Bisphenol A Removal by the Fungus Myrothecium roridumIM 6482—Analysis of the Cellular and Subcellular Level
Abstract
:1. Introduction
2. Results and Discussion
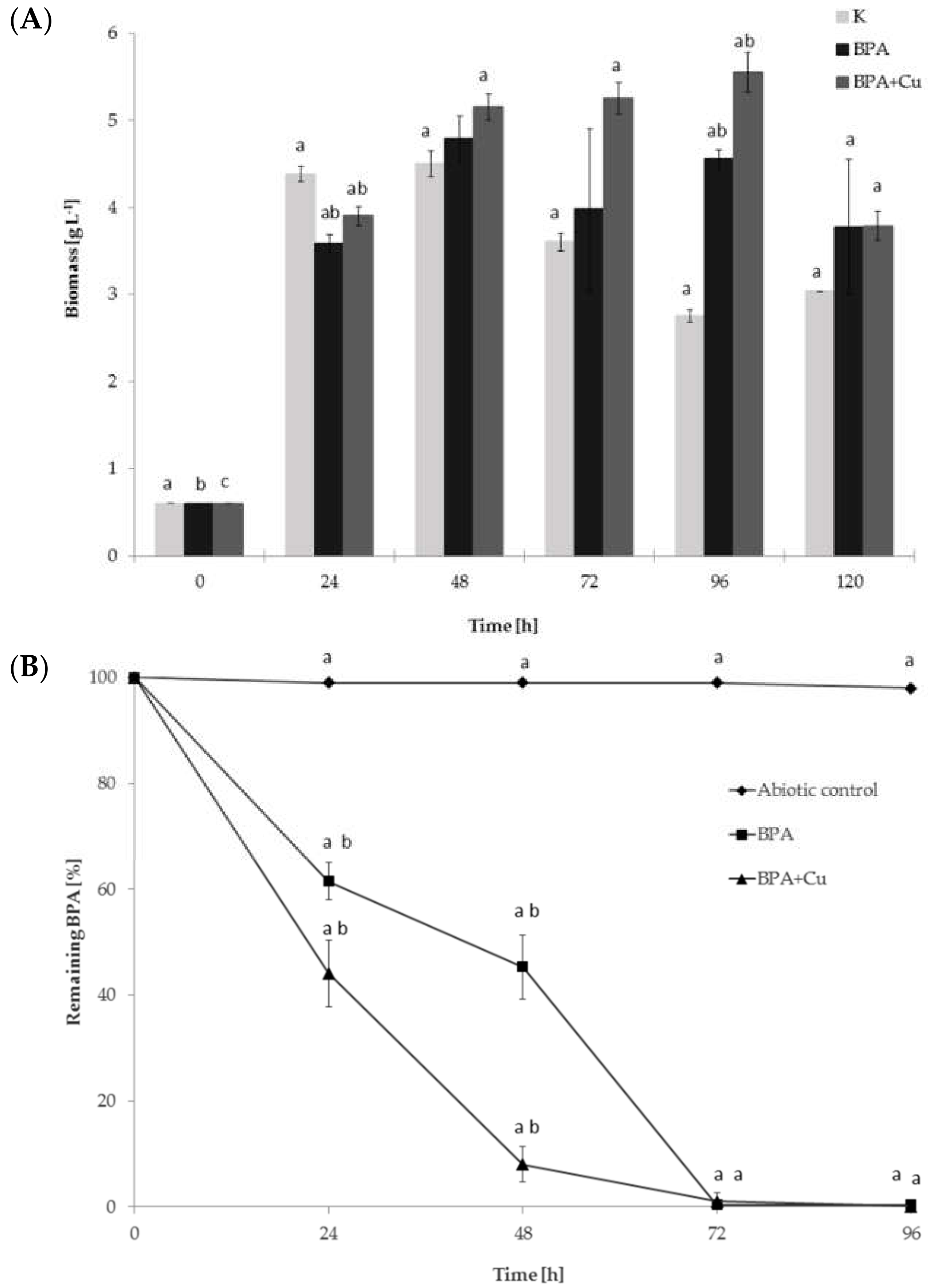
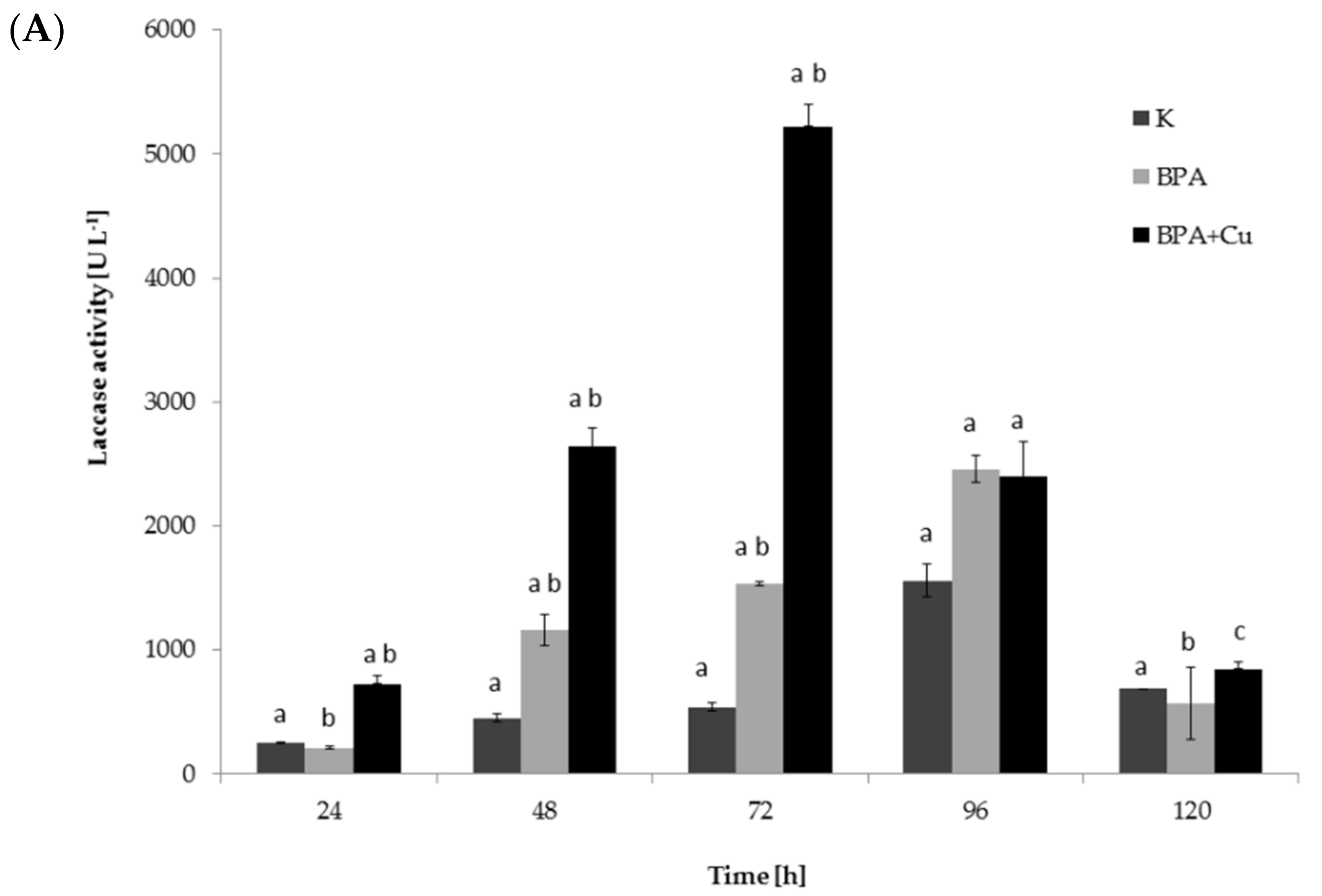
2.1. Fungal Growth, BPA Biodegradation and Laccase Involvement
2.2. Identification of the Intermediates of BPA Transformation
2.3. Analysis of Cellular Response to BPA
2.3.1. Permeability of Fungal Membrane
2.3.2. ROS Production
2.3.3. Cellular Lipids Peroxidation
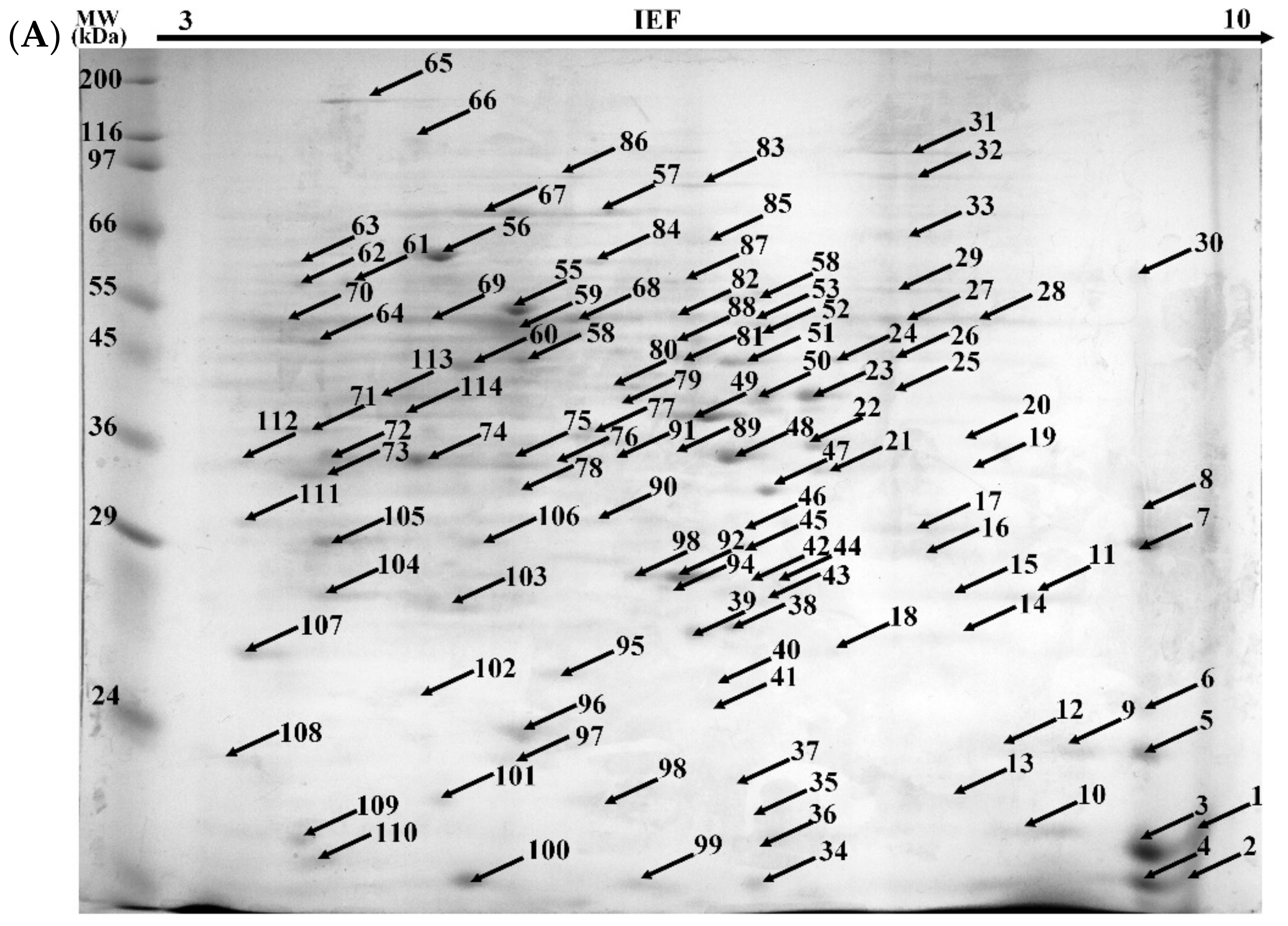
2.3.4. Proteomic Analysis
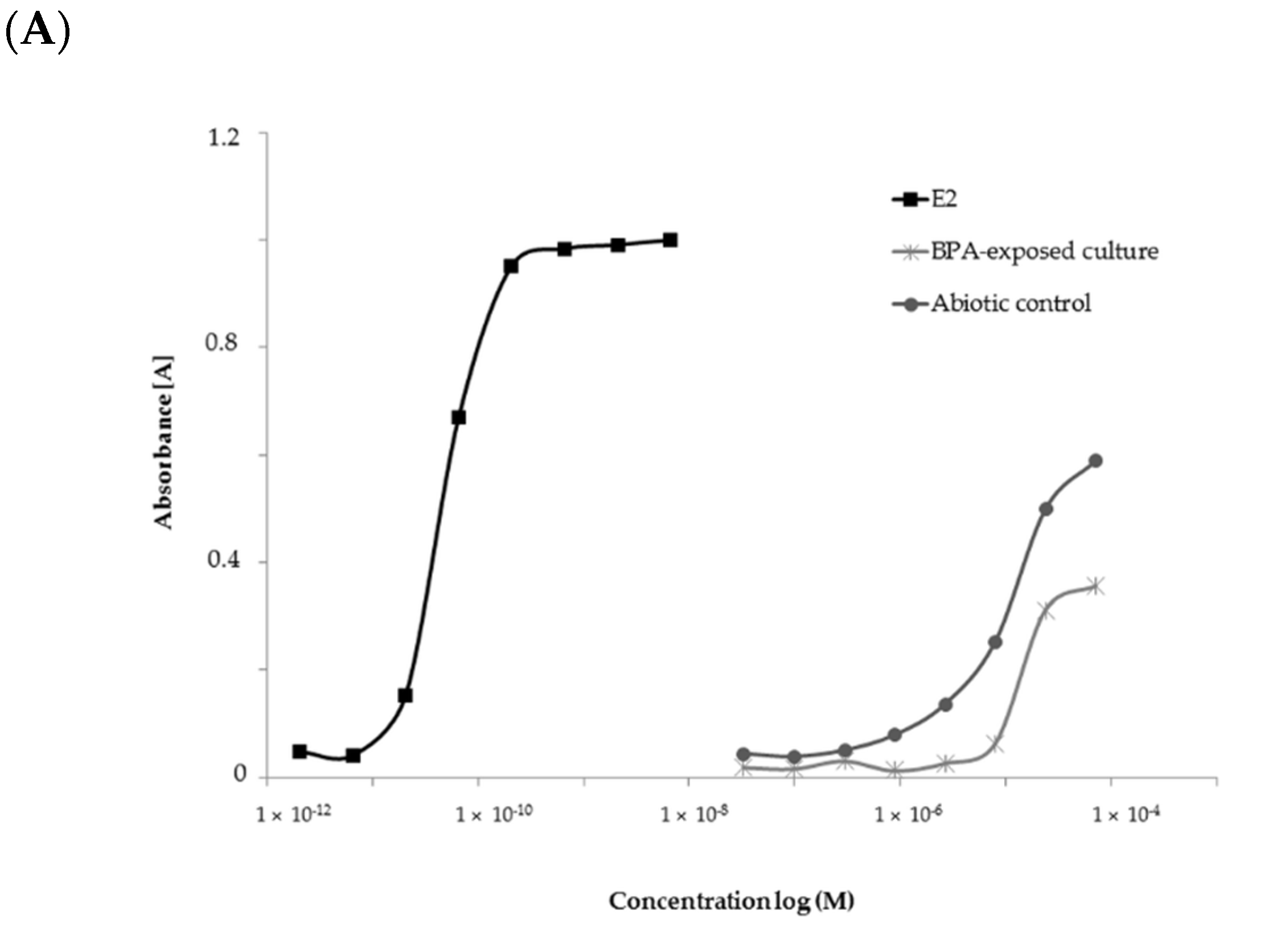
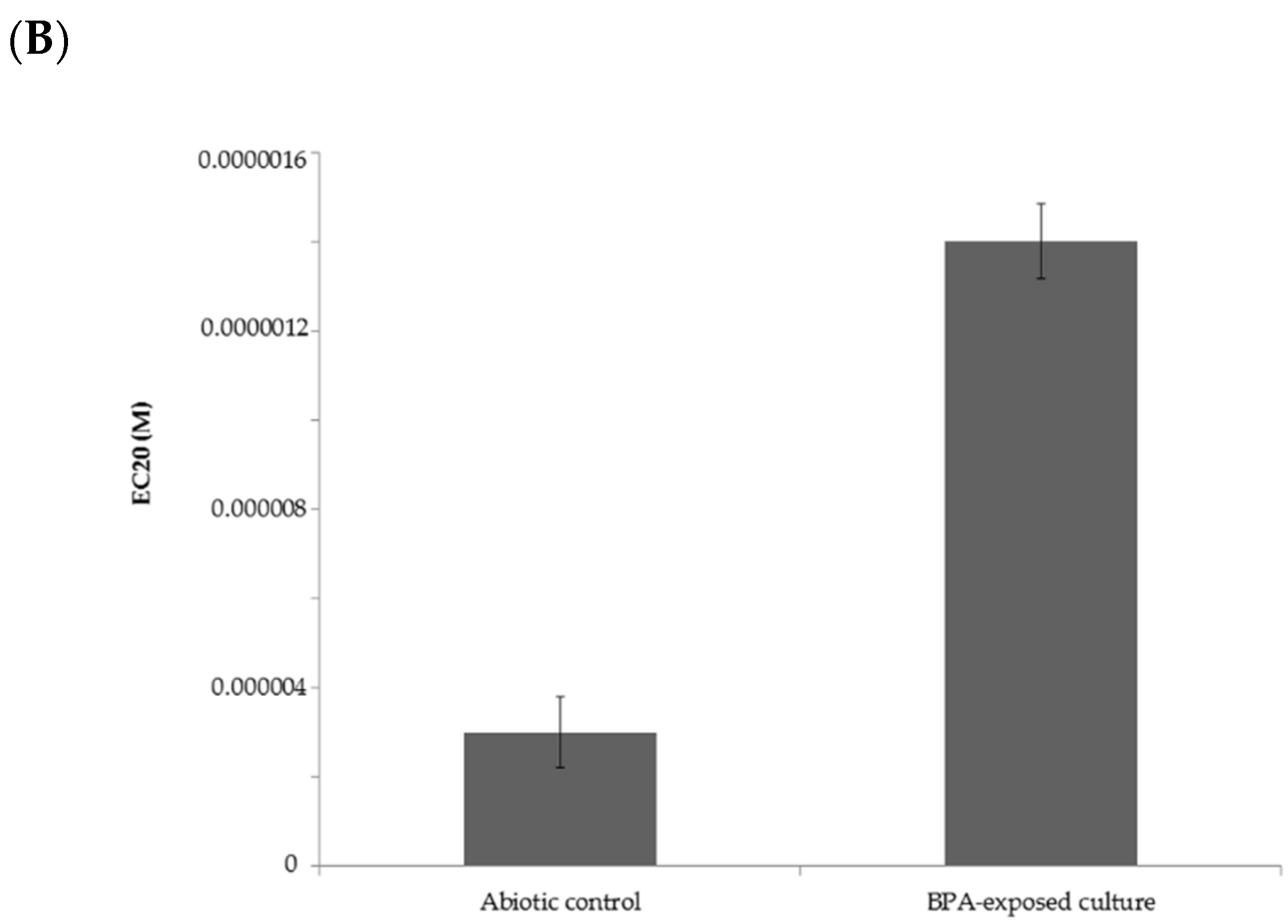
2.4. Estrogenic Activity of BPA and Its Biotransformation Products
3. Materials and Methods
3.1. Chemicals and Microorganisms
3.2. Batch Experiments
3.3. Dry Weight and Laccase Activity Determination
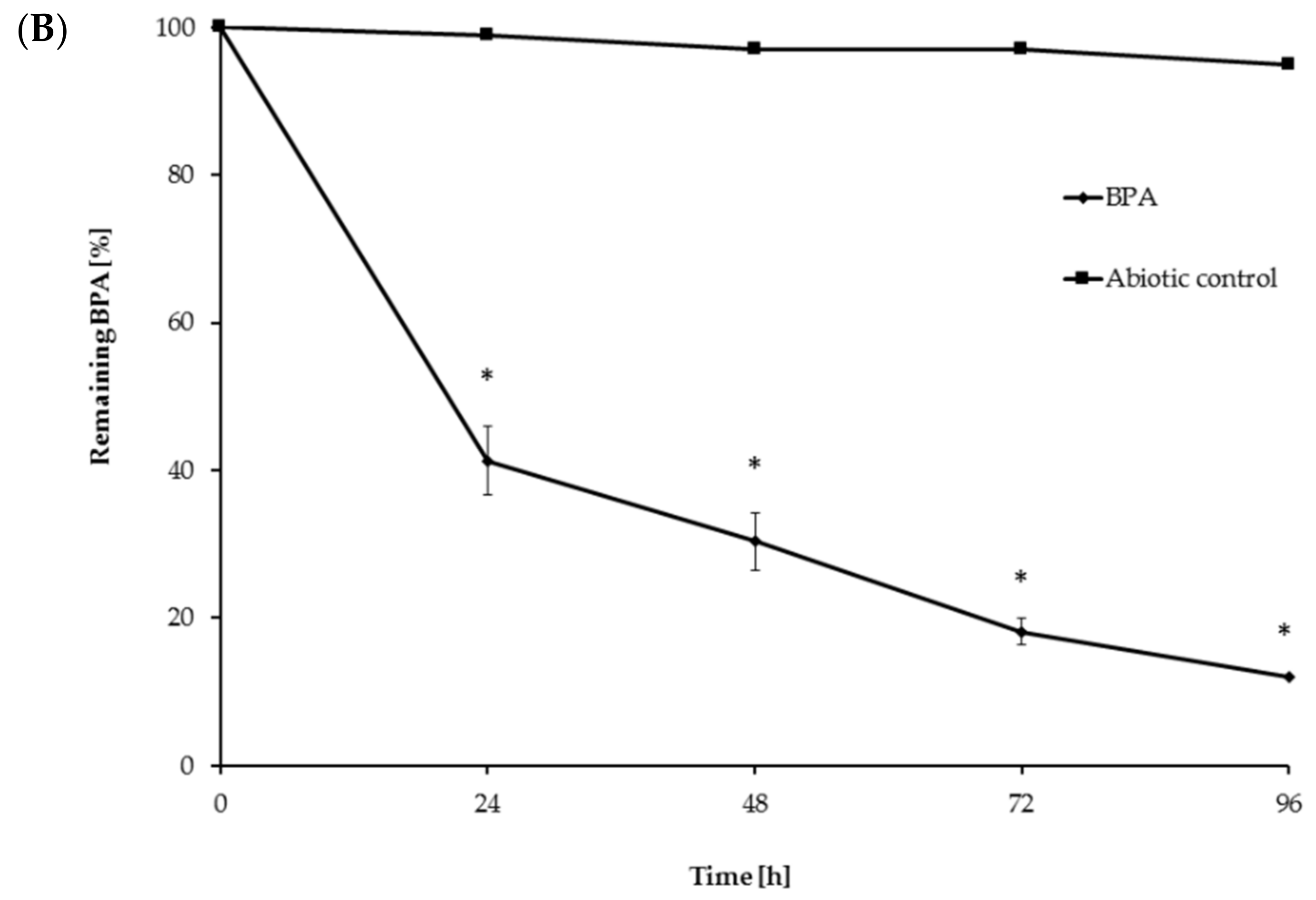
3.4. BPA Degradation Using Crude Laccase
3.5. Determination of BPA and Its Metabolites Using LC-MS/MS
3.6. Protein Extraction
3.7. 2-D Electrophoresis and Protein Digestion
3.8. MALDI-TOF/TOF Analysis, Protein Homology Identification
3.9. Lipid Peroxidation Assay
3.10. Membrane Permeability
3.11. ROS Measurement
3.12. Yeast Estrogen Bioassay
3.13. Data Acquisition and Statistical Analysis
4. Conclusions
- M. roridum IM 6482 is able to completely eliminate BPA from the growth environment within 72 h of incubation;
- the main biotransformation products are: hydroxylated derivatives, glucuronides and dimmers;
- BPA does not decrease biomass production by M. roridum IM 6482 but induces oxidative stress in fungal cells, which is manifested by increased membrane permeability, peroxidation of lipids, overproduction of ROS and oxidative stress enzymes. Oxidative stress is eliminated after BPA degradation;
- products of BPA biodegradation by M. roridum IM 6482 have lower estrogenic activity compared to the parent compound.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, S.; Tschudin, P.; Grob, K. Transfer of bisphenol A from thermal printer paper to the skin. Anal. Bioanal. Chem. 2010, 398, 571–576. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Mileva, G.; Baker, S.L.; Konkle, A.T.M.; Bielajew, C. Bisphenol-A: Epigenetic Reprogramming and Effects on Reproduction and Behavior. Int. J. Environ. Res. Public Health 2014, 11, 7537–7561. [Google Scholar] [CrossRef]
- Industry Experts. Bisphenol A—A Global Market Overview. 2016. Available online: https://industry-experts.com/verticals/chemicals-and-materials/bisphenol-a-a-global-market-overview (accessed on 3 September 2021).
- Nepalia, A.; Singh, A.; Mathur, N.; Kamath, R.; Pareek, S. Assessment of mutagenicity caused by popular baby foods and baby plastic-ware products: An imperative study using microbial bioassays and migration analysis. Ecotoxicol. Environ. Saf. 2018, 162, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Kwack, S.J.; Kim, K.B.; Kim, H.S.; Lee, B.M. Potential risk of bisphenol a migration from polycarbonate containers after heating, boiling, and microwaving. J. Toxicol. Environ. Health Part A 2009, 72, 1285–1291. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Park, J.; Moon, H.B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.J.; Eun, S.H.; Kim, G.H.; et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; de Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef]
- Lathi, R.B.; Liebert, C.; Brookfield, K.F.; Taylor, J.A.; Saal, F.S.V.; Fujimoto, V.Y.; Baker, V.L. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil. Steril. 2014, 102, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Yang, B.J.; Li, N.; Feng, L.M.; Shi, X.Y.; Zhao, W.H.; Liu, S.J. Bisphenol A and hormone-associated cancers: Current progress and perspectives. Medicine 2015, 94, e211. [Google Scholar] [CrossRef]
- Eve, L.; Fervers, B.; Le Romancer, M.; Etienne-Selloum, N. Exposure to endocrine disrupting chemicals and risk of breast cancer. Int. J. Mol. Sci. 2020, 21, 9139. [Google Scholar] [CrossRef]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential mechanisms of bisphenol A (BPA) contributing to human disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef] [PubMed]
- Erler, C.; Novak, J. Bisphenol A exposure: Human risk and health policy. J. Pediatr. Nurs. 2010, 25, 400–407. [Google Scholar] [CrossRef] [PubMed]
- European Comission. Comission Directive 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottlers. Off. J. Eur. Commun. 2011, 29–32. [Google Scholar]
- Commission regulation 2016/2235 of 12 December 2016 amending annex XVII to regulation (EC) No 1907/2006 of the European parliament and of the council concerning the registration, evaluation, authorisation and restriction of chemicals (REACH) as regards bisphenol A. Off. J. Eur. Union 2016, L337, 3–5.
- Santhi, V.A.; Sakai, N.; Ahmad, E.D.; Mustafa, A.M. Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci. Total Environ. 2012, 427–428, 332–338. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, C.; Zhu, L.; Liang, N.; Liu, X.; Jia, J.; Zhang, C.; Zhai, S.; Zhang, B. Adsorption of bisphenol A to a carbon nanotube reduced its endocrine disrupting effect in mice male offspring. Int. J. Mol. Sci. 2014, 15, 15981–15993. [Google Scholar] [CrossRef] [Green Version]
- Zahari, A.M.; Shuo, C.W.; Sathishkumar, P.; Yusoff, A.R.M.; Gu, F.L.; Buang, N.A.; Lau, W.J.; Gohari, R.J.; Yusop, Z. A reusable electrospun PVDF-PVP-MnO2 nanocomposite membrane for bisphenol A removal from drinking water. J. Environ. Chem. Eng. 2018, 6, 5801–5811. [Google Scholar] [CrossRef]
- Peng, Y.H.; Chen, Y.J.; Chang, Y.J.; Shih, Y.H. Biodegradation of bisphenol A with diverse microorganisms from river sediment. J. Hazard. Mater. 2015, 286, 285–290. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, A.; Torres-Sánchez, A.; Acuña, I.; Suárez, A.; Aguilera, M. Representative Bacillus sp. AM1 from gut microbiota harbor versatile molecular pathways for Bisphenol A biodegradation. Int. J. Mol. Sci. 2021, 22, 4952. [Google Scholar] [CrossRef]
- Xiong, J.; An, T.; Li, G.; Peng, P. Accelerated biodegradation of BPA in water-sediment microcosms with Bacillus sp. GZB and the associated bacterial community structure. Chemosphere 2017, 184, 120–126. [Google Scholar] [CrossRef]
- Jasińska, A.; Góralczyk, A.; Soboń, A.; Długoński, J. Novel laccase-like multicopper oxidases from the Myrothecium roridumfungus—Production enhancement, identification and application in the dye removal process. Acta Biochim. Pol. 2018, 65, 287–295. [Google Scholar] [CrossRef]
- Jasińska, A.; Góralczyk-Bińkowska, A.; Soboń, A.; Długoński, J. Lignocellulose resources for the Myrothecium roridum laccase production and their integrated application for dyes removal. Int J. Environ. Sci. Technol. 2019, 16, 4811–4822. [Google Scholar] [CrossRef] [Green Version]
- Jasińska, A.; Soboń, A.; Góralczyk-Bińkowska, A.; Długoński, J. Analysis of decolorization potential of Myrothecium roridum in the light of its secretome and toxicological studies. Environ. Sci. Pollut. Res. 2019, 26, 26313–26323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, S.; Chattopadhyay, P.; Pan, I.; Chatterjee, S.; Chanda, P.; Bandyopadhyay, D.; Das, K.; Sen, S.K. Microbial transformation of xenobiotics for environmental bioremediation. Afr. J. Biotechnol. 2009, 8, 6016–6027. [Google Scholar] [CrossRef]
- Babatabar, S.; Zamir, S.M.; Shojaosadati, S.A.; Yakhchali, B.; Zarch, A.B. Cometabolic degradation of bisphenol A by pure culture of Ralstonia eutropha and metabolic pathway analysis. J. Biosci. Bioeng. 2019, 127, 732–737. [Google Scholar] [CrossRef]
- Jia, Y.; Eltoukhy, A.; Wang, J.; Li, X.; Hlaing, T.S.; Aung, M.M.; Nwe, M.T.; Lamraoui, I.; Yan, Y. Biodegradation of bisphenol A by Sphingobium sp. YC-JY1 and the essential role of cytochrome P450 monooxygenase. Int. J. Mol. Sci. 2020, 21, 3588. [Google Scholar] [CrossRef]
- Conejo-Saucedo, U.; Ledezma-Villanueva, A.; de Paz, G.Á.; Herrero-Cervera, M.; Calvo, C.; Aranda, E. Evaluation of the potential of sewage sludge mycobiome to degrade high diclofenac and bisphenol-A concentrations. Toxics 2021, 9, 115. [Google Scholar] [CrossRef]
- Kyrila, G.; Katsoulas, A.; Schoretsaniti, V.; Rigopoulos, A.; Rizou, E.; Doulgeridou, S.; Sarli, V.; Samanidou, V.; Touraki, M. Bisphenol A removal and degradation pathways in microorganisms with probiotic properties. J. Hazard. Mater. 2021, 413, 125363. [Google Scholar] [CrossRef]
- De Freitas, E.N.; Bubna, G.A.; Brugnari, T.; Kato, C.G.; Nolli, M.; Rauen, T.G.; Moreira, R.d.F.P.M.; Peralta, R.A.; Bracht, A.; Souza, C.G.M.; et al. Removal of bisphenol A by laccases from Pleurotus ostreatus and Pleurotus pulmonarius and evaluation of ecotoxicity of degradation products. Chem. Eng. J. 2017, 330, 1361–1369. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.; Nejad, Z.G.; Ghasemi, S.; Khafaji, M.; Borghei, S.M. Removal of bisphenol A in aqueous solution using magnetic cross-linked laccase aggregates from Trametes hirsute. Bioresour. Technol. 2020, 306, 123169. [Google Scholar] [CrossRef]
- Mtibaà, R.; Olicón-Hernández, D.R.; Pozo, C.; Nasri, M.; Mechichi, T.; González, J.; Aranda, E. Degradation of bisphenol A and acute toxicity reduction by different thermo-tolerant ascomycete strains isolated from arid soils. Ecotoxicol. Environ. Saf. 2018, 156, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kato, K.; Yokogawa, Y.; Nishida, M.; Yamashita, N. Detoxification of bisphenol A and nonylphenol by purified extracellular laccase from a fungus isolated from soil. J. Biosci. Bioeng. 2004, 98, 64–66. [Google Scholar] [CrossRef]
- Daâssi, D.; Prieto, A.; Zouari-Mechichi, H.; Martínez, M.J.; Nasri, M.; Mechichi, T. Degradation of bisphenol A by different fungal laccases and identification of its degradation products. Int. Biodeterior. Biodegrad. 2016, 110, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Zhao, J.; Xia, L. Simultaneous production of laccase and degradation of bisphenol A with Trametes versicolor cultivated on agricultural wastes. Bioprocess Biosyst. Eng. 2017, 40, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Mokhtar, A.; Nishioka, T.; Matsumoto, H.; Kitada, S.; Ryuno, N.; Okobira, T. Novel biodegradation system for bisphenol A using laccase-immobilized hollow fiber membranes. Int. J. Biol. Macromol. 2019, 130, 737–744. [Google Scholar] [CrossRef]
- Fukuda, T.; Uchida, H.; Suzuki, M.; Miyamoto, H.; Morinaga, H.; Nawata, H.; Uwajima, T. Transformation products of bisphenol A by a recombinant Trametes villosa laccase and their estrogenic activity. J. Chem. Technol. Biotechnol. 2004, 79, 1212–1218. [Google Scholar] [CrossRef]
- Kabiersch, G.; Rajasärkkä, J.; Ullrich, R.; Tuomela, M.; Hofrichter, M.; Virta, M.; Hatakka, A.; Steffen, K. Fate of bisphenol A during treatment with the litter-decomposing fungi Stropharia rugosoannulata and Stropharia coronilla. Chemosphere 2011, 83, 226–232. [Google Scholar] [CrossRef]
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci. Total Environ. 2018, 615, 784–795. [Google Scholar] [CrossRef]
- Nicolaou, S.A.; Gaida, S.M.; Papoutsakis, E.T. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: From biofuels and chemicals, to biocatalysis and bioremediation. Metab. Eng. 2010, 12, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Heipieper, H.J.; Weber, F.J.; Sikkema, J.; Keweloh, H.; de Bont, J.A.M. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994, 12, 409–415. [Google Scholar] [CrossRef]
- Bernat, P.; Nykiel-Szymańska, J.; Stolarek, P.; Słaba, M.; Szewczyk, R.; Różalska, S. 2,4-dichlorophenoxyacetic acid-induced oxidative stress: Metabolome and membrane modifications in Umbelopsis isabellina, a herbicide degrader. PLoS ONE 2018, 13, e0199677. [Google Scholar] [CrossRef]
- Nowak, M.; Bernat, P.; Mrozińska, J.; Różalska, S. Acetamiprid affects destruxins production but its accumulation in Metarhizium sp. spores increases infection ability of fungi. Toxins 2020, 12, 587. [Google Scholar] [CrossRef]
- Szewczyk, R.; Różalska, S.; Mironenka, J.; Bernat, P. Atrazine biodegradation by mycoinsecticide Metarhizium robertsii: Insights into its amino acids and lipids profile. J. Environ. Manag. 2020, 262, 110304. [Google Scholar] [CrossRef]
- Słaba, M.; Szewczyk, R.; Piątek, M.A.; Długoński, J. Alachlor oxidation by the filamentous fungus Paecilomyces marquandii. J. Hazard. Mater. 2013, 261, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The fungicidal activity of thymol against Fusarium graminearum via Inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [Green Version]
- Rong, S.; Xu, H.; Li, L.; Chen, R.; Gao, X.; Xu, Z. Antifungal activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pestic. Biochem. Physiol. 2020, 162, 69–77. [Google Scholar] [CrossRef]
- Szewczyk, R.; Soboń, A.; Słaba, M.; Długoński, J. Mechanism study of alachlor biodegradation by Paecilomyces marquandii with proteomic and metabolomic methods. J. Hazard. Mater. 2015, 291, 52–64. [Google Scholar] [CrossRef]
- Soboń, A.; Szewczyk, R.; Długoński, J. Tributyltin (TBT) biodegradation induces oxidative stress of Cunninghamella echinulate. Int. Biodeterior. Biodegrad. 2016, 107, 92–101. [Google Scholar] [CrossRef]
- Nykiel-Szymańska, J.; Różalska, S.; Bernat, P.; Słaba, M. Assessment of oxidative stress and phospholipids alterations in chloroacetanilides-degrading Trichoderma spp. Ecotoxicol. Environ. Saf. 2019, 184, 109629. [Google Scholar] [CrossRef]
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagenes. 2017, 58, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Liu, B.; Farooq, M.A.; Islam, F.; Azizullah, A.; Yu, C.; Su, W.; Gan, Y. Toxicological effects of bisphenol A on growth and antioxidant defense system in Oryza sativa as revealed by ultrastructure analysis. Ecotoxicol. Environ. Saf. 2016, 124, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Maćczak, A.; Cyrkler, M.; Bukowska, B.; Michałowicz, J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol. In Vitro 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Meng, Z.; Tian, S.; Yan, J.; Jia, M.; Yan, S.; Li, R.; Zhang, R.; Zhu, W.; Zhou, Z. Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ. Pollut. 2019, 247, 935–943. [Google Scholar] [CrossRef]
- Szewczyk, R.; Soboń, A.; Różalska, S.; Dzitko, K.; Waidelich, D.; Długoński, J. Intracellular proteome expression during 4-n-nonylphenol biodegradation by the filamentous fungus Metarhizium robertsii. Int. Biodeterior. Biodegrad. 2014, 93, 44–53. [Google Scholar] [CrossRef]
- Vega-Morales, T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Evaluation of the presence of endocrine-disrupting compounds in dissolved and solid wastewater treatment plant samples of Gran Canaria Island (Spain). Biomed Res. Int. 2013, 2013, 790570. [Google Scholar] [CrossRef] [PubMed]
- Borgert, C.J.; Baker, S.P.; Matthews, J.C. Potency matters: Thresholds govern endocrine activity. Regul. Toxicol. Pharmacol. 2013, 67, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef]
- Gramec Skledar, D.; Peterlin Mašič, L. Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environ. Toxicol. Pharmacol. 2016, 47, 182–199. [Google Scholar] [CrossRef]
- Tapia-Orozco, N.; Meléndez-Saavedra, F.; Figueroa, M.; Gimeno, M.; García-Arrazola, R. Removal of bisphenol A in canned liquid food by enzyme-based nanocomposites. Appl. Nanosci. 2018, 8, 427–434. [Google Scholar] [CrossRef]
- Jasińska, A.; Różalska, S.; Bernat, P.; Paraszkiewicz, K.; Długoński, J. Malachite green decolorization by non-basidiomycete filamentous fungi of Penicillium pinophilum and Myrothecium roridum. Int. Biodeter. Biodegr. 2012, 73, 33–40. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Jasińska, A.; Długoński, A.; Płociński, P.; Długoński, J. Laccase activity of the ascomycete fungus Nectriella pironii and innovative strategies for its production on leaf litter of an urban park. PLoS ONE 2020, 15, e0231453. [Google Scholar] [CrossRef] [Green Version]
- Siewiera, P.; Bernat, P.; Różalska, S.; Długoński, J. Estradiol improves tributyltin degradation by the filamentous fungus Metarhizium robertsii. Int. Biodeter. Biodegr. 2015, 104, 258–263. [Google Scholar] [CrossRef]
- Słaba, M.; Różalska, S.; Bernat, P.; Szewczyk, R.; Piątek, M.A.; Długoński, J. Efficient alachlor degradation by the filamentous fungus Paecilomyces marquandii with simultaneous oxidative stress reduction. Bioresour. Technol. 2015, 197, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Routledge, E.; Sumpter, J. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ. Toxicol. Chem. 1996, 15, 241–248. [Google Scholar] [CrossRef]
- Roszko, M.; Kamińska, M.; Szymczyk, K.; Piasecka-Jóźwiak, K.; Chabłowska, B. Optimized yeast-based in vitro bioassay for determination of estrogenic and androgenic activity of hydroxylated/methoxylated metabolites of BDEs/CBs and related lipophilic organic pollutants. J. Environ. Sci. Health. B 2018, 53, 692–706. [Google Scholar] [CrossRef]

| ID | (M − H) −m/z | Rt (min) | Structure |
|---|---|---|---|
| M1 *• | 259 | 8.46 | 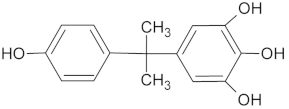 |
| M2 *• | 307 | 8.16 | 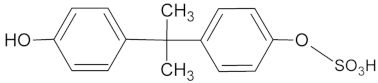 |
| M3 *•# | 453 | 11.31 11.80 | 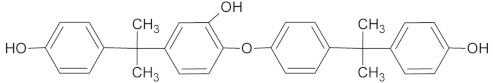 |
| M4 *• | 415 | 12.932 | 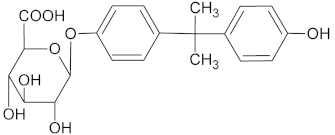 |
| M5 • | 243 | 8.846 |  |
| Time of Cultivation (h) | ||||
|---|---|---|---|---|
| Parameter | 24 | 72 | ||
| Control | BPA | Control | BPA | |
| Propidium iodide fluorescence intensity (U·mg−1) | 3.12 ± 0.55 | 25.52 ± 4.02 * | 17.19 ± 0.15 | 24.84 ± 1.36 * |
| TBARS level (µM·g−1) | 1.43 ± 0.36 | 4.02 ± 0,09 * | 1.42 ± 0.37 | 1.45 ± 0.04 |
| ROS ratio (%) | 0.27 ± 0.07 | 38.12 ± 6.53 * | 0.53 ± 0.21 | 3.26 ± 1.24 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasińska, A.; Soboń, A.; Różalska, S.; Średnicka, P. Bisphenol A Removal by the Fungus Myrothecium roridumIM 6482—Analysis of the Cellular and Subcellular Level. Int. J. Mol. Sci. 2021, 22, 10676. https://doi.org/10.3390/ijms221910676
Jasińska A, Soboń A, Różalska S, Średnicka P. Bisphenol A Removal by the Fungus Myrothecium roridumIM 6482—Analysis of the Cellular and Subcellular Level. International Journal of Molecular Sciences. 2021; 22(19):10676. https://doi.org/10.3390/ijms221910676
Chicago/Turabian StyleJasińska, Anna, Adrian Soboń, Sylwia Różalska, and Paulina Średnicka. 2021. "Bisphenol A Removal by the Fungus Myrothecium roridumIM 6482—Analysis of the Cellular and Subcellular Level" International Journal of Molecular Sciences 22, no. 19: 10676. https://doi.org/10.3390/ijms221910676
APA StyleJasińska, A., Soboń, A., Różalska, S., & Średnicka, P. (2021). Bisphenol A Removal by the Fungus Myrothecium roridumIM 6482—Analysis of the Cellular and Subcellular Level. International Journal of Molecular Sciences, 22(19), 10676. https://doi.org/10.3390/ijms221910676






