BCL-2 Inhibitor ABT-737 Effectively Targets Leukemia-Initiating Cells with Differential Regulation of Relevant Genes Leading to Extended Survival in a NRAS/BCL-2 Mouse Model of High Risk-Myelodysplastic Syndrome
Abastract
1. Introduction
2. Results
2.1. ABT-737 Treatment Prolongs Survival in HR-MDS Transgenic Mice
2.2. ABT-737 Treatment Targets Leukemia Initiating Cells (LICs) and Primitive Progenitors
2.3. ABT-737 Treatment Induced Reduced Apoptosis in the BM, Increased Apoptosis and Inhibition of Cell Proliferation in the Liver and Spleen of HR-MDS Transgenic Mice
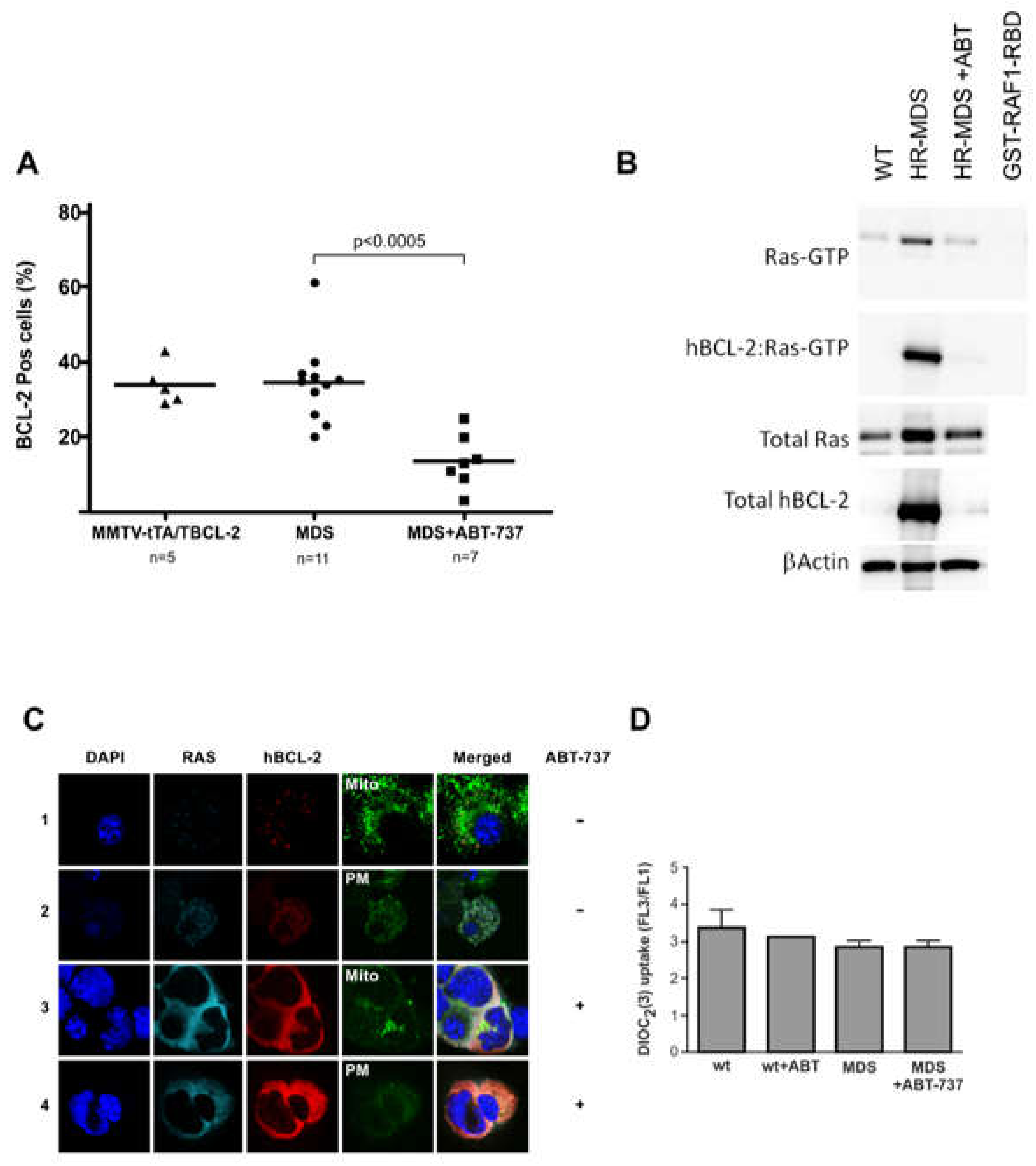
2.4. ABT-737 Induced Inhibition of BCL-2 Reduces RAS Activity in Sca1+ Cells
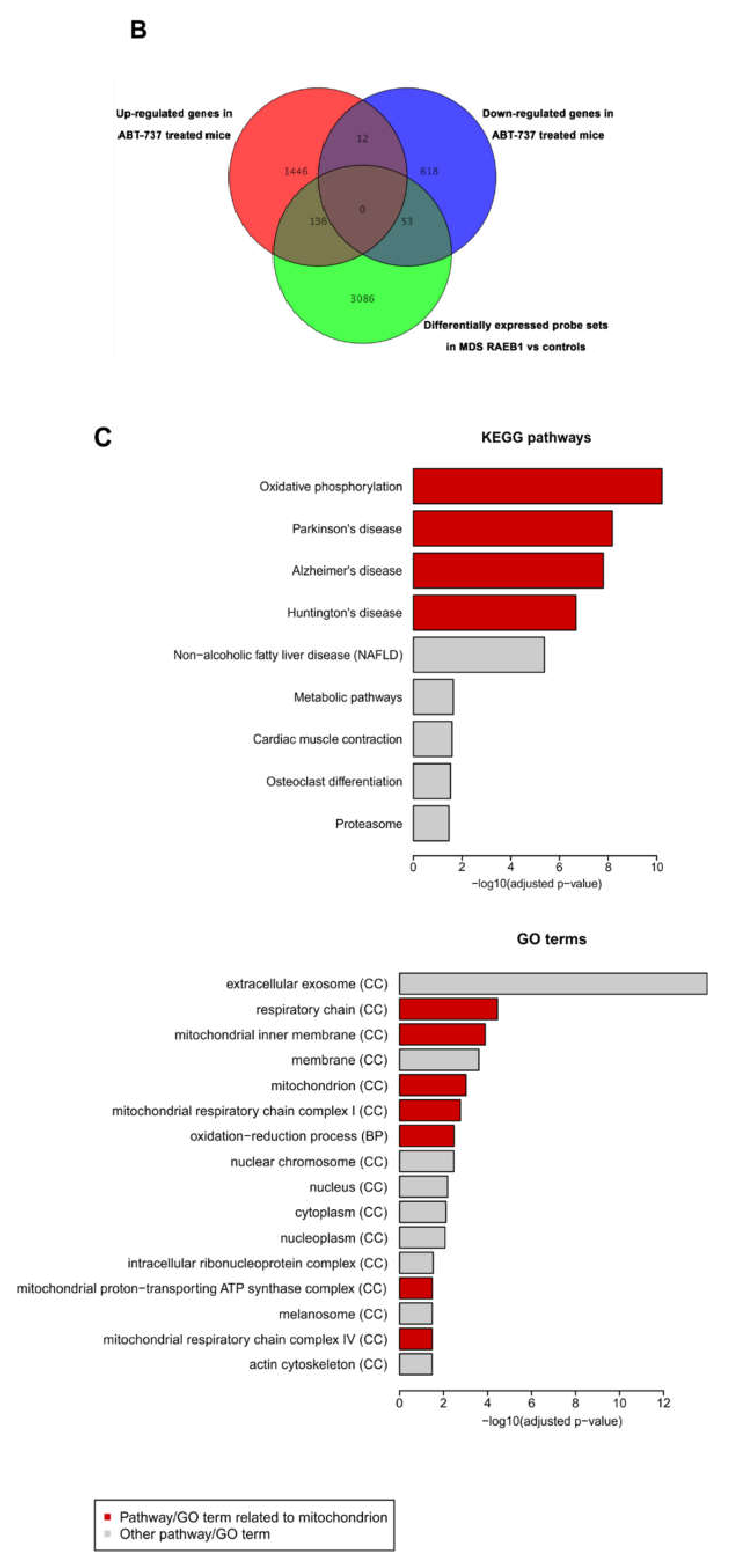
2.5. ABT-737 Treatment Induces Regulation of Pathways Implicated in Cell Survival, Proliferation and Stem Cell Regulation in HR-MDS Mice
3. Discussion
4. Materials and Methods
4.1. Transgenic Mice
4.2. ABT-737
4.3. Tissue and Cell Preparation, Flow Cytometry, Incucyte
4.4. Secondary Transplantation
4.5. Progenitor Colony Assay
4.6. Immunofluorescence and Confocal Microscopy
4.7. Mitochondrial Membrane Potential (MMP)
4.8. SPECT
4.8.1. ANX-Labeling
4.8.2. ANX-Scintigraphy
4.9. TUNEL
4.10. RAS Activation Assays and Western Blotting
4.11. Cell Preparation and RNA Extraction
4.12. Affymetrix Exon Array Hybridization
4.13. Array Data
4.14. RQ-PCR
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Padua, R.A.; Guinn, B.A.; Al-Sabah, A.; Smith, M.; Taylor, C.; Pettersson, T.; Ridge, S.; Carter, G.; White, D.; Oscier, D.; et al. RAS, FMS and p53 mutations and poor clinical outcome in myelodysplasias: A 10-year follow-up. Leukemia 1998, 12, 887–892. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Loo, P.V.; Yoon, C.J.; Ellis, P.; Wedge, D.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.E.; Mufti, G.J.; Rasool, F.; Mijovic, A.; Devereux, S.; Pagliuca, A. The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood 2000, 96, 3932–3938. [Google Scholar] [CrossRef]
- Karakas, T.; Maurer, U.; Weidmann, E.; Miething, C.C.; Hoelzer, D.; Bergmann, L. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Ann. Oncol. 1998, 9, 159–165. [Google Scholar] [CrossRef]
- Karakas, T.; Miething, C.C.; Maurer, U.; Weidmann, E.; Ackermann, H.; Hoelzer, D.; Bergmann, L. The coexpression of the apoptosis-related genes bcl-2 and wt1 in predicting survival in adult acute myeloid leukemia. Leukemia 2002, 16, 846–854. [Google Scholar] [CrossRef]
- Omidvar, N.; Kogan, S.; Beurlet, S.; Le Pogam, C.; Janin, A.; West, R.; Noguera, M.-E.; Reboul, M.; Soulié, A.; Leboeuf, C.; et al. BCL-2 and mutant NRAS interact physically and functionally in a mouse model of progressive myelodysplasia. Cancer Res. 2007, 67, 11657–11667. [Google Scholar] [CrossRef]
- Le Pogam, C.; Krief, P.; Beurlet, S.; Soulie, A.; Balitrand, N.; Cassinat, B.; Cavé, H.; Kosmider, O.; Setterblad, N.; Setterblad, N.; et al. Localization of the NRAS:BCL-2 complex determines anti-apoptotic features associated with progressive disease in myelodysplastic syndromes. Leuk. Res. 2013, 37, 312–319. [Google Scholar] [CrossRef]
- Beurlet, S.; Omidvar, N.; Gorombei, P.; Krief, P.; Le Pogam, C.; Setterblad, N.; De La Grange, P.; Leboeuf, C.; Janin, A.; Noguera, M.-E.; et al. BCL-2 inhibition with ABT-737 prolongs survival in an NRAS/BCL-2 mouse model of AML by targeting primitive LSK and progenitor cells. Blood 2013, 122, 2864–2876. [Google Scholar] [CrossRef]
- Roberts, A.W.; Seymour, J.F.; Brown, J.R.; Wierda, W.G.; Kipps, T.J.; Khaw, S.L.; Carney, D.A.; He, S.Z.; Huang, D.C.; Xiong, H.; et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL-2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 2012, 30, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; O’Connor, O.A.; Czuczman, M.S.; LaCasce, A.S.; Gerecitano, J.F.; Leonard, J.P.; Tulpule, A.; Dunleavy, K.; Xiong, H.; Chiu, Y.-L.; et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010, 11, 1149–1159. [Google Scholar] [CrossRef]
- Zhang, H.; Nimmer, P.M.; Tahir, S.K.; Chen, J.; Fryer, R.M.; Hahn, K.R.; A Iciek, L.; Morgan, S.J.; Nasarre, M.C.; Nelson, R.J.; et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007, 14, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Pellagatti, A.; Cazzola, M.; Giagounidis, A.; Perry, J.; Malcovati, L.; Della Porta, M.G.; Jädersten, M.; Killick, S.; Verma, A.; Norbury, C.J.; et al. Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia 2010, 24, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Van Delft, M.F.; Wei, A.H.; Mason, K.D.; Vandenberg, C.J.; Chen, L.; Czabotar, P.E.; Willis, S.N.; Scott, C.L.; Day, C.; Cory, S.; et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006, 10, 389–399. [Google Scholar] [CrossRef]
- Konopleva, M.; Contractor, R.; Tsao, T.; Samudio, I.; Ruvolo, P.P.; Kitada, S.; Deng, X.; Zhai, D.; Shi, Y.-X.; Sneed, T.; et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 2006, 10, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.D.; Vandenberg, C.J.; Scott, C.L.; Wei, A.H.; Cory, S.; Huang, D.C.; Roberts, A.W. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc. Natl. Acad. Sci. USA 2008, 105, 17961–17966. [Google Scholar] [CrossRef]
- Hann, C.L.; Daniel, V.C.; Sugar, E.A.; Dobromilskaya, I.; Murphy, S.C.; Cope, L.; Lin, X.; Hierman, J.S.; Wilburn, D.L.; Watkins, D.N.; et al. Therapeutic Efficacy of ABT-737, a Selective Inhibitor of BCL-2, in Small Cell Lung Cancer. Cancer Res. 2008, 68, 2321–2328. [Google Scholar] [CrossRef]
- Andreu-Fernandez, V.; Genoves, A.; Messeguer, A.; Orzaez, M.; Sancho, M.; Perez-Paya, E. BH3-mimetics- and cisplatin-induced cell death proceeds through different pathways depending on the availability of death-related cellular components. PLoS ONE 2013, 8, e56881. [Google Scholar] [CrossRef]
- Gersuk, G.M.; Lee, J.W.; Beckham, C.A.; Anderson, J.; Deeg, H.J. Fas (CD95) receptor and Fas-ligand expression in bone marrow cells from patients with myelodysplastic syndrome. Blood 1996, 88, 1122–1123. [Google Scholar] [CrossRef]
- Hyde, R.K.; Kamikubo, Y.; Anderson, S.; Kirby, M.; Alemu, L.; Zhao, L.; Liu, P.P. Cbfb/Runx1 repression-independent blockage of differentiation and accumulation of Csf2rb-expressing cells by Cbfb-MYH11. Blood 2010, 115, 1433–1443. [Google Scholar] [CrossRef]
- Fleischman, A.G. ALDH marks leukemia stem cell. Blood 2012, 119, 3376–3377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rong, Y.P.; Bultynck, G.; Aromolaran, A.S.; Zhong, F.; Parys, J.B.; Smedt, D.H.; Mignery, G.A.; Roderick, H.L.; Bootman, M.T.; Distelhorst, C.W. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc. Natl. Acad. Sci. USA 2009, 106, 14397–14402. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kikuchi, J.; Nakamura, M.; Iwase, S.; Yamada, H.; Matsuda, M. Lineage-specific regulation of cell cycle control gene expression during haematopoietic cell differentiation. Br. J. Haematol. 2000, 110, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Shen, H.; Jiang, H.; Hu, D.; Wang, J.; Wu, X. External Qi of Yan Xin Qigong inhibits activation of Akt, Erk1/2 and NF-kB and induces cell cycle arrest and apoptosis in colorectal cancer cells. Cell Physiol. Biochem. 2013, 31, 113–122. [Google Scholar] [CrossRef]
- Janku, F.; Lee, J.J.; Tsimberidou, A.M.; Hong, D.S.; Naing, A.; Falchook, G.S.; Fu, S.; Luthra, R.; Garrido-Laguna, I.; Kurzrock, R. PIK3CA Mutations Frequently Coexist with RAS and BRAF Mutations in Patients with Advanced Cancers. PLoS ONE 2011, 6, e22769. [Google Scholar] [CrossRef]
- Talab, F.; Allen, J.C.; Thompson, V.; Lin, K.; Slupsky, J.R. LCK is an important mediator of B-cell receptor signaling in chronic lymphocytic leukemia cells. Mol. Cancer Res. 2013, 11, 541–554. [Google Scholar] [CrossRef]
- Gandhi, L.; Camidge, D.R.; Ribeiro De Oliveira, M.M.R.; Bonomi, P.; Gandara, D.; Khaira, D.; Hann, C.L.; McKeegan, E.M.; Litvinovich, E.; Hemken, P.M.; et al. Phase I Study of Navitoclax (ABT-263), a Novel Bcl-2 Family Inhibitor, in Patients With Small-Cell Lung Cancer and Other Solid Tumors. J. Clin. Oncol. 2011, 29, 909–916. [Google Scholar] [CrossRef]
- Hallek, M.; Pflug, N. State of the art treatment of chronic lymphocytic leukaemia. Blood Rev. 2011, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef]
- Kallam, A.; Armitage, J.O. Venetoclax in chronic lymphocytic leukaemia: A possible cure? Lancet Oncol. 2018, 19, 1143–1144. [Google Scholar] [CrossRef]
- Valentin, R.; Grabow, S.; Davids, M.S. The rise of apoptosis: Targeting apoptosis in hematologic malignancies. Blood 2018, 132, 1248–1264. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Pratz, K.W.; Letai, A.; Jonas, B.; Wei, A.H.; Thirman, M.; Arellano, M.; Frattini, M.G.; Kantarjian, H.; Popovic, R.; et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: A non-randomised, open-label, phase 1b study. Lancet Oncol. 2018, 19, 216–228. [Google Scholar] [CrossRef]
- Hennighausen, L.; Wall, R.J.; Tillmann, U.; Li, M.; Furth, P.A. Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. J. Cell Biochem. 1995, 59, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Kogan, S.C.; Ward, J.M.; Anver, M.R.; Berman, J.J.; Brayton, C.; Cardiff, R.D.; Carter, J.S.; de Coronado, S.; Downing, J.R.; Fredrickson, T.N.; et al. Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood 2002, 100, 238–245. [Google Scholar] [CrossRef]
- Guerenne, L.; Beurlet, S.; Said, M.; Gorombei, P.; Le Pogam, C.; Guidez, F.; De La Grange, P.; Omidvar, N.; Vanneaux, V.; Mills, K.; et al. GEP analysis validates high risk MDS and acute myeloid leukemia post MDS mice models and highlights novel dysregulated pathways. J. Hematol. Oncol. 2016, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Novo, D.; Perlmutter, N.G.; Hunt, R.H.; Shapiro, H.M. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 1999, 35, 55–63. [Google Scholar] [CrossRef]
- De la Grange, P.; Gratadou, L.; Delord, M.; Dutertre, M.; Auboeuf, D. Splicing factor and exon profiling across human tissues. Nucleic. Acids. Res. 2010, 38, 2825–2838. [Google Scholar] [CrossRef] [PubMed]
- De la Grange, P.; Dutertre, M.; Martin, N.; Auboeuf, D. FAST DB: A website resource for the study of the expression regulation of human gene products. Nucleic. Acids. Res. 2005, 33, 4276–4284. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic. Acids. Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- High, L.M.; Szymanska, B.; Wilczynska-Kalak, U.; Barber, N.; O’Brien, R.; Khaw, S.L.; Vikstrom, I.B.; Roberts, A.W.; Lock, R.B. The Bcl-2 Homology Domain 3 Mimetic ABT-737 Targets the Apoptotic Machinery in Acute Lymphoblastic Leukemia Resulting in Synergistic in Vitro and in Vivo Interactions with Established Drugs. Mol. Pharmacol. 2009, 77, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Olberding, K.E.; Wang, X.; Zhu, Y.; Pan, J.; Rai, S.N.; Li, C. Actinomycin D synergistically enhances the efficacy of the BH3 mimetic ABT-737 by downregulating Mcl-1 expression. Cancer Biol. Ther. 2010, 10, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, T.-Y.; Zhu, H.; Yang, L.-Q.; Jiang, H.; Dong, X.-W.; Hu, Y.-Z.; Lin, N.-M.; He, Q.-J.; Yang, B. Synergistic Antitumor Activity of Gemcitabine and ABT-737 In Vitro and In Vivo through Disrupting the Interaction of USP9X and Mcl-1. Mol. Cancer Ther. 2011, 10, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.A.; Goldstein, N.; Johannes, W.U.; Walton, C.H.; Fujita, M.; Norris, D.A.; Shellman, Y.G. BH3 Mimetic ABT-737 and a Proteasome Inhibitor Synergistically Kill Melanomas through Noxa-Dependent Apoptosis. J. Investig. Dermatol. 2009, 129, 964–971. [Google Scholar] [CrossRef]
- Zhang, W.; Konopleva, M.; Ruvolo, V.R.; McQueen, T.; Evans, R.L.; Bornmann, W.G.; McCubrey, J.; Cortes, J.; Andreeff, M. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia 2008, 22, 808–818. [Google Scholar] [CrossRef]
- Konopleva, M.; Letai, A. BCL-2 inhibition in AML: An unexpected bonus? Blood 2018, 132, 1007–1012. [Google Scholar] [CrossRef]





| Apoptosis related genes regulated | |||
|---|---|---|---|
| Gene symbol | Regulation | Fold change | p value |
| Sgk1 | up | 1.67 | 2.05 × 10−2 |
| E2f2 | up | 2.06 | 2.12 × 10−2 |
| Osm | up | 2.26 | 3.18 × 10−2 |
| Rybp | up | 1.85 | 3.48 × 10−2 |
| Ripk3 | up | 1.68 | 3.62 × 10−2 |
| Rnf130 | up | 1.57 | 4.72 × 10−3 |
| Gpx1 | up | 1.76 | 7.96 × 10−3 |
| Bcl2l12 | up | 1.79 | 1.63 × 10−2 |
| App | up | 2.46 | 3.79 × 10−2 |
| Tnfrsf21 | up | 2.02 | 4.42 × 10−2 |
| Pdcd5 | up | 1.65 | 1.82 × 10−2 |
| Apaf1 | up | 1.62 | 3.56 × 10−2 |
| Mfsd10 | up | 1.53 | 1.33 × 10−2 |
| Pdcl3 | up | 1.50 | 2.26 × 10−3 |
| Ckap2 | up | 1.91 | 2.50 × 10−2 |
| Casp1 | up | 1.77 | 2.25 × 10−3 |
| Bcl2a1a | up | 2.00 | 2.85 × 10−2 |
| G2e3 | up | 1.89 | 1.74 × 10−3 |
| Birc5 | up | 2.45 | 2.10 × 10−2 |
| C1d | up | 1.52 | 1.07 × 10−2 |
| Sgpl1 | up | 1.56 | 3.36 × 10−2 |
| Bak1 | up | 1.97 | 1.49 × 10−2 |
| Hipk2 | up | 1.51 | 3.38 × 10−2 |
| Naip2 | up | 1.81 | 2.77 × 10−3 |
| Rnf144b | up | 1.51 | 1.28 × 10−2 |
| Pten | up | 1.98 | 4.07 × 10−3 |
| Csf2rb | up | 2.48 | 1.18 × 10−2 |
| Pik3cg | up | 1.62 | 3.48 × 10−3 |
| Chp1 | up | 1.85 | 5.80 × × 10−4 |
| Csf2rb2 | up | 1.73 | 2.07 × 10−2 |
| Cxcr2 | up | 4.98 | 1.37 × 10−2 |
| Wwox | down | 1.60 | 5.05 × 10−3 |
| Eif2ak3 | down | 1.54 | 9.38 × 10−3 |
| Fasl | down | 2.33 | 3.27 × 10−2 |
| Lck | down | 1.68 | 3.46 × 10−2 |
| Dyrk2 | down | 1.63 | 8.96 × × 10−4 |
| Trib3 | down | 1.51 | 1.59 × 10−2 |
| Sod1 | down | 1.54 | 4.83 × × 10−4 |
| Fas | down | 1.54 | 2.01 × 10−2 |
| Csrnp2 | down | 1.7 | 2.16 × 10−2 |
| Splice related genes regulated | |||
| Gene Symbol | Regulation | Fold-Change | p-Value |
| Rsrc1 | up | 1.55 | 3.59 × 10−2 |
| Gemin6 | up | 1.97 | 2.50 × 10−2 |
| Gemin7 | up | 1.66 | 5.08 × 10−3 |
| Lgals3 | up | 4.29 | 1.75 × 10−2 |
| Lsm6 | up | 1.62 | 3.69 × 10−2 |
| Zrsr2 | up | 1.71 | 4.37 × 10−2 |
| Snrnp27 | up | 1.59 | 3.39 × 10−2 |
| Snrpd2 | up | 1.76 | 4.59 × 10−2 |
| Lsm10 | up | 1.90 | 1.28 × 10−3 |
| Wbp4 | up | 1.85 | 1.67 × 10−2 |
| Isy1 | down | 1.62 | 3.51 × 10−2 |
| Pnn | down | 1.91 | 1.19 × 10−2 |
| Prpf38b | down | 1.54 | 1.56 × 10−2 |
| Rbm20 | down | 1.73 | 1.36 × 10−2 |
| Rbfox1 | down | 1.50 | 6.72 × 10−3 |
| Snrnp48 | down | 1.50 | 3.85 × 10−2 |
| Tut1 | down | 1.73 | 2.19 × 10−2 |
| Cell cycle related genes regulated | |||
| Gene symbol | Regulation | Fold change | p value |
| Cdk2 | up | 1.72 | 1.10 × 10−2 |
| Ccne2 | up | 1.76 | 2.18 × 10−2 |
| Cdc6 | up | 1.51 | 4.07 × 10−2 |
| E2f2 | up | 2.06 | 2.12 × 10−2 |
| Mapk13 | up | 3.06 | 3.22 × 10−2 |
| Ccnb2 | up | 2.25 | 3.62 × 10−2 |
| Cdca8 | up | 1.90 | 4.16 × 10−2 |
| Ccna2 | up | 2.51 | 2.04 × 10−2 |
| Cdk1 | up | 2.27 | 9.20 × 10−3 |
| Cdkn2d | up | 2.01 | 5.12 × 10−3 |
| Cdkn2c | up | 2.08 | 1.20 × 10−2 |
| Ccnd3 | up | 1.88 | 2.09 × 10−2 |
| Cdca3 | up | 2.24 | 1.91 × 10−2 |
| Cdc25a | up | 1.92 | 1.95 × 10−2 |
| Cdkn3 | up | 2.43 | 2.91 × 10−2 |
| Mapk3 | up | 1.89 | 2.36 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorombei, P.; Guidez, F.; Ganesan, S.; Chiquet, M.; Pellagatti, A.; Goursaud, L.; Tekin, N.; Beurlet, S.; Patel, S.; Guerenne, L.; et al. BCL-2 Inhibitor ABT-737 Effectively Targets Leukemia-Initiating Cells with Differential Regulation of Relevant Genes Leading to Extended Survival in a NRAS/BCL-2 Mouse Model of High Risk-Myelodysplastic Syndrome. Int. J. Mol. Sci. 2021, 22, 10658. https://doi.org/10.3390/ijms221910658
Gorombei P, Guidez F, Ganesan S, Chiquet M, Pellagatti A, Goursaud L, Tekin N, Beurlet S, Patel S, Guerenne L, et al. BCL-2 Inhibitor ABT-737 Effectively Targets Leukemia-Initiating Cells with Differential Regulation of Relevant Genes Leading to Extended Survival in a NRAS/BCL-2 Mouse Model of High Risk-Myelodysplastic Syndrome. International Journal of Molecular Sciences. 2021; 22(19):10658. https://doi.org/10.3390/ijms221910658
Chicago/Turabian StyleGorombei, Petra, Fabien Guidez, Saravanan Ganesan, Mathieu Chiquet, Andrea Pellagatti, Laure Goursaud, Nilgun Tekin, Stephanie Beurlet, Satyananda Patel, Laura Guerenne, and et al. 2021. "BCL-2 Inhibitor ABT-737 Effectively Targets Leukemia-Initiating Cells with Differential Regulation of Relevant Genes Leading to Extended Survival in a NRAS/BCL-2 Mouse Model of High Risk-Myelodysplastic Syndrome" International Journal of Molecular Sciences 22, no. 19: 10658. https://doi.org/10.3390/ijms221910658
APA StyleGorombei, P., Guidez, F., Ganesan, S., Chiquet, M., Pellagatti, A., Goursaud, L., Tekin, N., Beurlet, S., Patel, S., Guerenne, L., Le Pogam, C., Setterblad, N., de la Grange, P., LeBoeuf, C., Janin, A., Noguera, M.-E., Sarda-Mantel, L., Merlet, P., Boultwood, J., ... Padua, R. A. (2021). BCL-2 Inhibitor ABT-737 Effectively Targets Leukemia-Initiating Cells with Differential Regulation of Relevant Genes Leading to Extended Survival in a NRAS/BCL-2 Mouse Model of High Risk-Myelodysplastic Syndrome. International Journal of Molecular Sciences, 22(19), 10658. https://doi.org/10.3390/ijms221910658






