An Exon Skipping in CRS1 Is Associated with Perturbed Chloroplast Development in Maize
Abstract
:1. Introduction
2. Results
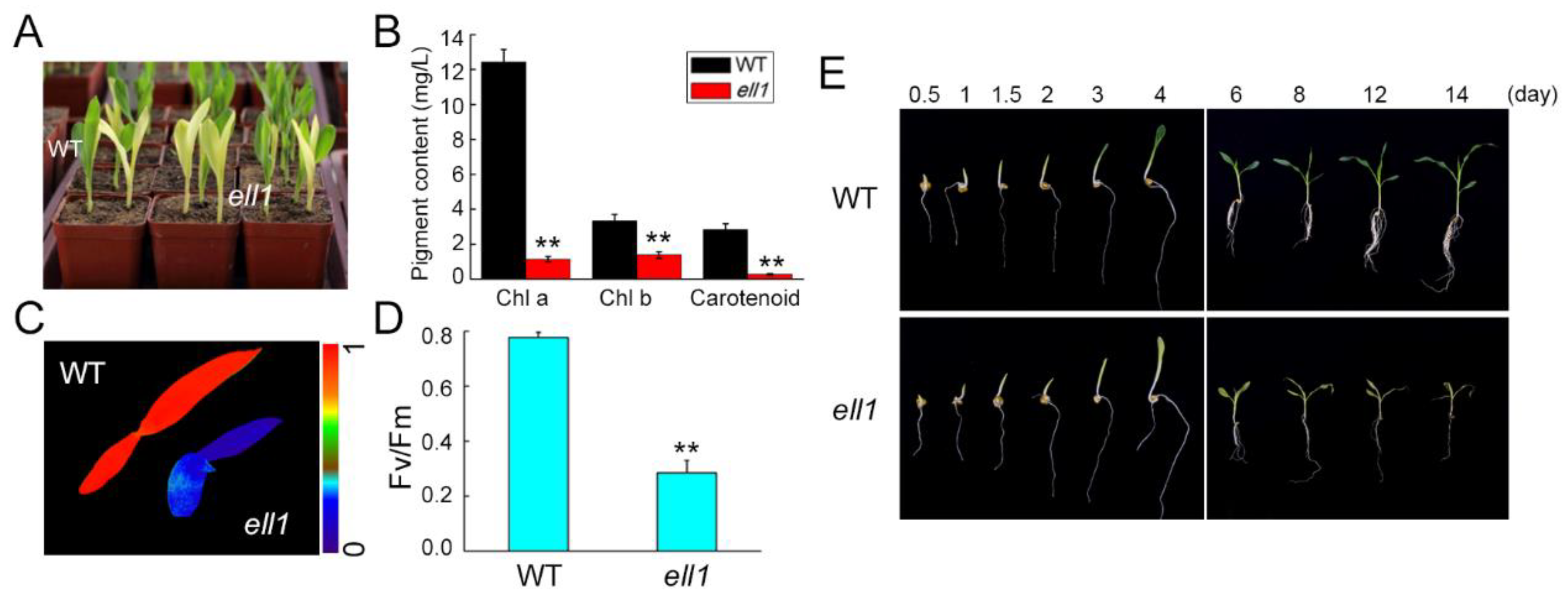
2.1. Identification and Characterization of the ell1 Mutant
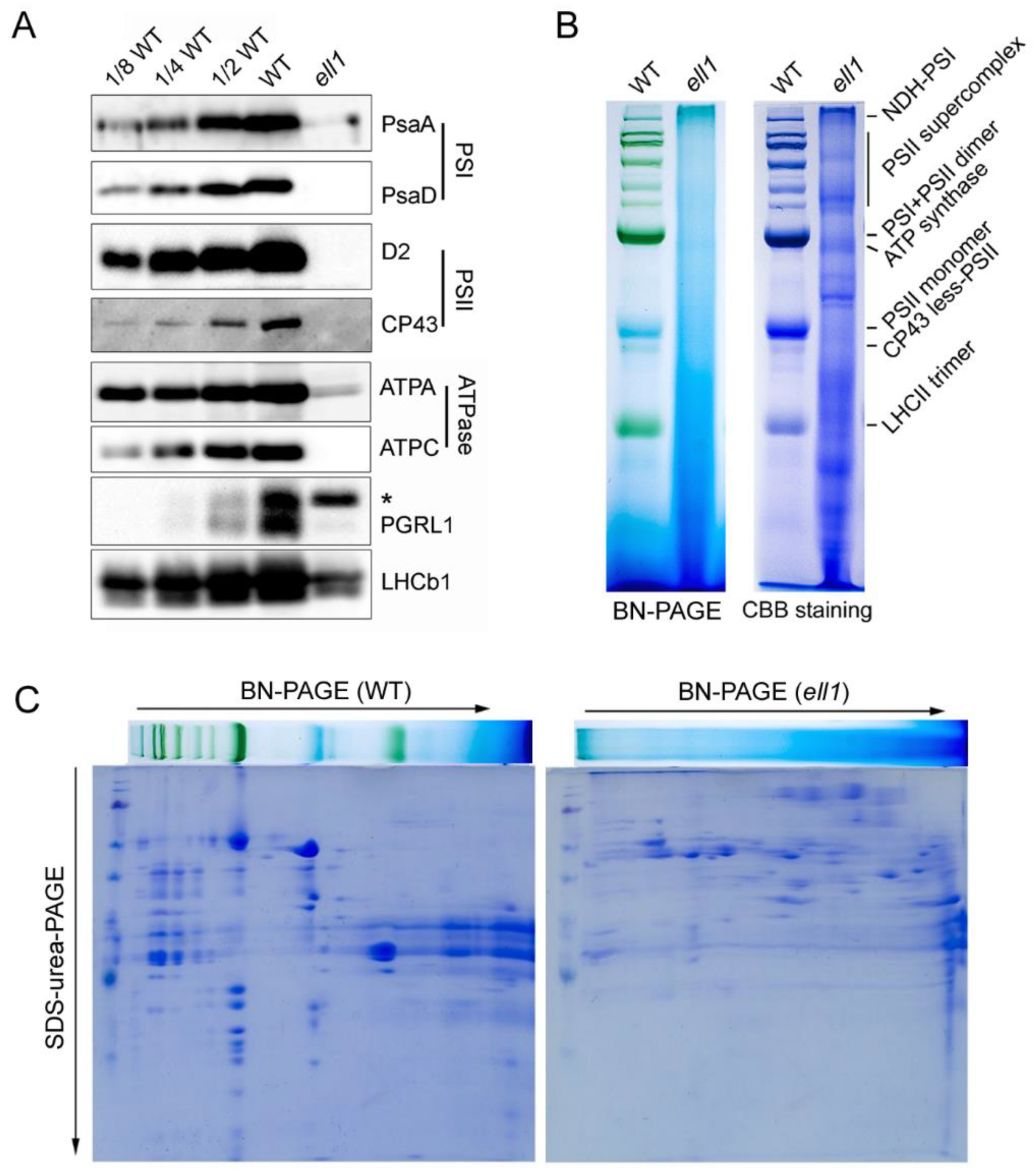
2.2. Accumulation of Photosynthetic Complexes Is Severely Impaired in the ell1 Mutant
2.3. Drastic Morphological Defects in ell1 Mutant Chloroplasts
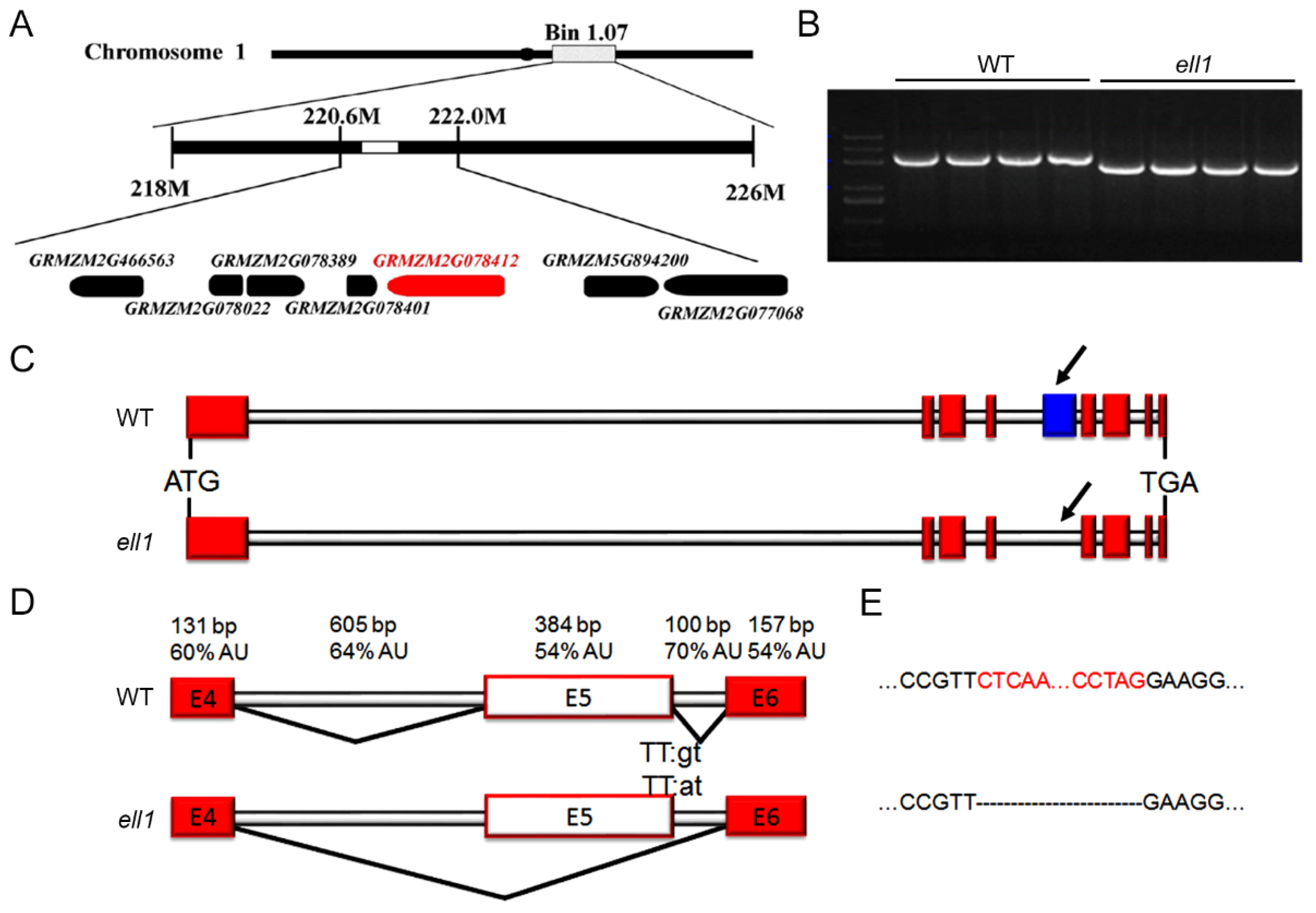
2.4. Map-Based Cloning of ZmELL1 Gene
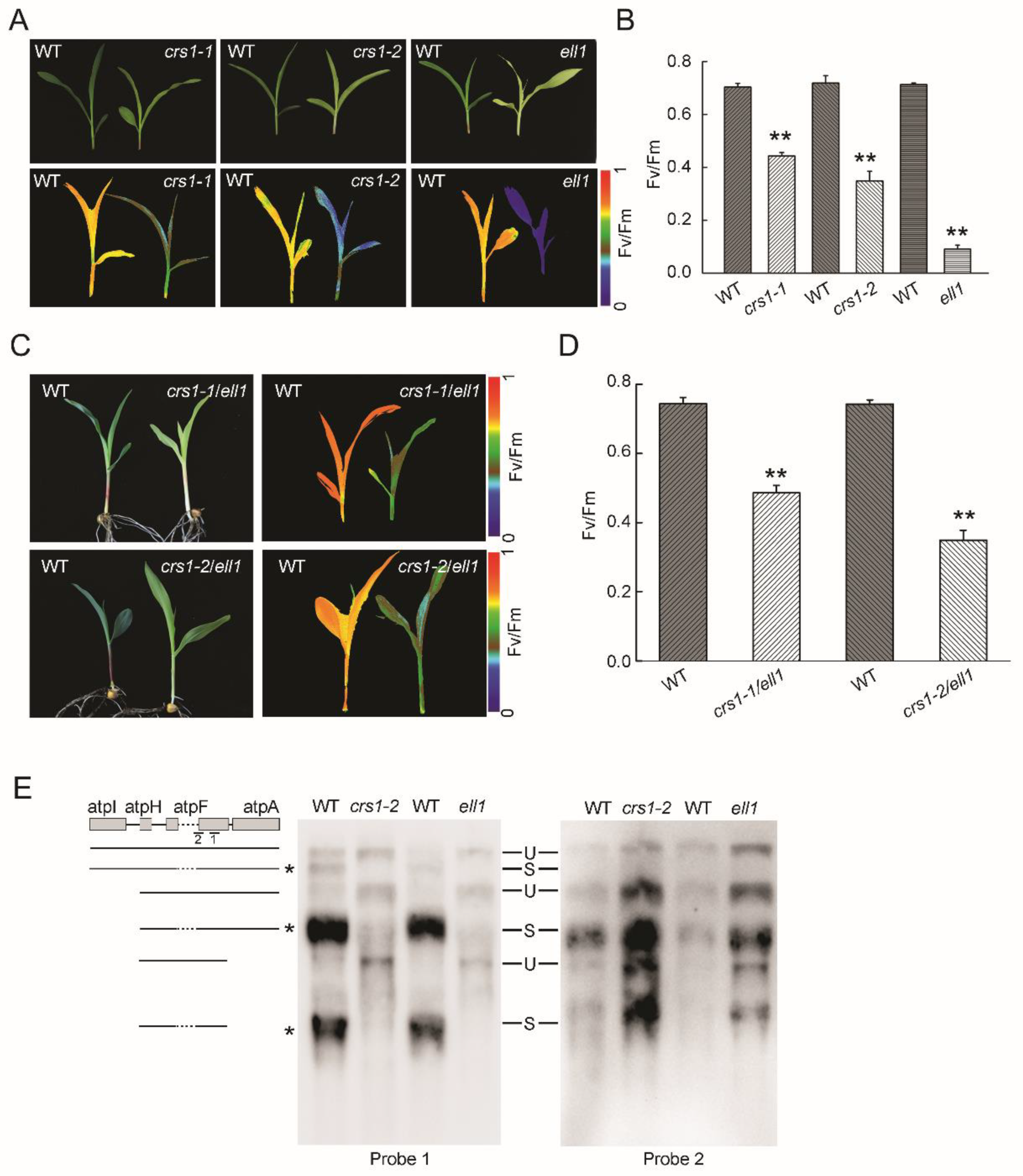
2.5. The Incorrectly Spliced CRS1 Is Responsible for Phenotypes of ell1 Mutant
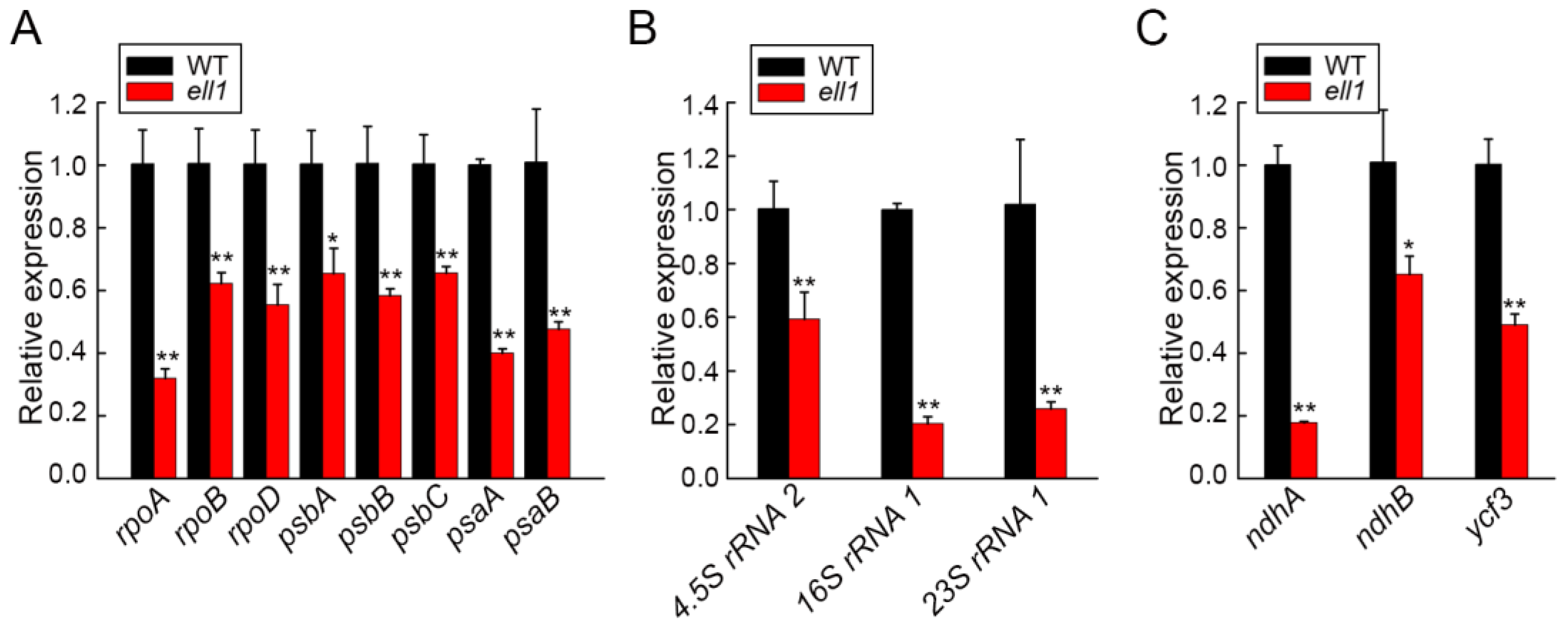
2.6. Chloroplast Transcription Is Affected in the ell1 Mutant
3. Discussion
3.1. ZmCRS1 Is Required for Chloroplast Development in Maize
3.2. Exon Skipping Induced Incorrect Splicing of CRS1 Is Responsible for ell1 Phenotypes
3.3. ZmCRS1 Participates in Chloroplast Transcription to Regulate Chloroplast Development
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Photosynthetic Pigments Measurements
4.3. Chlorophyll Fluorescence Measurements
4.4. Blue-Native PAGE and Immunoblot Analysis
4.5. Transmission Electron Microscopy
4.6. Nucleic Acid Analysis
4.7. Marker Development and Fine Mapping
4.8. Northern-Blot Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, N.; Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 2004, 5, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.; Lopez-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 2013, 14, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Chloroplast research in the genomic age. Trends Genet. 2003, 19, 47–56. [Google Scholar] [CrossRef]
- Li, H.-M.; Chiu, C.-C. Protein Transport into Chloroplasts. Annu. Rev. Plant Biol. 2010, 61, 157–180. [Google Scholar] [CrossRef]
- Yu, Q.B.; Huang, C.; Yang, Z.N. Nuclear-encoded factors associated with the chloroplast transcription machinery of higher plants. Front. Plant Sci. 2014, 5, 316. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Zhang, W.; Wen, K.; Chen, G.; Sun, J.; Tian, Y.; Tang, W.; Yu, J.; An, H.; Wu, T.; et al. OsPPR6, a pentatricopeptide repeat protein involved in editing and splicing chloroplast RNA, is required for chloroplast biogenesis in rice. Plant Mol. Biol. 2017, 95, 345–357. [Google Scholar] [CrossRef]
- Shiina, T.; Tsunoyama, Y.; Nakahira, Y.; Khan, M.S. Plastid RNA Polymerases, Promoters, and Transcription Regulators in Higher Plants. Int. Rev. Cytol. 2005, 244, 1–68. [Google Scholar] [CrossRef]
- Zubo, Y.O.; Kusnetsov, V.V.; Börner, T.; Liere, K. Reverse protection assay: A tool to analyze transcriptional rates from individual promoters. Plant Methods 2011, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Stern, D.B.; Goldschmidt-Clermont, M.P.; Hanson, M. Chloroplast RNA Metabolism. Annu. Rev. Plant Biol. 2010, 61, 125–155. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Y.; Wu, W.; Li, X.; Zhang, D.; Yin, P.; Huang, J. The Pentatricopeptide Repeat Protein SOT5/EMB2279 Is Required for Plastid rpl2 and trnK Intron Splicing. Plant Physiol. 2018, 177, 684–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Longevialle, A.F.; Small, I.D.; Lurin, C. Nuclearly encoded splicing factors implicated in RNA splicing in higher plant or-ganelles. Mol. Plant 2010, 3, 691–705. [Google Scholar] [CrossRef] [Green Version]
- Bonen, L.; Vogel, J. The ins and outs of group II introns. Trends Genet. 2001, 17, 322–331. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, H.; Xing, Y.; Tan, J.; Chen, X.; Zhang, J.; Peng, H.; Xie, Q.; Zhang, Z. Albino Leaf 2is involved in the splicing of chloroplast group I and II introns in rice. J. Exp. Bot. 2016, 67, 5339–5347. [Google Scholar] [CrossRef]
- Lehmann, K.; Schmidt, U. Group II Introns: Structure and Catalytic Versatility of Large Natural Ribozymes. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 249–303. [Google Scholar] [CrossRef]
- Lambowitz, A.M.; Zimmerly, S. Mobile Group II Introns. Annu. Rev. Genet. 2004, 38, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Bird, C.; Koller, B.; Auffret, A.; Huttly, A.; Howe, C.; Dyer, T.; Gray, J. The wheat chloroplast gene for CF0 subunit I of ATP synthase contains a large intron. EMBO J. 1985, 4, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, W.; Che, L.P.; Rochaix, J.D.; Lu, C.M.; Li, W.J.; Peng, L.W. PPR Protein BFA2 Is Essential for the Accumulation of the atpH/F Transcript in Chloroplasts. Front. Plant Sci. 2019, 10, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Till, B.; Schmitz-Linneweber, C.; Williams-Carrier, R.; Barken, A. CRS1 is a novel group II intron splicing factor that was de-rived from a domain of ancient origin. RNA 2001, 7, 1227–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostersetzer, O.; Cooke, A.M.; Watkins, K.P.; Barkan, A. CRS1, a Chloroplast Group II Intron Splicing Factor, Promotes Intron Folding through Specific Interactions with Two Intron Domains. Plant Cell 2005, 17, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonen, L. Cis- and trans-splicing of group II introns in plant mitochondria. Mitochondrion 2008, 8, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Staley, J.P.; Guthrie, C. Mechanical Devices of the Spliceosome: Motors, Clocks, Springs, and Things. Cell 1998, 92, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Wahl, M.C.; Will, C.L.; Lührmann, R. The Spliceosome: Design Principles of a Dynamic RNP Machine. Cell 2009, 136, 701–718. [Google Scholar] [CrossRef] [Green Version]
- Berget, S.M. Exon Recognition in Vertebrate Splicing. J. Biol. Chem. 1995, 270, 2411–2414. [Google Scholar] [CrossRef] [Green Version]
- Reed, R. Initial splice-site recognition and pairing during pre-mRNA splicing. Curr. Opin. Genet. Dev. 1996, 6, 215–220. [Google Scholar] [CrossRef]
- Simpson, C.G.; McQuade, C.; Lyon, J.; Brown, J.W. Characterization of exon skipping mutants of the COP1 gene from Ara-bidopsis. Plant J. 1998, 15, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gao, D.L.; Sun, J.J.; Liu, M.; Lun, Y.Y.; Zheng, J.S.; Wang, S.H.; Cui, Q.Z.; Wang, X.F.; Huang, S.W. An exon skipping in a SEPALLATA-Like gene is associated with perturbed floral and fruits development in cucumber. J. Integr. Plant Biol. 2016, 58, 766–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogson, B.; Albrecht, V. Genetic Dissection of Chloroplast Biogenesis and Development: An Overview. Plant Physiol. 2011, 155, 1545–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.L.; Wang, C.M.; Zheng, M.; Lyu, J.; Xu, Y.; Li, X.H.; Niu, M.; Long, W.H.; Wang, D.; Wang, H.Y.; et al. WHITE PANICLE1, a Val-tRNA synthetase regulating chloroplast ribosome biogenesis in rice, is essential for early chloroplast development. Plant Physiol. 2016, 170, 2110–2123. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.; Klipcan, L.; Ostersetzer, O.; Kawamura, T.; Asakura, Y.; Watkins, K.P. The CRM domain: An RNA binding module derived from an ancient ribosome-associated protein. RNA 2006, 13, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Pu, H.; Duan, Z.; Li, Y.; Liu, B.; Zhang, Q.; Li, W.; Rochaix, J.-D.; Liu, L.; Peng, L. Nucleus-Encoded Protein BFA1 Promotes Efficient Assembly of the Chloroplast ATP Synthase Coupling Factor 1. Plant Cell 2018, 30, 1770–1788. [Google Scholar] [CrossRef] [Green Version]
- Asakura, Y.; Barkan, A. Arabidopsis Orthologs of Maize Chloroplast Splicing Factors Promote Splicing of Orthologous and Species-Specific Group II Introns. Plant Physiol. 2006, 142, 1656–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakura, Y.; Barkan, A. A CRM domain protein functions dually in group I and group II intron splicing in land plant chlo-roplasts. Plant Cell 2007, 19, 3864–3875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectros-copy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, H.; Wang, H.; Li, N.; Wang, D.; Song, Y.; Miao, Y.; Song, C. Generation of the genetic mutant population for the screening and characterization of the mutants in response to drought in maize. Chin. Sci. Bull. 2014, 59, 766–775. [Google Scholar] [CrossRef]
- Duan, Z.; Kong, F.; Zhang, L.; Li, W.; Zhang, J.; Peng, L. A bestrophin-like protein modulates the proton motive force across the thylakoid membrane in Arabidopsis. J. Integr. Plant Biol. 2016, 58, 848–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.-C.; Chen, Z.; Shen, Y.; Zhang, Y.; Lai, J.; Sun, S.S.M. Identification of an Active New Mutator Transposable Element in Maize. G3 Genes Genome Genet. 2011, 1, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−Δ∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, Y.; Subhash, C.; Yan, J.; Song, C.; Zhao, J.; Li, J. Maize leaf temperature responses to drought: Thermal imaging and quantitative trait loci (QTL) mapping. Environ. Exp. Bot. 2011, 71, 158–165. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, Z.; Zhang, J.; Peng, L. Biogenesis Factor Required for Atp Synthase 3 Facilitates Assembly of the Chloroplast ATP Synthase Complex. Plant Physiol. 2016, 171, 1291–1306. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, K.; Li, Y.; Mi, L.; Hu, Z.; Guo, S.; Song, C.-P.; Duan, Z. An Exon Skipping in CRS1 Is Associated with Perturbed Chloroplast Development in Maize. Int. J. Mol. Sci. 2021, 22, 10668. https://doi.org/10.3390/ijms221910668
Wang M, Li K, Li Y, Mi L, Hu Z, Guo S, Song C-P, Duan Z. An Exon Skipping in CRS1 Is Associated with Perturbed Chloroplast Development in Maize. International Journal of Molecular Sciences. 2021; 22(19):10668. https://doi.org/10.3390/ijms221910668
Chicago/Turabian StyleWang, Mao, Kaiwen Li, Yang Li, Lingyu Mi, Zhubing Hu, Siyi Guo, Chun-Peng Song, and Zhikun Duan. 2021. "An Exon Skipping in CRS1 Is Associated with Perturbed Chloroplast Development in Maize" International Journal of Molecular Sciences 22, no. 19: 10668. https://doi.org/10.3390/ijms221910668





