Multiplex Site-Directed Gene Editing Using Polyethylene Glycol-Mediated Delivery of CRISPR gRNA:Cas9 Ribonucleoprotein (RNP) Complexes to Carrot Protoplasts
Abstract
:1. Introduction
2. Results
2.1. Validation of the RNP Complex Activity
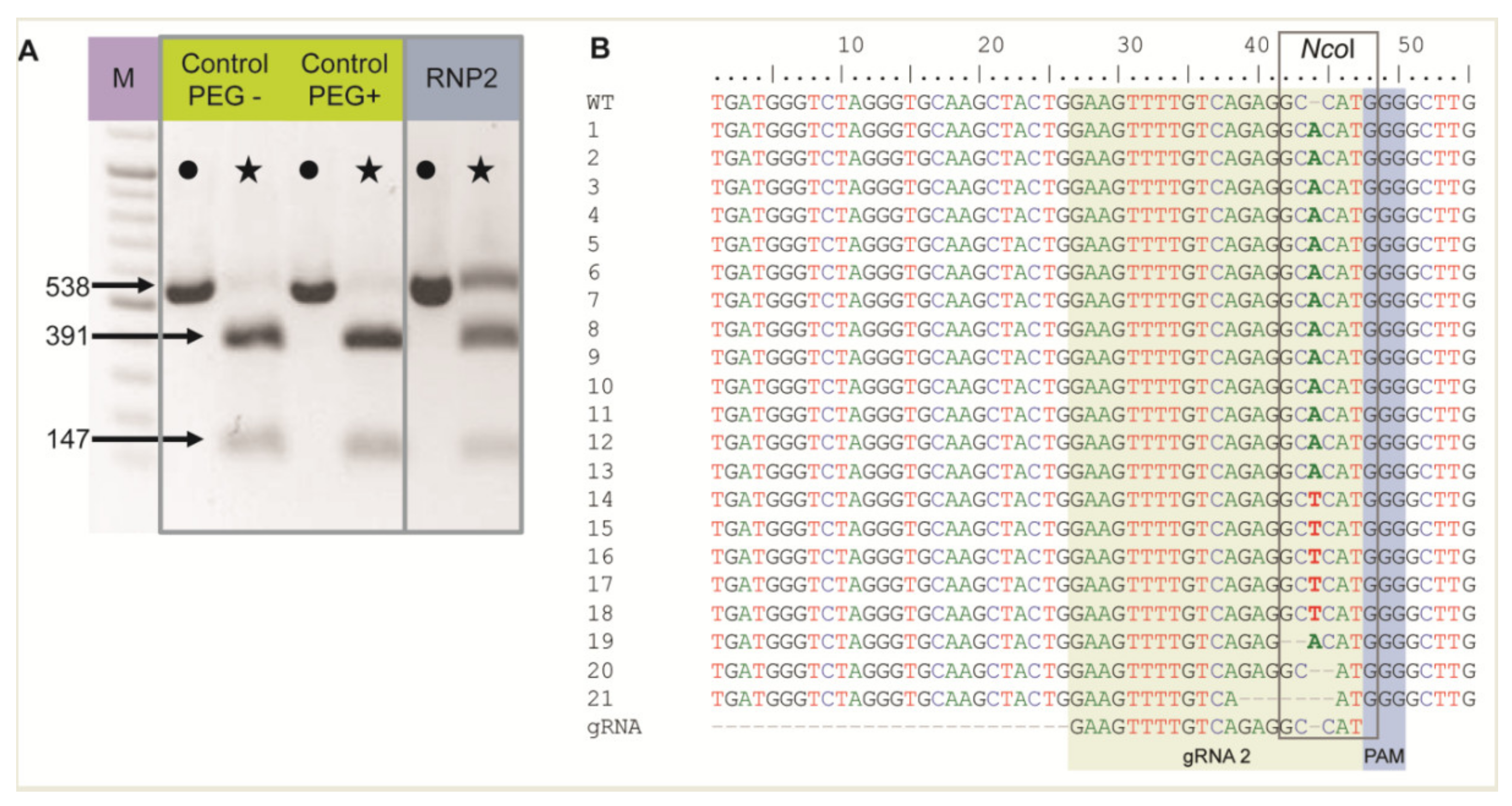
2.2. Single Site Editing
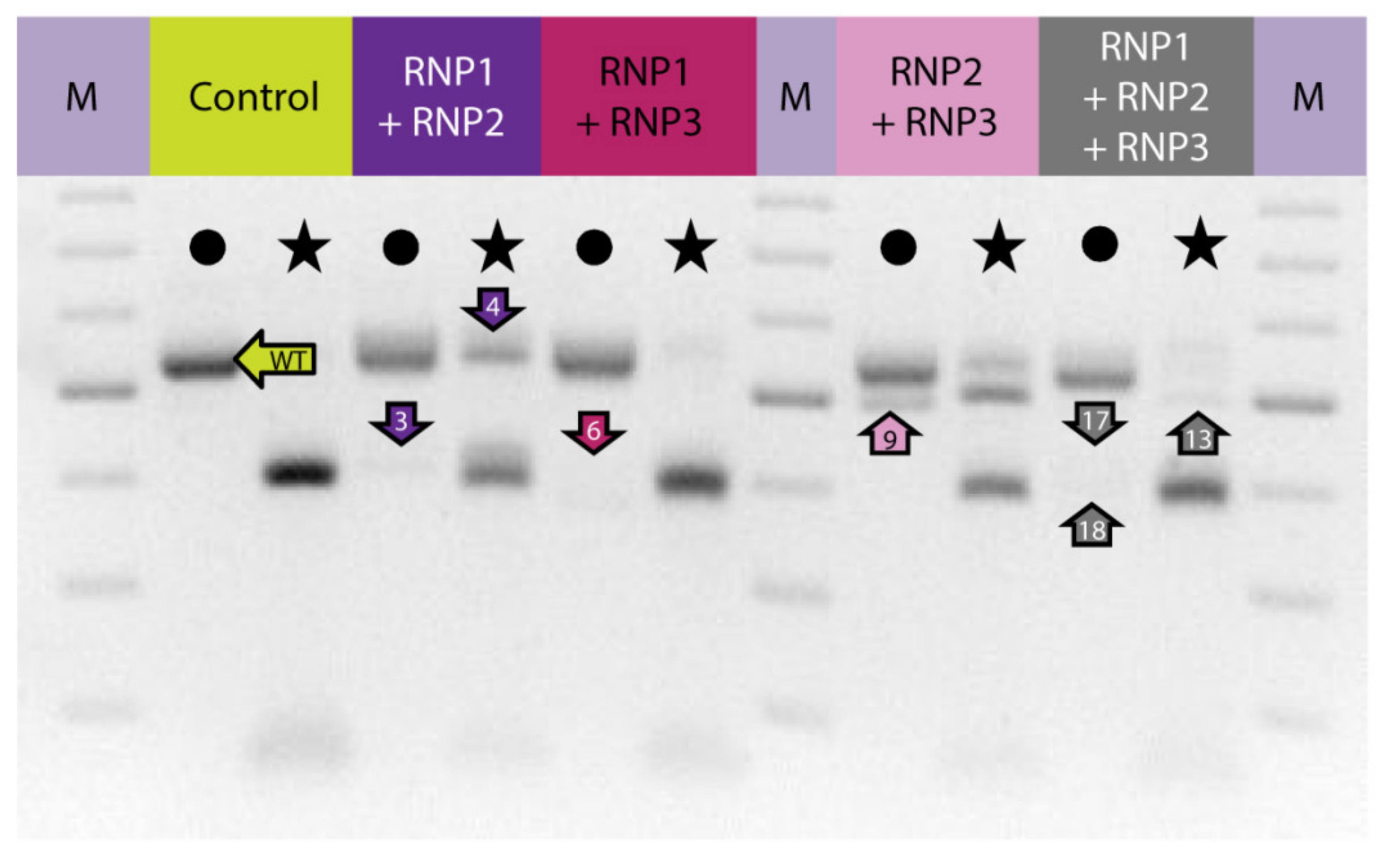
2.3. Multiplex Editing
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. gRNA Design and Synthesis
4.3. PCR Fragment Digestion In Vitro
4.4. Protoplast Isolation and PEG-Mediated Transformation
4.5. PCR and Restriction Fragment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nidhi, S.; Anand, U.; Oleksak, P.; Tripathi, P.; Lal, J.; Thomas, G.; Kuca, K.; Tripathi, V. Novel CRISPR–Cas Systems: An Updated Review of the Current Achievements, Applications, and Future Research Perspectives. Int. J. Mol. Sci. 2021, 22, 3327. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hua, K.; Lang, Z. Genome Editing for Horticultural Crop Improvement. Hortic. Res. 2019, 6, 113. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Chen, K.; Zhang, Y.; Liu, J.; Yin, K.; Qiu, J.; Gao, C. Genome Editing of Bread Wheat Using Biolistic Delivery of CRISPR/Cas9 In Vitro Transcripts or Ribonucleoproteins. Nat. Protocol. 2018, 13, 413–430. [Google Scholar] [CrossRef]
- Wang, H.; La Russa, M.; Qi, L. CRISPR/Cas9 in Genome Editing and Beyond. Annu. Rev. Biochem. 2016, 85, 227–264. [Google Scholar] [CrossRef] [Green Version]
- Pickar-Oliver, A.; Gersbach, C. The next Generation of CRISPR–Cas Technologies and Applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef]
- Ku, H.-K.; Ha, S.-H. Improving Nutritional and Functional Quality by Genome Editing of Crops: Status and Perspectives. Front. Plant Sci. 2020, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Menz, J.; Modrzejewski, D.; Hartung, F.; Wilhelm, R.; Sprink, T. Genome Edited Crops Touch the Market: A View on the Global Development and Regulatory Environment. Front. Plant Sci. 2020, 11, 586027. [Google Scholar] [CrossRef] [PubMed]
- Zess, E.; Begemann, M. CRISPR-Cas9 and Beyond: What’s Next in Plant Genome Engineering. Vitro Cell. Dev. Biol. Plant 2021. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Kim, H.; Lee, S.; Seo, J.; Choi, J.; Park, J.; Min, S.; Yoon, S.; Cho, S.; Kim, H. Prediction of the Sequence-Specific Cleavage Activity of Cas9 Variants. Nat. Biotechnol. 2020, 38, 1328–1336. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Neises, A.; Chen, W.; Hu, L.; Ji, G.; Yu, J.; Xu, J.; Yuan, W.; Cheng, T.; et al. Different Effects of sgRNA Length on CRISPR-Mediated Gene Knockout Efficiency. Sci. Rep. 2016, 6, 28566. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Puchta, H. Novel CRISPR/Cas Applications in Plants: From Prime Editing to Chromosome Engineering. Transgenic Res. 2021. [Google Scholar] [CrossRef]
- Yip, B. Recent Advances in CRISPR/Cas9 Delivery Strategies. Biomolecules 2020, 10, 839. [Google Scholar] [CrossRef]
- Lino, C.; Harper, J.; Carney, J.; Timlin, J. Delivering CRISPR: A Review of the Challenges and Approaches. Drug Deliv. 2018, 25, 1234–1257. [Google Scholar] [CrossRef] [Green Version]
- Mehravar, M.; Shirazi, A.; Nazari, M.; Banan, M. Mosaicism in CRISPR/Cas9-Mediated Genome Editing. Dev. Biol. 2019, 445, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Grützner, R.; Martin, P.; Horn, C.; Mortensen, S.; Cram, E.J.; Lee-Parsons, C.W.T.; Stuttmann, J.; Marillonnet, S. High-Efficiency Genome Editing in Plants Mediated by a Cas9 Gene Containing Multiple Introns. Plant Commun. 2021, 2, 100135. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Kim, J.; Kwon, S.; Corvalán, C.; Cho, S.; Kim, H.; Kim, S.; Kim, S.; Choe, S.; Kim, J. DNA-Free Genome Editing in Plants with Preassembled CRISPR-Cas9 Ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef]
- Demirer, G.; Silva, T.; Jackson, C.; Thomas, J.; Ehrhardt, D.W.; Rhee, S.; Mortimer, J.; Landry, M. Nanotechnology to Advance CRISPR–Cas Genetic Engineering of Plants. Nat. Nanotechnol. 2021, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Nekrasov, V. CRISPR/Cas Precision: Do We Need to Worry about Off-Targeting in Plants? Plant Cell Rep. 2018, 38, 437–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Choe, S. DNA-Free Genome Editing with Preassembled CRISPR/Cas9 Ribonucleoproteins in Plants. Transgenic Res. 2019, 28, 61–64. [Google Scholar] [CrossRef]
- Rostoks, N. Implications of the EFSA Scientific Opinion on Site Directed Nucleases 1 and 2 for Risk Assessment of Genome-Edited Plants in the EU. Agronomy 2021, 11, 572. [Google Scholar] [CrossRef]
- Naeem, M.; Majeed, S.; Hoque, M.; Ahmad, I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells 2020, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision Genome Editing in Plants: State-of-The-Art in CRISPR/Cas9-Based Genome Engineering. BMC Plant Biol. 2020, 20, 234. [Google Scholar] [CrossRef]
- Sandhya, D.; Jogam, P.; Allini, V.; Abbagani, S.; Alok, A. The Present and Potential Future Methods for Delivering CRISPR/Cas9 Components in Plants. Int. J. Genet. Eng. Biotechnol. 2020, 18, 25. [Google Scholar] [CrossRef]
- Baranski, R. Genetic Transformation of Carrot (Daucus carota) and Other Apiaceae Species. Trans. Plant J. 2008, 2, 18–38. [Google Scholar]
- Klimek-Chodacka, M.; Oleszkiewicz, T.; Lowder, L.G.; Qi, Y.; Baranski, R. Efficient CRISPR/Cas9-Based Genome Editing in Carrot Cells. Plant. Cell Rep. 2018, 37, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Subburaj, S.; Chung, S.; Lee, C.; Ryu, S.; Kim, D.; Kim, J.; Bae, S.; Lee, G. Site-Directed Mutagenesis in Petunia × hybrida Protoplast System Using Direct Delivery of Purified Recombinant Cas9 Ribonucleoproteins. Plant Cell Rep. 2016, 35, 1535–1544. [Google Scholar] [CrossRef]
- Ramlee, M.; Yan, T.; Cheung, A.; Chuah, C.; Li, S. High-Throughput Genotyping of CRISPR/Cas9-Mediated Mutants Using Fluorescent PCR-Capillary Gel Electrophoresis. Sci. Rep. 2015, 5, 15587. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Ryu, J.; Kang, B.; Kim, J.; Kim, S. CRISPR/Cpf1-Mediated DNA-Free Plant Genome Editing. Nat. Comm. 2017, 8, 14406. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, Y.; Liang, Z.; Ren, C.; Nishitani, C.; Osakabe, K.; Wada, M.; Komori, S.; Malnoy, M.; Velasco, R.; Poli, M.; et al. CRISPR–Cas9-Mediated Genome Editing in Apple and Grapevine. Nat. Protocol. 2018, 13, 2844–2863. [Google Scholar] [CrossRef] [PubMed]
- Murovec, J.; Guček, K.; Bohanec, B.; Avbelj, M.; Jerala, R. DNA-Free Genome Editing of Brassica oleracea and B. rapa Protoplasts Using CRISPR-Cas9 Ribonucleoprotein Complexes. Front. Plant Sci. 2018, 9, 1594. [Google Scholar] [CrossRef]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas Systems in Genome Editing: Methodologies and Tools for sgRNA Design, Off-Target Evaluation, and Strategies to Mitigate Off-Target Effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Andersson, M.; Turesson, H.; Olsson, N.; Fält, A.; Ohlsson, P.; Gonzalez, M.; Samuelsson, M.; Hofvander, P. Genome Editing in Potato via CRISPR-Cas9 Ribonucleoprotein Delivery. Physiol. Plantarum 2018, 164, 378–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Iaffaldano, B.; Qi, Y. CRISPR Ribonucleoprotein-Mediated Genetic Engineering in Plants. Plant Commun. 2021, 2, 100168. [Google Scholar] [CrossRef] [PubMed]
- Cheong, T.; Blasco, R.; Chiarle, R. The CRISPR/Cas9 System as a Tool to Engineer Chromosomal Translocation In Vivo. In Chromosome Translocation. Advances in Experimental Medicine and Biology; Zhang, Y., Ed.; Springer: Singapore, 2018; pp. 39–48. [Google Scholar] [CrossRef]
- Grzebelus, E.; Szklarczyk, M.; Baranski, R. An Improved Protocol for Plant Regeneration from Leaf- and Hypocotyl-Derived Protoplasts of Carrot. Plant Cell Tissue Organ. Cult. 2012, 109, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Brandt, K.; Gunn, H.; Moretti, N.; Zemetra, R. A Streamlined Protocol for Wheat (Triticum eestivum) Protoplast Isolation and Transformation with CRISPR-Cas Ribonucleoprotein Complexes. Front. Plant Sci. 2020, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.; Cho, S.; Kim, J.; Kim, J. Highly Efficient RNA-Guided Genome Editing in Human Cells via Delivery of Purified Cas9 Ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, A.; Cheng, Z.; Kong, L.; Zhu, Z.; Lin, S.; Gao, G.; Zhang, B. CasOT: A Genome-Wide Cas9/GRNA Off-Target Searching Tool. Bioinformatics 2014, 30, 1180–1182. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; Park, J.; Kim, J.-S. Cas-OFFinder: A Fast and Versatile Algorithm That Searches for Potential Off-Target Sites of Cas9 RNA-Guided Endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef] [Green Version]
- Rogers, S.; Bendich, A. Extraction of DNA from Plant Tissues. In Plant Molecular Biology Manual; Gelvin, S., Schilperoort, R., Eds.; Kluwer Academic Publishers: Boston, FL, USA, 1988; Volume A6, pp. 1–10. [Google Scholar]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]


| Name | Sequence 5′–3′ | Length | PAM |
|---|---|---|---|
| gRNA1 | GTAGTCCCGGGCCCTAATC | 19 | GGG |
| gRNA2 | GAAGTTTTGTCAGAGGCCAT | 20 | GGG |
| gRNA3 | GCTATTACAAAGGCCTGTG | 19 | TGG |
| Name | Sequence 5′–3′ | Annealing Temp. (°C) | Expected Product Length (bp) | Purpose |
|---|---|---|---|---|
| F3H_F | GCAAGATTGGCGAGAGATAG | 60 | 367 | In vitro digestion |
| F3H_R_FAM | AGTGATCCAGGTTTTTCCGC | |||
| F3H_FO | GAGAAACTCCGGTTCGATATG | 56 | 709 | I step nested PCR |
| F3H_RO | CTGAACAGTGATCCAGGTTT | |||
| F3H_FM | CGTGTTATCGTTGGGATCGG | 56 | 538 | II step nested PCR |
| F3H_RM | AGCAAGAGCGTAATTGTGCC | |||
| F3H_F_ROX | GCAAGATTGGCGAGAGATAG | 60 | 309 | fluorescent PCR |
| F3H_RM | AGCAAGAGCGTAATTGTGCC | |||
| gRNA_seq | GACTCGGTGCCACTTTTTCA | - | - | gRNA sequencing after in vitro transcription |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek-Chodacka, M.; Gieniec, M.; Baranski, R. Multiplex Site-Directed Gene Editing Using Polyethylene Glycol-Mediated Delivery of CRISPR gRNA:Cas9 Ribonucleoprotein (RNP) Complexes to Carrot Protoplasts. Int. J. Mol. Sci. 2021, 22, 10740. https://doi.org/10.3390/ijms221910740
Klimek-Chodacka M, Gieniec M, Baranski R. Multiplex Site-Directed Gene Editing Using Polyethylene Glycol-Mediated Delivery of CRISPR gRNA:Cas9 Ribonucleoprotein (RNP) Complexes to Carrot Protoplasts. International Journal of Molecular Sciences. 2021; 22(19):10740. https://doi.org/10.3390/ijms221910740
Chicago/Turabian StyleKlimek-Chodacka, Magdalena, Miron Gieniec, and Rafal Baranski. 2021. "Multiplex Site-Directed Gene Editing Using Polyethylene Glycol-Mediated Delivery of CRISPR gRNA:Cas9 Ribonucleoprotein (RNP) Complexes to Carrot Protoplasts" International Journal of Molecular Sciences 22, no. 19: 10740. https://doi.org/10.3390/ijms221910740






