Liquid-Liquid Chromatography Separation of Guaiane-Type Sesquiterpene Lactones from Ferula penninervis Regel & Schmalh. and Evaluation of Their In Vitro Cytotoxic and Melanin Inhibitory Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Liquid-Liquid Chromatography Separation of Sesquiterpene Lactones from Ferula penninervis Methanolic Root Extract
2.2. Structure Elucidation of the New Guaiane-Type Sesquiterpene Lactones
2.3. Effects on Gastrointestinal, Prostate and Skin Cancer and Non-Cancer Cell Viability
2.3.1. Cytotoxic Activity of Ferula penninervis Methanolic Root Extract
2.3.2. Cytotoxic Activity of Guaiane-Type Sesquiterpene Lactones
2.4. Influence on Melanin Synthesis and Release in αMSH-Stimulated Melanoma B16F10 Cells
3. Materials and Methods
3.1. Apparatus
3.2. Chemicals
3.3. Plant Material and Extraction
3.4. Isolation Procedures
3.4.1. Liquid-Liquid Chromatographic Experiments
3.4.2. Semi-Preparative HPLC Separations
3.4.3. Analytical HPLC Separations
3.5. Structure Elucidation
3.6. Cell Viability Assay
3.6.1. Cell Lines and Cell Cultures
3.6.2. Neutral Red Uptake Assay
3.7. Melanin Release/Content Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaqoob, U.; Nawchoo, I.A. Distribution and taxonomy of Ferula L.: A review. Res. Rev. J. Bot. 2016, 5, 15–23. [Google Scholar]
- Zhou, Y.; Xin, F.; Zhang, G.; Qu, H.; Yang, D.; Han, X. Recent advances on bioactive constituents in Ferula. Drug Dev. Res. 2017, 78, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Iranshahy, M.; Iranshahi, M. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis). Iran. J. Basic Med. Sci. 2015, 18, 1050. [Google Scholar] [PubMed]
- Gamal-Eldeen, A.M.; Hegazy, M.-E. A crystal lapiferin derived from Ferula vesceritensis induces apoptosis pathway in MCF-7 breast cancer cells. Nat. Prod. Res. 2010, 24, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, P.; Bisht, S. Ferula asafoetida: Traditional uses and pharmacological activity. Pharmacogn. Rev. 2012, 6, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.; Li, N.; Zhou, D.; Chen, G.; Jiao, K.; Wang, W.; Si, Y.; Hou, Y. Sesquiterpene coumarins from Ferula sinkiangensis act as neuroinflammation inhibitors. Planta Med. 2017, 83, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Nazari, Z.E.; Iranshahi, M. Biologically active sesquiterpene coumarins from Ferula species. Phytother. Res. 2011, 25, 315–323. [Google Scholar] [CrossRef]
- Znati, M.; Ben Jannet, H.; Cazaux, S.; Souchard, J.P.; Harzallah Skhiri, F.; Bouajila, J. Antioxidant, 5-lipoxygenase inhibitory and cytotoxic activities of compounds isolated from the Ferula lutea flowers. Molecules 2014, 19, 16959–16975. [Google Scholar] [CrossRef] [Green Version]
- Maggi, F.; Papa, F.; Dall’Acqua, S.; Nicoletti, M. Chemical analysis of essential oils from different parts of Ferula communis L. growing in central Italy. Nat. Prod. Res. 2016, 30, 806–813. [Google Scholar] [CrossRef]
- Kogure, K.; Yamauchi, I.; Tokumura, A.; Kondou, K.; Tanaka, N.; Takaishi, Y.; Fukuzawa, K. Novel antioxidants isolated from plants of the genera Ferula, Inula, Prangos and Rheum collected in Uzbekistan. Phytomedicine 2004, 11, 645–651. [Google Scholar] [CrossRef]
- Sattar, Z.; Iranshahi, M. Phytochemistry and pharmacology of Ferula persica Boiss.: A review. Iran. J. Basic Med. Sci. 2017, 20, 1. [Google Scholar]
- Shikishima, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Tori, M.; Takaoka, S.; Kodzhimatov, O.K.; Ashurmetov, O. Sesquiterpenes from Ferula penninervis. J. Nat. Prod. 2002, 65, 1897–1903. [Google Scholar] [CrossRef]
- Ermatov, N.; Ban’kovskii, A.; Perel’son, M. Coumarins of Ferula penninervis. Chem. Nat. Compd. 1966, 2, 125–127. [Google Scholar] [CrossRef]
- Dzhumagalieva, F.D. Pharmacological Properties of Some Medicinal Plants in Kazakhstan; Alma-Ata: Almaty, Kazakhstan, 1971. [Google Scholar]
- Konovalova, O.; Rybalko, K.; Sheichenko, V. Sesquiterpene lactones of Ferula olgae. Chem. Nat. Compd. 1975, 11, 620–628. [Google Scholar] [CrossRef]
- Nurmukhamedova, M.; Kasymov, S.Z.; Abdullaev, N.; Sidyakin, G. Structure of fegolide. Chem. Nat. Compd. 1985, 21, 313–314. [Google Scholar] [CrossRef]
- Nurmukhamedova, M.; Kasymov, S.Z.; Sidyakin, G. Ferolide—A new lactone from Ferula penninervis. Chem. Nat. Compd. 1983, 19, 506–507. [Google Scholar] [CrossRef]
- Berthod, A. Countercurrent Chromatography; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Foucault, A.P. Centrifugal Partition Chromatography; M. Dekker: New York, NY, USA, 1995. [Google Scholar]
- Marston, A.; Hostettmann, K. Developments in the application of counter-current chromatography to plant analysis. J. Chromatogr. A 2006, 1112, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Brent Friesen, J.; Pauli, G.F. GUESS—A generally useful estimate of solvent systems for CCC. J. Liq. Chromatogr. Rel. Technol. 2005, 28, 2777–2806. [Google Scholar] [CrossRef]
- Luca, S.V.; Miron, A.; Ignatova, S.; Skalicka-Woźniak, K. An overview of the two-phase solvent systems used in the countercurrent separation of phenylethanoid glycosides and iridoids and their biological relevance. Phytochem. Rev. 2019, 18, 377–403. [Google Scholar] [CrossRef] [Green Version]
- Morley, R.; Minceva, M. Operating mode and parameter selection in liquid–liquid chromatography. J. Chromatogr. A 2020, 1617, 460479. [Google Scholar] [CrossRef]
- Luca, S.V.; Czerwińska, M.E.; Miron, A.; Aprotosoaie, A.C.; Marcourt, L.; Wolfender, J.-L.; Granica, S.; Skalicka-Woźniak, K. High-performance countercurrent chromatographic isolation of acylated iridoid diglycosides from Verbascum ovalifolium Donn ex Sims and evaluation of their inhibitory potential on IL-8 and TNF-α production. J. Pharm. Biomed. Anal. 2019, 166, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Ji, L.; Luo, Y.; Hu, Y. Preparative isolation and purification of three sesquiterpenoid lactones from Eupatorium lindleyanum DC. by high-speed counter-current chromatography. Molecules 2012, 17, 9002–9009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Su, Z.; Yang, Y.; Ba, H.; Aisa, H.A. Isolation of three sesquiterpene lactones from the roots of Cichorium glandulosum Boiss. et Huet. by high-speed counter-current chromatography. J. Chromatogr. A 2007, 1176, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Rychłewska, U.; Hodgson, D.J.; Holub, M.; Buděšínský, M.; Smítalová, Z. The structure of 2-oxo-8α-angeloyloxy-11α-acetoxy-5βH, 6αH, 7αH-guai-1 (10), 3-dien-6, 12-olide, a sesquiterpenic lactone from Laserpitium prutenicum L. Revision of the stereostructures of native 2-oxoguai-1 (10), 3-dien-6, 12-olides from the species of the Umbelliferae family. Coll. Czechoslov. Chem. Commun. 1985, 50, 2607–2624. [Google Scholar]
- Sallam, A.A.; Hitotsuyanagi, Y.; Mansour, E.S.S.; Ahmed, A.F.; Gedara, S.; Fukaya, H.; Takeya, K. Sesquiterpene Lactones from Daucus glaber. Helv. Chim. Acta 2010, 93, 48–57. [Google Scholar] [CrossRef]
- World Health Organization (W.H.O). 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 17 August 2021).
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Bioph. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef]
- Bagheri, S.M.; Sahebkar, A.; Gohari, A.R.; Saeidnia, S.; Malmir, M.; Iranshahi, M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm. Biol. 2010, 48, 242–246. [Google Scholar] [CrossRef]
- Eslami, J.B.; Dehpouri, A.; Nemati, F.; Rezaei, B. Cytotoxicity effects of the Ferula gummosa extract on the cancer cell line MCF7. J. Anim. Biol. 2013, 5, 1–7. [Google Scholar]
- Gudarzi, H.; Salimi, M.; Irian, S.; Amanzadeh, A.; Mostafapour Kandelous, H.; Azadmanesh, K.; Salimi, M. Ethanolic extract of Ferula gummosa is cytotoxic against cancer cells by inducing apoptosis and cell cycle arrest. Nat. Prod. Res. 2015, 29, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Hajimehdipoor, H.; Esmaeili, S.; Ramezani, R.; Jafari Anaraki, M.; Mosaddegh, M. The cytotoxic effects of Ferula persica var. persica and Ferula hezarlalehzarica against HepG2, A549, HT29, MCF7 and MDBK cell lines. Iran. J. Pharm. Sci. 2012, 8, 113–117. [Google Scholar]
- Hamzeloomoghadam, M.; Esmaeili, S.; Fotoohi, F.; Naghibi, F.; Pirani, A.; Hajimehdipoor, H. In vitro evaluation for cytotoxic activity of three Ferula species. Int. J. Pharm. Sci. Res. 2013, 4, 2673. [Google Scholar]
- Nizioł-Łukaszewska, Z.; Gaweł-Bęben, K.; Rybczyńska-Tkaczyk, K.; Jakubczyk, A.; Karaś, M.; Bujak, T. Biochemical properties, UV-protecting and fibroblast growth-stimulating activity of Plantago lanceolata L. extracts. Ind. Crops Prod. 2019, 138, 111453. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus× incanus L. and Cistus ladanifer L. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Czop, M.; Sakipova, Z.; Głowniak, K.; Kukula-Koch, W. Achillea millefolium L. and Achillea biebersteinii Afan. hydroglycolic extracts–bioactive ingredients for cosmetic use. Molecules 2020, 25, 3368. [Google Scholar] [CrossRef] [PubMed]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef] [PubMed]
- Aydoğan, F.; Baykan, Ş.; Bütüner, B.D. Cytotoxic Activity of sesquiterpenoids isolated from endemic Ferula tenuissima Hub.-Mor & Peşmen. Turk. J. Pharm. Sci. 2019, 16, 476. [Google Scholar]
- Kasaian, J.; Iranshahy, M.; Masullo, M.; Piacente, S.; Ebrahimi, F.; Iranshahi, M. Sesquiterpene lactones from Ferula oopoda and their cytotoxic properties. J. Asian Nat. Prod. Res. 2014, 16, 248–253. [Google Scholar] [CrossRef]
- Suzuki, K.; Okasaka, M.; Kashiwada, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; Sekiya, M. Sesquiterpene lactones from the roots of Ferula varia and their cytotoxic activity. J. Nat. Prod. 2007, 70, 1915–1918. [Google Scholar] [CrossRef]
- Mustapha, N.; Bzéouich, I.M.; Ghedira, K.; Hennebelle, T.; Chekir-Ghedira, L. Compounds isolated from the aerial part of Crataegus azarolus inhibit growth of B16F10 melanoma cells and exert a potent inhibition of the melanin synthesis. Biomed. Pharmacother. 2015, 69, 139–144. [Google Scholar] [CrossRef]
- Ullah, S.; Park, C.; Ikram, M.; Kang, D.; Lee, S.; Yang, J.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogues. Bioorg. Chem. 2019, 87, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Srisayam, M.; Weerapreeyakul, N.; Kanokmedhakul, K. Inhibition of two stages of melanin synthesis by sesamol, sesamin and sesamolin. Asian Pac. J. Tropic. Biomed. 2017, 7, 886–895. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Kim, K.; Kim, Y.-D.; Lee, S.-E. Antimelanogenic activities of piperlongumine derived from Piper longum on murine B16F10 melanoma cells in vitro and zebrafish embryos in vivo: Its molecular mode of depigmenting action. Appl. Biol. Chem. 2019, 62, 1–7. [Google Scholar] [CrossRef]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
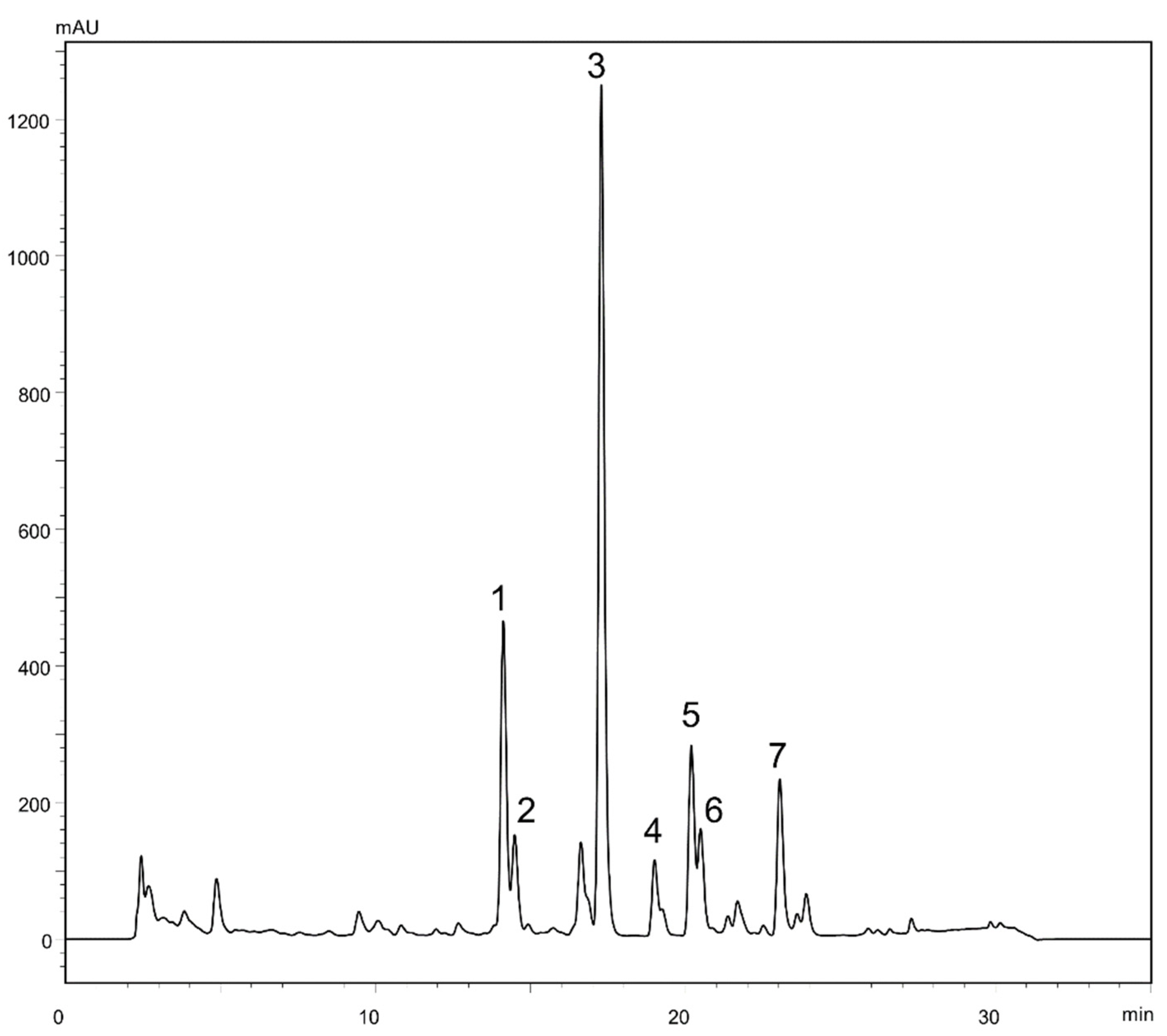

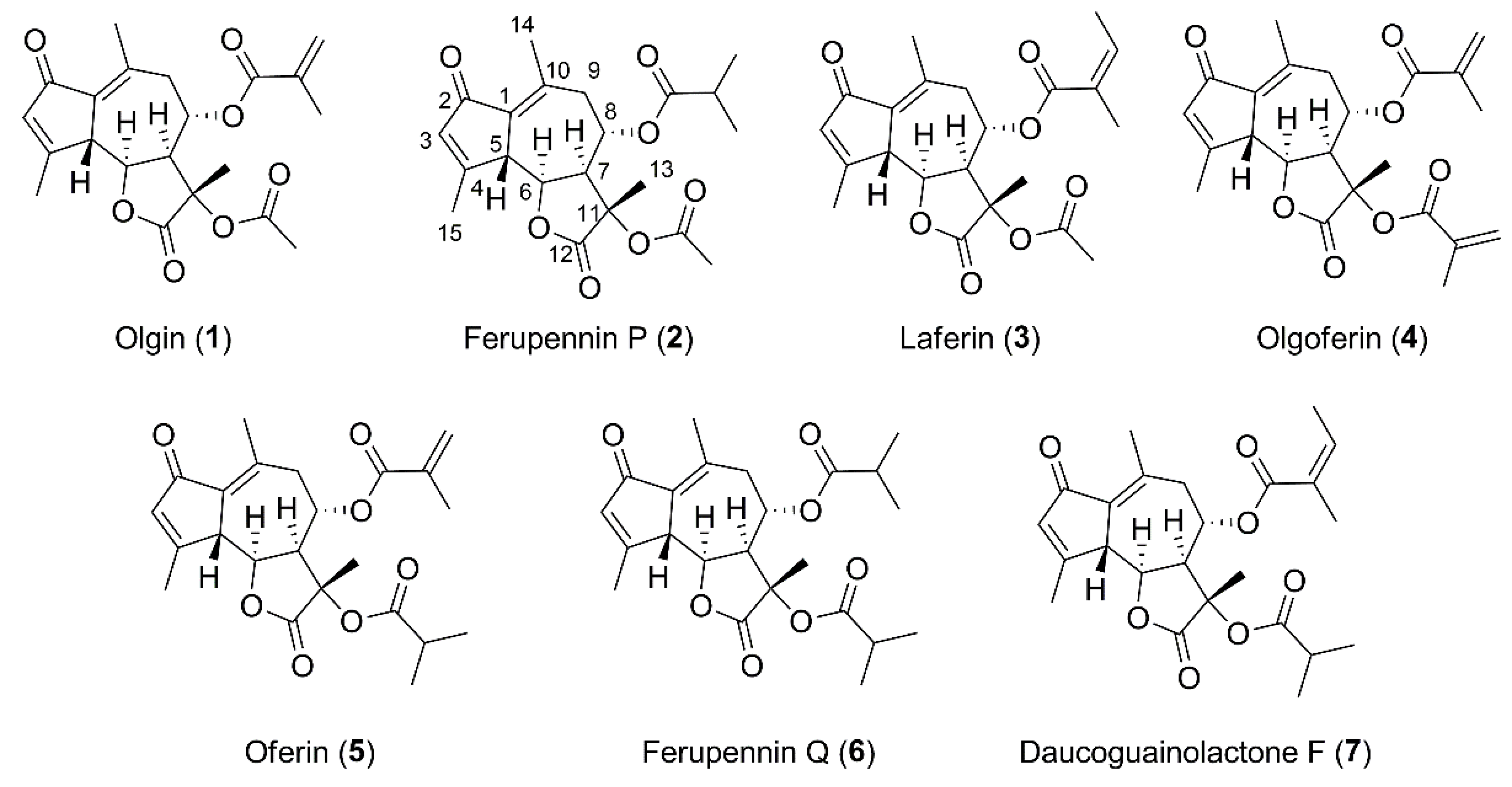
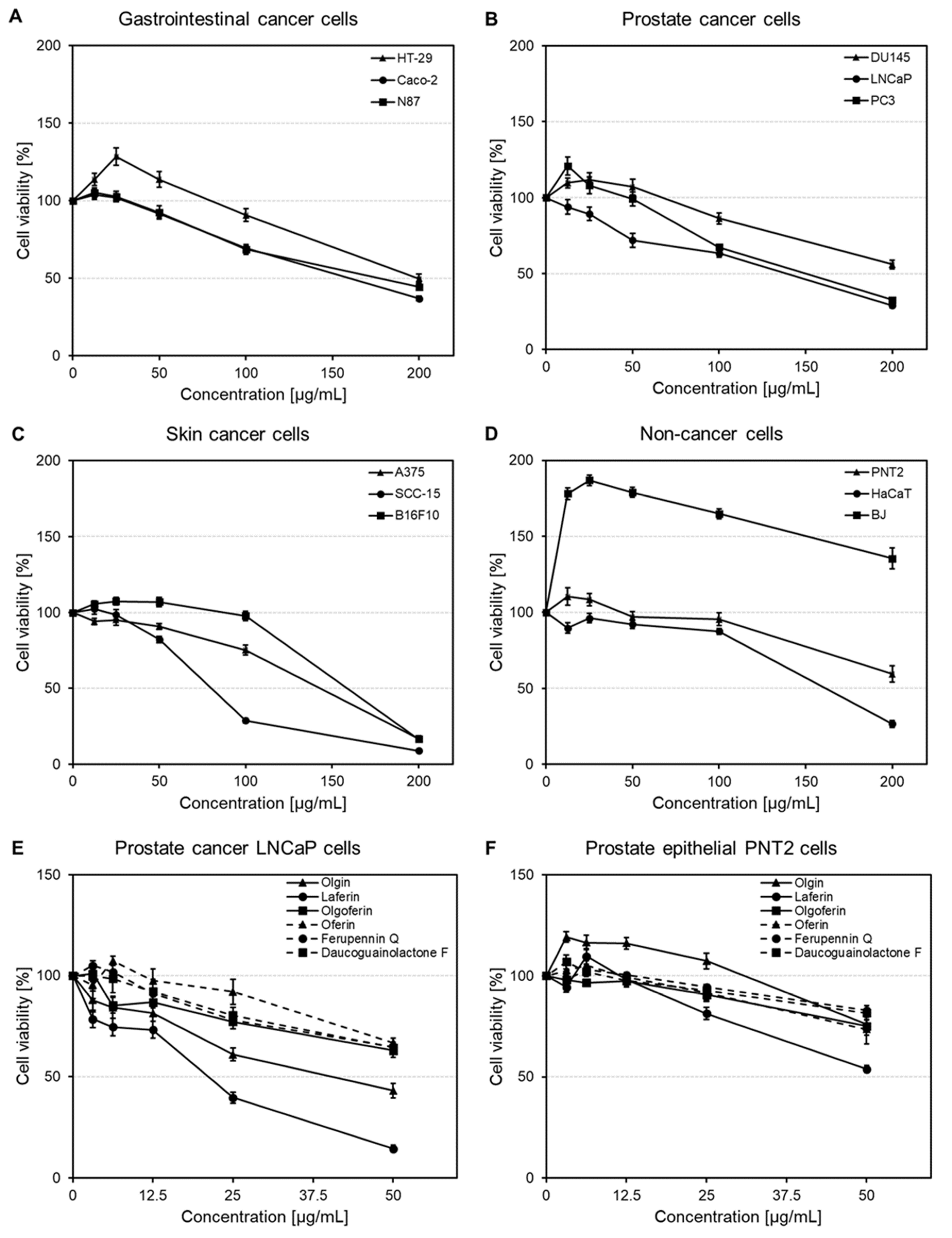

| No. | HEMWat | P1 | P2 | P3 | P4 | P5 | P6 | P7 |
|---|---|---|---|---|---|---|---|---|
| 1 | 5/6/5/6 | 5.20 | 5.28 | 8.18 | 10.13 | 14.20 | 15.30 | 21.77 |
| 2 | 1/1/1/1 | 1.91 | 2.04 | 3.03 | 4.69 | 5.34 | 5.48 | 7.95 |
| 3 | 6/5/6/5 | 0.95 | 1.04 | 1.66 | 2.43 | 2.89 | 2.98 | 4.49 |
| 4 | 3/2/3/2 | 0.44 | 0.48 | 0.70 | 1.02 | 1.27 | 1.32 | 1.89 |
| α | − | 1.1 | 1.5 | 1.5 | 1.3 | 1.0 | 1.4 |
| Position | Ferupennin P (2) | Ferupennin Q (6) | ||
|---|---|---|---|---|
| δH (Multiplicity, J, nH) | δC | δH (Multiplicity, J, nH) | δC | |
| Sesq. | ||||
| 1 | 130.6 | 130.6 | ||
| 2 | 197.6 | 197.7 | ||
| 3 | 6.19 (s, 1H) | 136.3 | 6.20 (p, 1.1 Hz, 1H) | 136.3 |
| 4 | 173.1 | 173.1 | ||
| 5 | 3.94 (d, 11.4 Hz, 1H) | 48.8 | 3.94 (d, 11.5 Hz, 1H) | 48.9 |
| 6 | 4.69 (t, 11.4, 9.7 Hz, 1H) | 80.2 | 4.70 (dd, 11.5, 9.7 Hz, 1H) | 80.2 |
| 7 | 3.63 (dd, 10.9, 9.7 Hz, 1H) | 48.5 | 3.55 (dd, 11.1, 9.7 Hz, 1H) | 48.8 |
| 8 | 5.60 (td, 10.9, 3.3 Hz, 1H) | 68.7 | 5.60 (td, 11.1, 3.3 Hz, 1H) | 68.8 |
| 9 | 2.79 (dd, 19.1, 3.3 Hz, 1H) 2.65 (dd, 19.1, 10.9 Hz, 1H) | 44.4 | 2.80 (dd, 19.1, 3.3 Hz, 1H) 2.64 (dd, 19.1, 10.8 Hz, 1H) | 44.4 |
| 10 | 147.7 | 147.7 | ||
| 11 | 79.4 | 79.3 | ||
| 12 | 175.6 | 175.6 | ||
| 13 | 1.61 (s, 3H) | 20.7 | 1.61 (s, 3H) | 20.5 |
| 14 | 2.25 (s, 3H) | 20.3 | 2.25 (s, 3H) | 20.3 |
| 15 | 2.25 (s, 3H) | 20.2 | 2.26 (t, 1.1 Hz, 3H) | 20.2 |
| Acyl-8 | ||||
| 8a | 177.3 | 177.3 | ||
| 8b | 2.61 (hept, 7.0 Hz, 1H) | 35.4 | 2.60 (hept, 6.9 Hz, 1H) | 35.4 |
| 8c | 1.24 (d, 7.0 Hz, 3H) | 19.3 | 1.23 (d, 6.9 Hz, 3H) | 19.3 |
| 8d | 1.21 (d, 7.0 Hz, 3H) | 19.0 | 1.20 (d, 6.9 Hz, 3H) | 19.1 |
| Acyl-11 | ||||
| 11a | 171.3 | 177.3 | ||
| 11b | 2.10 (s, 3H) | 20.7 | 2.60 (hept, 6.9 Hz, 1H) | 34.9 |
| 11c | 1.20 (d, 6.9 Hz, 3H) | 19.0 | ||
| 11d | 1.19 (d, 6.9 Hz, 3H) | 18.9 | ||
| Cell Line-Type | Cell Line | IC50 (μg/mL) |
|---|---|---|
| Gastrointestinal cancer cells | N87 | 154.54 ± 4.13 |
| Caco-2 | 145.08 ± 6.82 | |
| HT-29 | 190.53 ± 9.90 | |
| Prostate cancer cells | LNCaP | 112.12 ± 3.82 |
| DU145 | >200 | |
| PC3 | 112.28 ± 3.98 | |
| Skin cancer cells | SCC-15 | 77.84 ± 2.55 |
| A375 | 138.48 ± 9.45 | |
| B16F10 | 110.04 ± 1.34 | |
| Non-cancer cells | PNT2 | >200 |
| HaCaT | 159.28 ± 5.70 | |
| BJ | >200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, S.V.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Jumabayeva, A.; Sakipova, Z.; Xiao, J.; Marcourt, L.; Wolfender, J.-L.; Skalicka-Woźniak, K. Liquid-Liquid Chromatography Separation of Guaiane-Type Sesquiterpene Lactones from Ferula penninervis Regel & Schmalh. and Evaluation of Their In Vitro Cytotoxic and Melanin Inhibitory Potential. Int. J. Mol. Sci. 2021, 22, 10717. https://doi.org/10.3390/ijms221910717
Luca SV, Gaweł-Bęben K, Strzępek-Gomółka M, Jumabayeva A, Sakipova Z, Xiao J, Marcourt L, Wolfender J-L, Skalicka-Woźniak K. Liquid-Liquid Chromatography Separation of Guaiane-Type Sesquiterpene Lactones from Ferula penninervis Regel & Schmalh. and Evaluation of Their In Vitro Cytotoxic and Melanin Inhibitory Potential. International Journal of Molecular Sciences. 2021; 22(19):10717. https://doi.org/10.3390/ijms221910717
Chicago/Turabian StyleLuca, Simon Vlad, Katarzyna Gaweł-Bęben, Marcelina Strzępek-Gomółka, Ainur Jumabayeva, Zuriyadda Sakipova, Jianbo Xiao, Laurence Marcourt, Jean-Luc Wolfender, and Krystyna Skalicka-Woźniak. 2021. "Liquid-Liquid Chromatography Separation of Guaiane-Type Sesquiterpene Lactones from Ferula penninervis Regel & Schmalh. and Evaluation of Their In Vitro Cytotoxic and Melanin Inhibitory Potential" International Journal of Molecular Sciences 22, no. 19: 10717. https://doi.org/10.3390/ijms221910717






