spector, A.A.; Wanders, R.J. Identification of the Peroxisomal Beta-Oxidation Enzymes Involved in the Biosynthesis of Docosahexaenoic Acid. J. Lipid Res. 2001, 42, 1987–1995. [Google Scholar] [CrossRef]
- Nury, T.; Zarrouk, A.; Yammine, A.; Mackrill, J.J.; Vejux, A.; Lizard, G. Oxiapoptophagy: A Type of Cell Death Induced by Some Oxysterols. Br. J. Pharmacol. 2021, 178, 3115–3123. [Google Scholar] [CrossRef] [PubMed]
- Debbabi, M.; Zarrouk, A.; Bezine, M.; Meddeb, W.; Nury, T.; Badreddine, A.; Karym, E.M.; Sghaier, R.; Bretillon, L.; Guyot, S.; et al. Comparison of the Effects of Major Fatty Acids Present in the Mediterranean Diet (Oleic Acid, Docosahexaenoic Acid) and in Hydrogenated Oils (Elaidic Acid) on 7-Ketocholesterol-Induced Oxiapoptophagy in Microglial BV-2 Cells. Chem. Phys. Lipids 2017, 207, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Herr, D.R.; Yam, T.Y.A.; Tan, W.S.D.; Koh, S.S.; Wong, W.S.F.; Ong, W.-Y.; Chayaburakul, K. Ultrastructural Characteristics of DHA-Induced Pyroptosis. Neuromol. Med. 2020, 22, 293–303. [Google Scholar] [CrossRef]
- Srikanth, M.; Chandrasaharan, K.; Zhao, X.; Chayaburakul, K.; Ong, W.-Y.; Herr, D.R. Metabolism of Docosahexaenoic Acid (DHA) Induces Pyroptosis in BV-2 Microglial Cells. Neuromol. Med. 2018, 20, 504–514. [Google Scholar] [CrossRef]
- Geng, X.; Yang, B.; Li, R.; Teng, T.; Ladu, M.J.; Sun, G.Y.; Greenlief, C.M.; Lee, J.C. Effects of Docosahexaenoic Acid and Its Peroxidation Product on Amyloid-β Peptide-Stimulated Microglia. Mol. Neurobiol. 2020, 57, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, R.; Michael Greenlief, C.; Fritsche, K.L.; Gu, Z.; Cui, J.; Lee, J.C.; Beversdorf, D.Q.; Sun, G.Y. Unveiling Anti-Oxidative and Anti-Inflammatory Effects of Docosahexaenoic Acid and Its Lipid Peroxidation Product on Lipopolysaccharide-Stimulated BV-2 Microglial Cells. J. Neuroinflamm. 2018, 15, 202. [Google Scholar] [CrossRef]
- Sun, G.Y.; Li, R.; Yang, B.; Fritsche, K.L.; Beversdorf, D.Q.; Lubahn, D.B.; Geng, X.; Lee, J.C.; Greenlief, C.M. Quercetin Potentiates Docosahexaenoic Acid to Suppress Lipopolysaccharide-Induced Oxidative/Inflammatory Responses, Alter Lipid Peroxidation Products, and Enhance the Adaptive Stress Pathways in BV-2 Microglial Cells. Int. J. Mol. Sci. 2019, 20, 932. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Tsao, Y.-Y.; Leung, Y.-M.; Su, K.-P. Docosahexaenoic Acid Suppresses Neuroinflammatory Responses and Induces Heme Oxygenase-1 Expression in BV-2 Microglia: Implications of Antidepressant Effects for ω-3 Fatty Acids. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 2238–2248. [Google Scholar] [CrossRef]
- Inoue, T.; Tanaka, M.; Masuda, S.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Wada, H.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; et al. Omega-3 Polyunsaturated Fatty Acids Suppress the Inflammatory Responses of Lipopolysaccharide-Stimulated Mouse Microglia by Activating SIRT1 Pathways. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 552–560. [Google Scholar] [CrossRef]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic Acid-Containing Choline Phospholipid Modulates LPS-Induced Neuroinflammation in Vivo and in Microglia in Vitro. J. Neuroinflamm. 2017, 14, 170. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Pu, H.; Wang, G.; Li, W.; Leak, R.K.; Chen, J.; Liou, A.K.; Hu, X. N-3 PUFA Supplementation Benefits Microglial Responses to Myelin Pathology. Sci. Rep. 2014, 4, 7458. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.-O.; Basun, H.; et al. Omega-3 Fatty Acids Enhance Phagocytosis of Alzheimer’s Disease-Related Amyloid-β 42 by Human Microglia and Decrease Inflammatory Markers. J. Alzheimers Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Kuan, Y.-H.; Li, J.-R.; Chen, W.-Y.; Ou, Y.-C.; Pan, H.-C.; Liao, S.-L.; Raung, S.-L.; Chang, C.-J.; Chen, C.-J. Docosahexaenoic Acid Reduces Cellular Inflammatory Response Following Permanent Focal Cerebral Ischemia in Rats. J. Nutr. Biochem. 2013, 24, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Pettit, L.K.; Varsanyi, C.; Tadros, J.; Vassiliou, E. Modulating the Inflammatory Properties of Activated Microglia with Docosahexaenoic Acid and Aspirin. Lipids Health Dis. 2013, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Wu, C.-C.; Wang, J.-D.; Li, J.-R.; Wang, Y.-Y.; Lin, S.-Y.; Chen, W.-Y.; Liao, S.-L.; Chen, C.-J. DHA Attenuated Japanese Encephalitis Virus Infection-Induced Neuroinflammation and Neuronal Cell Death in Cultured Rat Neuron/Glia. Brain. Behav. Immun. 2021, 93, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Mancera, P.; Wappenhans, B.; Cordobilla, B.; Virgili, N.; Pugliese, M.; Rueda, F.; Espinosa-Parrilla, J.F.; Domingo, J.C. Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice. Nutrients 2017, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Plastina, P.; Vincken, J.-P.; Jansen, R.; Balvers, M.; Ten Klooster, J.P.; Gruppen, H.; Witkamp, R.; Meijerink, J. N-Docosahexaenoyl Dopamine, an Endocannabinoid-like Conjugate of Dopamine and the n-3 Fatty Acid Docosahexaenoic Acid, Attenuates Lipopolysaccharide-Induced Activation of Microglia and Macrophages via COX-2. ACS Chem. Neurosci. 2017, 8, 548–557. [Google Scholar] [CrossRef]
- Park, T.; Chen, H.; Kevala, K.; Lee, J.-W.; Kim, H.-Y. N-Docosahexaenoylethanolamine Ameliorates LPS-Induced Neuroinflammation via CAMP/PKA-Dependent Signaling. J. Neuroinflamm. 2016, 13, 284. [Google Scholar] [CrossRef]
- Park, T.; Chen, H.; Kim, H.-Y. GPR110 (ADGRF1) Mediates Anti-Inflammatory Effects of N-Docosahexaenoylethanolamine. J. Neuroinflamm. 2019, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Teeling, J. Microglia and Macrophages of the Central Nervous System: The Contribution of Microglia Priming and Systemic Inflammation to Chronic Neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Chataigner, M.; Martin, M.; Lucas, C.; Pallet, V.; Layé, S.; Mehaignerie, A.; Bouvret, E.; Dinel, A.-L.; Joffre, C. Fish Hydrolysate Supplementation Containing N-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation. Nutrients 2021, 13, 824. [Google Scholar] [CrossRef]
- Caputo, M.P.; Radlowski, E.C.; Lawson, M.; Antonson, A.; Watson, J.E.; Matt, S.M.; Leyshon, B.J.; Das, A.; Johnson, R.W. Herring Roe Oil Supplementation Alters Microglial Cell Gene Expression and Reduces Peripheral Inflammation After Immune Activation in a Neonatal Piglet Model. Brain. Behav. Immun. 2019, 81, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Trépanier, M.-O.; Hopperton, K.E.; Giuliano, V.; Masoodi, M.; Bazinet, R.P. Increased Brain Docosahexaenoic Acid Has No Effect on the Resolution of Neuroinflammation Following Intracerebroventricular Lipopolysaccharide Injection. Neurochem. Int. 2018, 118, 115–126. [Google Scholar] [CrossRef]
- Zhao, Y.; Calon, F.; Julien, C.; Winkler, J.W.; Petasis, N.A.; Lukiw, W.J.; Bazan, N.G. Docosahexaenoic Acid-Derived Neuroprotectin D1 Induces Neuronal Survival via Secretase- and PPARγ-Mediated Mechanisms in Alzheimer’s Disease Models. PLoS ONE 2011, 6, e15816. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Trépanier, M.-O.; Giuliano, V.; Bazinet, R.P. Brain Omega-3 Polyunsaturated Fatty Acids Modulate Microglia Cell Number and Morphology in Response to Intracerebroventricular Amyloid-β 1-40 in Mice. J. Neuroinflamm. 2016, 13, 257. [Google Scholar] [CrossRef]
- Abdelmeguid, N.E.; Khalil, M.I.M.; Elhabet, R.; Sultan, A.S.; Salam, S.A. Combination of Docosahexaenoic Acid and Ginko Biloba Extract Improves Cognitive Function and Hippocampal Tissue Damages in a Mouse Model of Alzheimer’s Disease. J. Chem. Neuroanat. 2021, 116, 101995. [Google Scholar] [CrossRef]
- Parrott, M.D.; Winocur, G.; Bazinet, R.P.; Ma, D.W.L.; Greenwood, C.E. Whole-Food Diet Worsened Cognitive Dysfunction in an Alzheimer’s Disease Mouse Model. Neurobiol. Aging 2015, 36, 90–99. [Google Scholar] [CrossRef][Green Version]
- Sharman, M.J.; Gyengesi, E.; Liang, H.; Chatterjee, P.; Karl, T.; Li, Q.-X.; Wenk, M.R.; Halliwell, B.; Martins, R.N.; Münch, G. Assessment of Diets Containing Curcumin, Epigallocatechin-3-Gallate, Docosahexaenoic Acid and α-Lipoic Acid on Amyloid Load and Inflammation in a Male Transgenic Mouse Model of Alzheimer’s Disease: Are Combinations More Effective? Neurobiol. Dis. 2019, 124, 505–519. [Google Scholar] [CrossRef]
- Adkins, Y.; Soulika, A.M.; Mackey, B.; Kelley, D.S. Docosahexaenoic Acid (22:6n-3) Ameliorated the Onset and Severity of Experimental Autoimmune Encephalomyelitis in Mice. Lipids 2019, 54, 13–23. [Google Scholar] [CrossRef]
- Cai, W.; Liu, S.; Hu, M.; Sun, X.; Qiu, W.; Zheng, S.; Hu, X.; Lu, Z. Post-Stroke DHA Treatment Protects Against Acute Ischemic Brain Injury by Skewing Macrophage Polarity Toward the M2 Phenotype. Transl. Stroke Res. 2018, 9, 669–680. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Zhang, R.; Qiao, S.; Fan, J. Resolvin D2 Recovers Neural Injury by Suppressing Inflammatory Mediators Expression in Lipopolysaccharide-Induced Parkinson’s Disease Rat Model. Biochem. Biophys. Res. Commun. 2015, 460, 799–805. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.A.; Egorova, E.L.; Starinets, A.A.; Ponomarenko, A.I.; Ermolenko, E.V.; Manzhulo, I.V. N-Docosahexaenoylethanolamine Attenuates Neuroinflammation and Improves Hippocampal Neurogenesis in Rats with Sciatic Nerve Chronic Constriction Injury. Mar. Drugs 2020, 18, 516. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Li, X.; Li, K.; Chen, L.; Zhang, Z.; Peng, M. Neuroprotectin D1 Protects Against Postoperative Delirium-Like Behavior in Aged Mice. Front. Aging Neurosci. 2020, 12, 582674. [Google Scholar] [CrossRef]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional Intervention to Prevent Alzheimer’s Disease: Potential Benefits of Xanthophyll Carotenoids and Omega-3 Fatty Acids Combined. J. Alzheimers Dis. JAD 2018, 64, 367–378. [Google Scholar] [CrossRef]
- Karimi, M.; Vedin, I.; Freund Levi, Y.; Basun, H.; Faxén Irving, G.; Eriksdotter, M.; Wahlund, L.-O.; Schultzberg, M.; Hjorth, E.; Cederholm, T.; et al. DHA-Rich n-3 Fatty Acid Supplementation Decreases DNA Methylation in Blood Leukocytes: The OmegAD Study. Am. J. Clin. Nutr. 2017, 106, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Eriksdotter, M.; Vedin, I.; Falahati, F.; Freund-Levi, Y.; Hjorth, E.; Faxen-Irving, G.; Wahlund, L.-O.; Schultzberg, M.; Basun, H.; Cederholm, T.; et al. Plasma Fatty Acid Profiles in Relation to Cognition and Gender in Alzheimer’s Disease Patients During Oral Omega-3 Fatty Acid Supplementation: The OmegAD Study. J. Alzheimers Dis. JAD 2015, 48, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hjorth, E.; Vedin, I.; Eriksdotter, M.; Freund-Levi, Y.; Wahlund, L.-O.; Cederholm, T.; Palmblad, J.; Schultzberg, M. Effects of N-3 FA Supplementation on the Release of Proresolving Lipid Mediators by Blood Mononuclear Cells: The OmegAD Study. J. Lipid Res. 2015, 56, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Freund-Levi, Y.; Vedin, I.; Hjorth, E.; Basun, H.; Faxén Irving, G.; Schultzberg, M.; Eriksdotter, M.; Palmblad, J.; Vessby, B.; Wahlund, L.-O.; et al. Effects of Supplementation with Omega-3 Fatty Acids on Oxidative Stress and Inflammation in Patients with Alzheimer’s Disease: The OmegAD Study. J. Alzheimers Dis. JAD 2014, 42, 823–831. [Google Scholar] [CrossRef]
- Freund Levi, Y.; Vedin, I.; Cederholm, T.; Basun, H.; Faxén Irving, G.; Eriksdotter, M.; Hjorth, E.; Schultzberg, M.; Vessby, B.; Wahlund, L.-O.; et al. Transfer of Omega-3 Fatty Acids across the Blood-Brain Barrier after Dietary Supplementation with a Docosahexaenoic Acid-Rich Omega-3 Fatty Acid Preparation in Patients with Alzheimer’s Disease: The OmegAD Study. J. Intern. Med. 2014, 275, 428–436. [Google Scholar] [CrossRef]
- Faxén-Irving, G.; Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Basun, H.; Hjorth, E.; Palmblad, J.; Vedin, I.; Cederholm, T.; Wahlund, L.-O. Effects on Transthyretin in Plasma and Cerebrospinal Fluid by DHA-Rich n-3 Fatty Acid Supplementation in Patients with Alzheimer’s Disease: The OmegAD Study. J. Alzheimers Dis. JAD 2013, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vedin, I.; Cederholm, T.; Freund-Levi, Y.; Basun, H.; Garlind, A.; Irving, G.F.; Eriksdotter-Jönhagen, M.; Wahlund, L.-O.; Dahlman, I.; Palmblad, J. Effects of DHA-Rich n-3 Fatty Acid Supplementation on Gene Expression in Blood Mononuclear Leukocytes: The OmegAD Study. PLoS ONE 2012, 7, e35425. [Google Scholar] [CrossRef]
- Vedin, I.; Cederholm, T.; Freund-Levi, Y.; Basun, H.; Hjorth, E.; Irving, G.F.; Eriksdotter-Jönhagen, M.; Schultzberg, M.; Wahlund, L.-O.; Palmblad, J. Reduced Prostaglandin F2 Alpha Release from Blood Mononuclear Leukocytes after Oral Supplementation of Omega3 Fatty Acids: The OmegAD Study. J. Lipid Res. 2010, 51, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Vedin, I.; Cederholm, T.; Freund Levi, Y.; Basun, H.; Garlind, A.; Faxén Irving, G.; Jönhagen, M.E.; Vessby, B.; Wahlund, L.-O.; Palmblad, J. Effects of Docosahexaenoic Acid-Rich n-3 Fatty Acid Supplementation on Cytokine Release from Blood Mononuclear Leukocytes: The OmegAD Study. Am. J. Clin. Nutr. 2008, 87, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Childs, C.E.; Calder, P.C.; Rogers, P.J. No Effect of Omega-3 Fatty Acid Supplementation on Cognition and Mood in Individuals with Cognitive Impairment and Probable Alzheimer’s Disease: A Randomised Controlled Trial. Int. J. Mol. Sci. 2015, 16, 24600–24613. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M.; et al. Docosahexaenoic Acid Supplementation and Cognitive Decline in Alzheimer Disease: A Randomized Trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef]
- Freund-Levi, Y.; Basun, H.; Cederholm, T.; Faxén-Irving, G.; Garlind, A.; Grut, M.; Vedin, I.; Palmblad, J.; Wahlund, L.-O.; Eriksdotter-Jönhagen, M. Omega-3 Supplementation in Mild to Moderate Alzheimer’s Disease: Effects on Neuropsychiatric Symptoms. Int. J. Geriatr. Psychiatry 2008, 23, 161–169. [Google Scholar] [CrossRef]
- Kotani, S.; Sakaguchi, E.; Warashina, S.; Matsukawa, N.; Ishikura, Y.; Kiso, Y.; Sakakibara, M.; Yoshimoto, T.; Guo, J.; Yamashima, T. Dietary Supplementation of Arachidonic and Docosahexaenoic Acids Improves Cognitive Dysfunction. Neurosci. Res. 2006, 56, 159–164. [Google Scholar] [CrossRef]
- Manes, M.; Alberici, A.; Di Gregorio, E.; Boccone, L.; Premi, E.; Mitro, N.; Pasolini, M.P.; Pani, C.; Paghera, B.; Perani, D.; et al. Docosahexaenoic Acid Is a Beneficial Replacement Treatment for Spinocerebellar Ataxia 38. Ann. Neurol. 2017, 82, 615–621. [Google Scholar] [CrossRef]
- Manes, M.; Alberici, A.; Di Gregorio, E.; Boccone, L.; Premi, E.; Mitro, N.; Pasolini, M.P.; Pani, C.; Paghera, B.; Orsi, L.; et al. Long-Term Efficacy of Docosahexaenoic Acid (DHA) for Spinocerebellar Ataxia 38 (SCA38) Treatment: An Open Label Extension Study. Parkinsonism Relat. Disord. 2019, 63, 191–194. [Google Scholar] [CrossRef]
- Hernando, S.; Herran, E.; Hernandez, R.M.; Igartua, M. Nanostructured Lipid Carriers Made of Ω-3 Polyunsaturated Fatty Acids: In Vitro Evaluation of Emerging Nanocarriers to Treat Neurodegenerative Diseases. Pharmaceutics 2020, 12, 928. [Google Scholar] [CrossRef]
- Hernando, S.; Herran, E.; Figueiro-Silva, J.; Pedraz, J.L.; Igartua, M.; Carro, E.; Hernandez, R.M. Intranasal Administration of TAT-Conjugated Lipid Nanocarriers Loading GDNF for Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 145–155. [Google Scholar] [CrossRef]
- Huang, J.; Lu, Y.; Wang, H.; Liu, J.; Liao, M.; Hong, L.; Tao, R.; Ahmed, M.M.; Liu, P.; Liu, S.; et al. The Effect of Lipid Nanoparticle PEGylation on Neuroinflammatory Response in Mouse Brain. Biomaterials 2013, 34, 7960–7970. [Google Scholar] [CrossRef]
- Illum, L. Nasal Drug Delivery—Possibilities, Problems and Solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Song, Q.; Huang, M.; Yao, L.; Wang, X.; Gu, X.; Chen, J.; Chen, J.; Huang, J.; Hu, Q.; Kang, T.; et al. Lipoprotein-Based Nanoparticles Rescue the Memory Loss of Mice with Alzheimer’s Disease by Accelerating the Clearance of Amyloid-Beta. ACS Nano 2014, 8, 2345–2359. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Song, H.; Xu, J.; Huang, J.; Hu, M.; Gu, X.; Chen, J.; Zheng, G.; Chen, H.; Gao, X. Biomimetic ApoE-Reconstituted High Density Lipoprotein Nanocarrier for Blood-Brain Barrier Penetration and Amyloid Beta-Targeting Drug Delivery. Mol. Pharm. 2016, 13, 3976–3987. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Hu, M.; Song, Q.; Song, H.; Huang, J.; Gu, X.; Wang, X.; Chen, J.; Kang, T.; Feng, X.; et al. GM1-Modified Lipoprotein-like Nanoparticle: Multifunctional Nanoplatform for the Combination Therapy of Alzheimer’s Disease. ACS Nano 2015, 9, 10801–10816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Daniels, R.; Schluesener, H.J. Oridonin Ameliorates Neuropathological Changes and Behavioural Deficits in a Mouse Model of Cerebral Amyloidosis. J. Cell. Mol. Med. 2013, 17, 1566–1576. [Google Scholar] [CrossRef]
- Bernardi, A.; Frozza, R.L.; Meneghetti, A.; Hoppe, J.B.; Battastini, A.M.O.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C.G. Indomethacin-Loaded Lipid-Core Nanocapsules Reduce the Damage Triggered by Aβ1-42 in Alzheimer’s Disease Models. Int. J. Nanomed. 2012, 7, 4927–4942. [Google Scholar] [CrossRef]
- Sruthi, S.; Loiseau, A.; Boudon, J.; Sallem, F.; Maurizi, L.; Mohanan, P.V.; Lizard, G.; Millot, N. In Vitro Interaction and Biocompatibility of Titanate Nanotubes with Microglial Cells. Toxicol. Appl. Pharmacol. 2018, 353, 74–86. [Google Scholar] [CrossRef]
- Sela, H.; Cohen, H.; Elia, P.; Zach, R.; Karpas, Z.; Zeiri, Y. Spontaneous Penetration of Gold Nanoparticles through the Blood Brain Barrier (BBB). J. Nanobiotechnol. 2015, 13, 71. [Google Scholar] [CrossRef]
- De Astis, S.; Corradini, I.; Morini, R.; Rodighiero, S.; Tomasoni, R.; Lenardi, C.; Verderio, C.; Milani, P.; Matteoli, M. Nanostructured TiO2 Surfaces Promote Polarized Activation of Microglia, but Not Astrocytes, toward a Proinflammatory Profile. Nanoscale 2013, 5, 10963–10974. [Google Scholar] [CrossRef]
- Sobska, J.; Waszkielewicz, M.; Podleśny-Drabiniok, A.; Olesiak-Banska, J.; Krężel, W.; Matczyszyn, K. Gold Nanoclusters Display Low Immunogenic Effect in Microglia Cells. Nanomaterials 2021, 11, 1066. [Google Scholar] [CrossRef]
- Xiao, L.; Wei, F.; Zhou, Y.; Anderson, G.J.; Frazer, D.M.; Lim, Y.C.; Liu, T.; Xiao, Y. Dihydrolipoic Acid-Gold Nanoclusters Regulate Microglial Polarization and Have the Potential To Alter Neurogenesis. Nano Lett. 2020, 20, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, T.; Liu, Y.; Jiang, Y.; Seshadri, V.D.D.; Mohan, S.K.; Ling, L. Neuroprotective Effect of Biosynthesised Gold Nanoparticles Synthesised from Root Extract of Paeonia Moutan against Parkinson Disease – In Vitro & In Vivo Model. J. Photochem. Photobiol. B 2019, 200, 111635. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yi, E.H.; Kim, Y.; Park, G. Anti-Neuroinflammatory Effects of Ephedra Sinica Stapf Extract-Capped Gold Nanoparticles in Microglia. Int. J. Nanomed. 2019, 14, 2861–2877. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, Z.D.; Sahmetlioglu, E.; Narin, I.; Cumaoglu, A. Synthesis of Gold and Silver Nanoparticles Using Flavonoid Quercetin and Their Effects on Lipopolysaccharide Induced Inflammatory Response in Microglial Cells. 3 Biotech 2019, 9, 212. [Google Scholar] [CrossRef]
- Yuan, Q.; Yao, Y.; Zhang, X.; Yuan, J.; Sun, B.; Gao, X. The Gold Nanocluster Protects Neurons Directly or via Inhibiting Cytotoxic Secretions of Microglia Cell. J. Nanosci. Nanotechnol. 2019, 19, 1986–1995. [Google Scholar] [CrossRef]
- Liu, R.; Yang, J.; Liu, L.; Lu, Z.; Shi, Z.; Ji, W.; Shen, J.; Zhang, X. An “Amyloid-β Cleaner” for the Treatment of Alzheimer’s Disease by Normalizing Microglial Dysfunction. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2020, 7, 1901555. [Google Scholar] [CrossRef]
- Glat, M.; Skaat, H.; Menkes-Caspi, N.; Margel, S.; Stern, E.A. Age-Dependent Effects of Microglial Inhibition in Vivo on Alzheimer’s Disease Neuropathology Using Bioactive-Conjugated Iron Oxide Nanoparticles. J. Nanobiotechnol. 2013, 11, 32. [Google Scholar] [CrossRef]
- Ameruoso, A.; Palomba, R.; Palange, A.L.; Cervadoro, A.; Lee, A.; Di Mascolo, D.; Decuzzi, P. Ameliorating Amyloid-β Fibrils Triggered Inflammation via Curcumin-Loaded Polymeric Nanoconstructs. Front. Immunol. 2017, 8, 1411. [Google Scholar] [CrossRef]
- Yao, L.; Gu, X.; Song, Q.; Wang, X.; Huang, M.; Hu, M.; Hou, L.; Kang, T.; Chen, J.; Chen, H.; et al. Nanoformulated Alpha-Mangostin Ameliorates Alzheimer’s Disease Neuropathology by Elevating LDLR Expression and Accelerating Amyloid-Beta Clearance. J. Control. Release Off. J. Control. Release Soc. 2016, 226, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sriramoju, B.; Kanwar, R.K.; Kanwar, J.R. Nanoformulated Mutant SurR9-C84A: A Possible Key for Alzheimer’s and Its Associated Inflammation. Pharm. Res. 2015, 32, 2787–2797. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.K.; Chmielowski, R.; Abdelhamid, D.S.; Faig, J.J.; Francis, N.; Baum, J.; Pang, Z.P.; Uhrich, K.E.; Moghe, P.V. Polymer Brain-Nanotherapeutics for Multipronged Inhibition of Microglial α-Synuclein Aggregation, Activation, and Neurotoxicity. Biomaterials 2016, 111, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. ROS Responsive Resveratrol Delivery from LDLR Peptide Conjugated PLA-Coated Mesoporous Silica Nanoparticles across the Blood-Brain Barrier. J. Nanobiotechnol. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.-H.; Wu, J.; Mou, F.-F.; Xie, W.-H.; Wang, F.-B.; Wang, Q.-L.; Fang, J.; Xu, Y.-W.; Dong, Y.-R.; Liu, J.-R.; et al. Exosomes Derived from Hypoxia-Preconditioned Mesenchymal Stromal Cells Ameliorate Cognitive Decline by Rescuing Synaptic Dysfunction and Regulating Inflammatory Responses in APP/PS1 Mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 654–668. [Google Scholar] [CrossRef]
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Sakai, S.; Mitsutake, S.; Okada, M.; Tahara, H.; Furukawa, J.; Fujitani, N.; Shinohara, Y.; Igarashi, Y. Decreased Amyloid-β Pathologies by Intracerebral Loading of Glycosphingolipid-Enriched Exosomes in Alzheimer Model Mice. J. Biol. Chem. 2014, 289, 24488–24498. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Usuki, S.; Sakai, S.; Hanamatsu, H.; Mioka, T.; Kimura, N.; Okada, M.; Tahara, H.; Furukawa, J.; et al. A Potential Function for Neuronal Exosomes: Sequestering Intracerebral Amyloid-β Peptide. FEBS Lett. 2015, 589, 84–88. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-Inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A Novel Nanoparticle Drug Delivery System: The Anti-Inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; El-Aleem, S.A.A.; El-Tahawy, N.F.G. Neuroprotective Effect of Quercetin Nanoparticles: A Possible Prophylactic and Therapeutic Role in Alzheimer’s Disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yang, X.; Calvelli, H.R.; Cao, Y.; Francis, N.L.; Chmielowski, R.A.; Joseph, L.B.; Pang, Z.P.; Uhrich, K.E.; Baum, J.; et al. Antioxidant Nanoparticles for Concerted Inhibition of α-Synuclein Fibrillization, and Attenuation of Microglial Intracellular Aggregation and Activation. Front. Bioeng. Biotechnol. 2020, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Motavaf, M.; Sadeghizadeh, M.; Babashah, S.; Zare, L.; Javan, M. Protective Effects of a Nano-Formulation of Curcumin against Cuprizone-Induced Demyelination in the Mouse Corpus Callosum. Iran. J. Pharm. Res. IJPR 2020, 19, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Grayson, S.M.; Fréchet, J.M. Convergent Dendrons and Dendrimers: From Synthesis to Applications. Chem. Rev. 2001, 101, 3819–3868. [Google Scholar] [CrossRef]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting Mitochondrial Dysfunction and Oxidative Stress in Activated Microglia Using Dendrimer-Based Therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Navath, R.S.; Balakrishnan, B.; Guru, B.R.; Mishra, M.K.; Romero, R.; Kannan, R.M.; Kannan, S. Intrinsic Targeting of Inflammatory Cells in the Brain by Polyamidoamine Dendrimers upon Subarachnoid Administration. Nanomedicine 2010, 5, 1317–1329. [Google Scholar] [CrossRef]
- Bertero, A.; Boni, A.; Gemmi, M.; Gagliardi, M.; Bifone, A.; Bardi, G. Surface Functionalisation Regulates Polyamidoamine Dendrimer Toxicity on Blood-Brain Barrier Cells and the Modulation of Key Inflammatory Receptors on Microglia. Nanotoxicology 2014, 8, 158–168. [Google Scholar] [CrossRef]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci. Transl. Med. 2012, 4, 130ra46. [Google Scholar] [CrossRef]
- Zhang, F.; Mastorakos, P.; Mishra, M.K.; Mangraviti, A.; Hwang, L.; Zhou, J.; Hanes, J.; Brem, H.; Olivi, A.; Tyler, B.; et al. Uniform Brain Tumor Distribution and Tumor Associated Macrophage Targeting of Systemically Administered Dendrimers. Biomaterials 2015, 52, 507–516. [Google Scholar] [CrossRef]
- DeRidder, L.; Sharma, A.; Liaw, K.; Sharma, R.; John, J.; Kannan, S.; Kannan, R.M. Dendrimer-Tesaglitazar Conjugate Induces a Phenotype Shift of Microglia and Enhances β-Amyloid Phagocytosis. Nanoscale 2021, 13, 939–952. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; An, S.; Kuang, Y.; He, X.; Ma, H.; Li, J.; Lu, J.; Lv, J.; Zhang, N.; et al. Targeting Caspase-3 as Dual Therapeutic Benefits by RNAi Facilitating Brain-Targeted Nanoparticles in a Rat Model of Parkinson’s Disease. PLoS ONE 2013, 8, e62905. [Google Scholar] [CrossRef]
- Kahn, E.; Vejux, A.; Ménétrier, F.; Maiza, C.; Hammann, A.; Sequeira-Le Grand, A.; Frouin, F.; Tourneur, Y.; Brau, F.; Riedinger, J.-M.; et al. Analysis of CD36 Expression on Human Monocytic Cells and Atherosclerotic Tissue Sections with Quantum Dots: Investigation by Flow Cytometry and Spectral Imaging Microscopy. Anal. Quant. Cytol. Histol. 2006, 28, 14–26. [Google Scholar] [PubMed]
- Kahn, E.; Lizard, G.; Monier, S.; Bessède, G.; Frouin, F.; Gambert, P.; Todd-Pokropek, A. Flow Cytometry and Factor Analysis Evaluation of Confocal Image Sequences of Morphologic and Functional Changes Occurring at the Mitochondrial Level during 7-Ketocholesterol-Induced Cell Death. Anal. Quant. Cytol. Histol. 2002, 24, 355–362. [Google Scholar] [PubMed]
- Ren, C.; Li, D.; Zhou, Q.; Hu, X. Mitochondria-Targeted TPP-MoS2 with Dual Enzyme Activity Provides Efficient Neuroprotection through M1/M2 Microglial Polarization in an Alzheimer’s Disease Model. Biomaterials 2020, 232, 119752. [Google Scholar] [CrossRef] [PubMed]
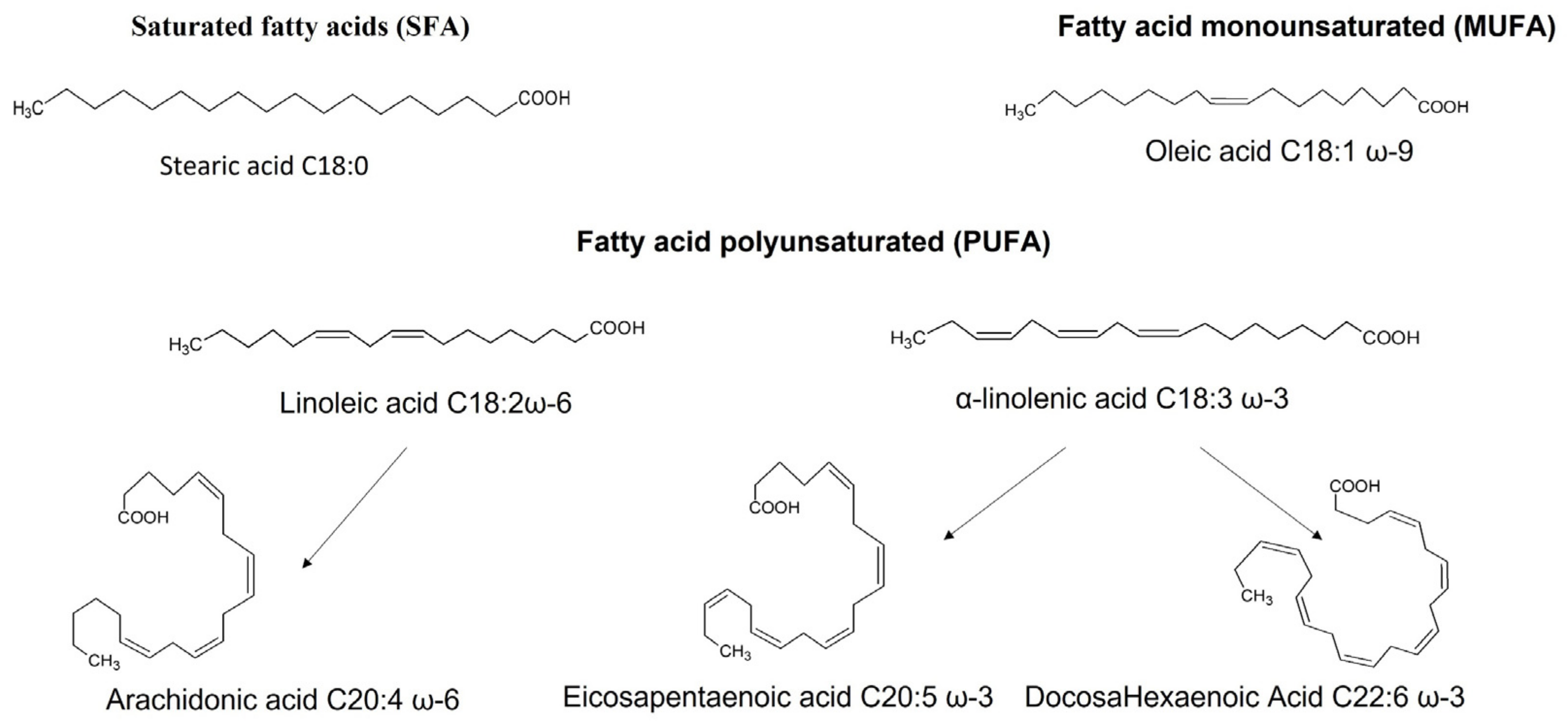

| Experimentation Type | Target | DHA Forms | Concentration or Dose | Effects | References |
|---|---|---|---|---|---|
| BV-2 cells treated with 7-ketocholesterol | Cell death oxidative stress | Chemical form | 12 µM | Protection against cell death and oxidative stress | [15] |
| BV-2 cells | Cell death | 200 µM | Induction of cell death (pyroptosis) | [16] | |
| BV-2 cells treated with oligomeric amyloid-β peptide | Oxidative stress | 10 µM | Reduce oxidative stress (involving Nrf2/HO-1) | [18] | |
| BV-2 cells treated with LPS | 1.25–10 μM | Enhance Nrf-2/HO-1 signaling | [19] | ||
| 10 µM DHA + quercetin (2.5 µM) | [20] | ||||
| BV-2 cells treated with interferon-γ | Inflammation | 30 µM | Reduce expression of proteins implicated in inflammation | [21] | |
| BV-2 cells and MG6 cells stimulated with LPS | 100 µM DHA and 100 µM EPA | Inhibition of inflammation by involving SIRT1 | [22] | ||
| BV-2 cells stimulated with LPS | 30 µM | Decrease cytokine expression | [17,23] | ||
| Primary cultures of mice microglial cells stimulated with LPS | 20 µM to 80 µM | Reduce NO and TNF-α release, modulation of phenotypic polarization of microglia | [24] | ||
| Human CHME3 microglial cells exposed to Amyloid-β42 | 0.1 to 1 µM | Decrease pro-inflammatory cytokine production, induction of a shift in phenotype away from pro-inflammatory M1 activation | [25] | ||
| Rat glial primary cell cultures, LPS/ IFN-γ stimulation | 100 µM | Regulation of the pro-inflammatory response | [26] | ||
| EOC20 microglia cells treated by Polyinosinic-Polycytidylic acid or 10 μg/mL Imiquimod | 50 µM | Reduction of the production of cytokines TNF-α and IL-6 | [27] | ||
| Primary neuron/glia rat primary cultures infected with Japanese Encephalitis virus | 25 or 50 µM | Increase of neurotoxin cytokines production | [28] | ||
| BV-2 cells activated with LPS and IFN-γ | Triglyceride forms | 20 µM | Reduce IL-6 and TNF-α production | [29] | |
| BV-2 cells treated with LPS | N-docosahexaenoyl dopamine (DHDA) | 2 µM | Decrease IL-6 and CCL-20 production | [30] | |
| Primary cultures of rat microglia and BV-2 cells treated with LPS | Synaptamide | 1–100 nM | Suppression of LPS-induced TNF-α and iNOS mRNA expression | [31,32] |
| Experimentation Type | Target | DHA Forms | Concentration or Dose | Effects | References |
|---|---|---|---|---|---|
| Injection of LPS intraperitoneally (i.p.) or in brain of C57Bl/6J mice | Inflammation | Synaptamide, endogenous metabolite derived from docosahexaenoic acid | 5 mg/kg, i.p | Decrease of TNF-α, IL-1β, IL-6, iNOS, and CCL2 mRNA involving orphan adhesion G-protein-coupled receptor 110 (GPR110) | [32] |
| C57Bl/6J mice intraperitoneally injected with LPS | Fish hydrolysate supplement | 143 µg in 150 µL of fish hydrolysate supplement/day | Reduce expression of TLR4, cytokines (IL-6, TNF-α, IL-1β,), CCL2, and IκB | [34] | |
| Chemical form | 10 mg/day | Reduce IL-6 expression | [34] | ||
| C57Bl6/J mice injected with LPS (i.p.). | 1-palmitoyl,2-docosahexaenoyl-PC (PC-DHA) | 4.33 μg/g of mouse | Decrease IL-6 production | [23] | |
| Piglets | Herring oil | 32.30% W/W total fatty acids | No attenuation of the LPS induced inflammation | [35] | |
| C57Bl/6 male mice with intracerebroventricular LPS injections | Fish oil | 1.4% of total fatty acids | No change in the expression of pro-inflammatory genes | [36] | |
| Triple-transgenic mouse model of AD | Neuroprotectin D1 (organic synthesis) | 50 nM | Downregulation of Aβ42-induced expression of COX-2, TNF-α and B-94 | [37] | |
| Male C57BL/6 mice with intracerebroventricular amyloid-β infusion (AD model) | Fish oil | 1.5% of total fatty acid | Decrease some elements of the inflammatory response | [38] | |
| Male albino Swiss mice, administration of AlCl3 (20 mg/kg) intragastrically (i.g.) then an intraperitoneal injection with D-gal (120 mg/kg) (AD model) | Chemical form | 200 mg/kg | Downregulation of TNF-α expression | [39] | |
| TgCRND8 mice (AD model) | Whole-food diet contained skinless freeze-dried Atlantic salmon and a proprietary mixture of powdered, freeze-dried vegetables and fruits | 0.246% of DHA (wt/wt) in a whole-food diet | Increase TNF-α expression | [40] | |
| Male Tg2576 (APPswe) transgenic mice (AD model) | Chemical form | 50 mg/kg body weight | Microglial activation | [41] | |
| Male C57/BL6 mice fed for 5 weeks with a diet containing 0.2% cuprizone (MS model) | Regular diet supplemented with n-3 PUFAs | DHA + EPA, 15 g/kg | Suppression of the increase of M1-associated genes and increase of the expression of M2-associated genes | [24] | |
| Female C57BL/6J mice immunization with >95% pure synthetic MOG35-55 peptide (MS model) | Development of the pathology | TG-DHA obtained by enzymatic synthesis | 250 mg/kg/day | Improve clinical score | [29] |
| C57BL/6J female/EAE model (MS model) | Phospholipid-DHA 0.3% or 1% and triacylglycerol-DHA | 0.3 or 1% either 0.48 or 1.6 mg DHA/g body weight/day respectively | Reduce EAE onset and severity | [42] |
| Experimentation Type | DHA Forms | Concentration or Dose | Effects | References |
|---|---|---|---|---|
| AD Patients | Fish oil | 1 g/day of fish oil (30 mg DHA, 90 mg EPA) | Slowing down AD | [47] |
| AD Patients | Capsule EPAX 1050TG | four capsules (One capsule: 430 mg DHA and 150 mg EPA) | Induction of DNA hypomethylation in blood cell, can be used as treatment in AD | [48] |
| AD patients | 2.3 g of omega-3 fatty acid | Positive correlation between plasma levels of omega-3 fatty acids and cognitive functions | [49] | |
| Peripheral blood mononuclear cells treated with the Aβ40 peptide | Capsule EPAX 1050TG | Prevention of the reduction of specialized proresolving mediators (lipoxin A4 and resolvin D1) released from PBMCs | [50] | |
| Moderate AD patients | Capsule EPAX 1050TG | four capsules (One capsule: 430 mg DHA and 150 mg EPA | No clear effect on oxidative stress but potential role in immunoregulation | [51] |
| AD patients | Capsule EPAX 1050TG | four capsules (One capsule: 430 mg DHA and 150 mg EPA | Increase in eicosapentaenoic acid (EPA), DHA, and total n-3 FA levels in cerebrospinal fluid | [52] |
| Increase plasma levels of transthyretin which could influence Aβ peptide deposits in the brain | [53] | |||
| Peripheral blood mononuclear cells of AD patients | Regulation of genes involved in inflammation regulation, neurodegeneration, and in ubiquitination processes | [54] | ||
| LPS-stimulated peripheral blood mononuclear cells | Anti-inflammatory effects, reduction in the release of IL-1β, IL-6, and granulocyte colony-stimulating factor | [55,56] | ||
| Cognitive impairment: no dementia and AD patients | Capsules | 625 mg DHA and 600 mg EPA | No beneficial effect on cognition and mood | [57] |
| Mild or moderate AD patients | Algal origin | 2 g of capsule containing 15% to 55% DHA | No slowdown in cognitive decline | [58] |
| Mild or moderate AD patients | Capsule EPAX 1050TG | four capsules (One capsule: 430 mg DHA and 150 mg EPA | No effect on neuropsychiatric symptoms, possible positive effects on depressive symptoms in non-ApoEω4 carriers and on agitation symptoms in ApoEω4 carriers | [59] |
| Patients with organic brain damage or mild cognitive impairment | Aravita capsules | 40 mg/capsule of arachidonic acid and DHA | Significant improvements in memory | [60] |
| Spinocerebellar ataxia 38 | Algal oil | 600 mg/day | Improvement in clinical symptoms and no degradation of neurophysiological parameters | [61,62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charrière, K.; Ghzaiel, I.; Lizard, G.; Vejux, A. Involvement of Microglia in Neurodegenerative Diseases: Beneficial Effects of Docosahexahenoic Acid (DHA) Supplied by Food or Combined with Nanoparticles. Int. J. Mol. Sci. 2021, 22, 10639. https://doi.org/10.3390/ijms221910639
Charrière K, Ghzaiel I, Lizard G, Vejux A. Involvement of Microglia in Neurodegenerative Diseases: Beneficial Effects of Docosahexahenoic Acid (DHA) Supplied by Food or Combined with Nanoparticles. International Journal of Molecular Sciences. 2021; 22(19):10639. https://doi.org/10.3390/ijms221910639
Chicago/Turabian StyleCharrière, Karine, Imen Ghzaiel, Gérard Lizard, and Anne Vejux. 2021. "Involvement of Microglia in Neurodegenerative Diseases: Beneficial Effects of Docosahexahenoic Acid (DHA) Supplied by Food or Combined with Nanoparticles" International Journal of Molecular Sciences 22, no. 19: 10639. https://doi.org/10.3390/ijms221910639
APA StyleCharrière, K., Ghzaiel, I., Lizard, G., & Vejux, A. (2021). Involvement of Microglia in Neurodegenerative Diseases: Beneficial Effects of Docosahexahenoic Acid (DHA) Supplied by Food or Combined with Nanoparticles. International Journal of Molecular Sciences, 22(19), 10639. https://doi.org/10.3390/ijms221910639








