Tauroursodeoxycholic Acid Decreases Keloid Formation by Reducing Endoplasmic Reticulum Stress as Implicated in the Pathogenesis of Keloid
Abstract
:1. Introduction
2. Results
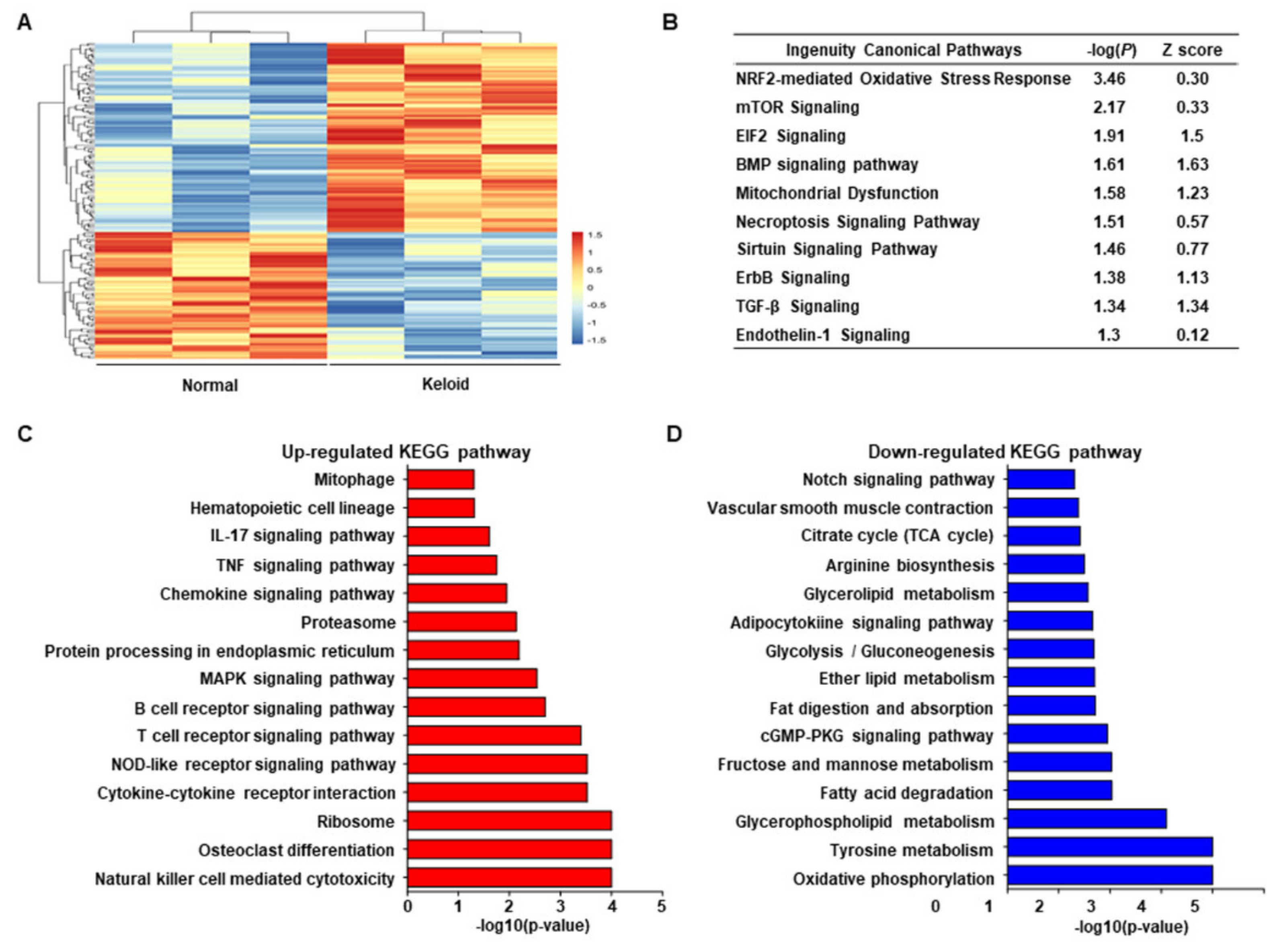
2.1. Transcriptome Sequencing of Keloid and Normal Tissues Revealing the Link between Stress Response of Cell Organelles, including Mitochondria and ER, with Keloids
2.2. ER Stress Signaling Was Upregulated in Keloid Tissues, Compared to Controls, in Western Blot Analysis and TEM
2.3. Tauroursodeoxycholic Acid (TUDCA), an ER Stress Inhibitor, Injection Treatment Was More Effective Compared to Steroid Injection, in Rabbit Ear Scar Models
2.4. Dysmorphic Mitochondria and Expanded ER Were Present in Rabbit Hypertrophic Model, Similar to That in Human Keloid Tissues, and ER Was Stabilized after Treatment with TUDCA
2.5. TUDCA Reduced Scarring in the Rabbit Model in Histological and Metrological Analyses through the Regulation of TGF-β1 Signaling
3. Discussion
4. Materials and Methods
4.1. Patient Population
4.2. RNA-Seq
4.3. Transcriptomics Analysis
4.4. Western Blot
4.5. TEM
4.6. Rabbit Ear Hypertrophic Scar Model
4.7. Histological Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bock, O.; Schmid-Ott, G.; Malewski, P.; Mrowietz, U. Quality of life of patients with keloid and hypertrophic scarring. Arch. Dermatol. Res. 2006, 297, 433. [Google Scholar] [CrossRef]
- Kouwenberg, C.A.; Bijlard, E.; Timman, R.; Hovius, S.E.; Busschbach, J.J.; Mureau, M.A. Emotional quality of life is severely affected by keloid disease: Pain and itch are the main determinants of burden. Plast. Reconstr. Surg. 2015, 136, 150–151. [Google Scholar] [CrossRef]
- Niessen, F.B.; Spauwen, P.H.; Schalkwijk, J.; Kon, M. On the nature of hypertrophic scars and keloids: A review. Plast. Reconstr. Surg. 1999, 104, 1435–1458. [Google Scholar] [CrossRef]
- Murray, J.C.; Pollack, S.V.; Pinnell, S.R. Keloids: A review. J. Am. Acad. Dermatol. 1981, 4, 461–470. [Google Scholar] [CrossRef]
- Omo-Dare, P. Genetic studies on keloid. J. Natl. Med Assoc. 1975, 67, 428. [Google Scholar] [PubMed]
- Ehrlich, H.P.; Needle, A.L.; McGrath, M.H. Wound healing in tight-skin mice: Delayed closure of excised wounds. Plast. Reconstr. Surg. 1983, 72, 197–198. [Google Scholar] [CrossRef]
- Bloch, E.F.; Hall, M.G., Jr.; Denson, M.J.; Slay-Solomon, V. General immune reactivity in keloid patients. Plast. Reconstr. Surg. 1984, 73, 448–451. [Google Scholar] [CrossRef]
- Lee, Y.; Minn, K.-W.; Baek, R.-M.; Hong, J.J. A new surgical treatment of keloid: Keloid core excision. Ann. Plast. Surg. 2001, 46, 135–140. [Google Scholar] [CrossRef]
- Berman, B.; Perez, O.A.; Konda, S.; Kohut, B.E.; Viera, M.H.; Delgado, S.; Zell, D.; Li, Q. A review of the biologic effects, clinical efficacy, and safety of silicone elastomer sheeting for hypertrophic and keloid scar treatment and management. Dermatol. Surg. 2007, 33, 1291–1303. [Google Scholar] [PubMed]
- Ardehali, B.; Nouraei, S.A.R.; Van Dam, H.; Dex, E.; Wood, S.; Nduka, C. Objective assessment of keloid scars with three-dimensional imaging: Quantifying response to intralesional steroid therapy. Plast. Reconstr. Surg. 2007, 119, 556–561. [Google Scholar] [CrossRef]
- Aggarwal, H.; Saxena, A.; Lubana, P.S.; Mathur, R.; Jain, D. Treatment of keloids and hypertrophic scars using bleomycin. J. Cosmet. Dermatol. 2008, 7, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.; Studd, D. “Oyster splints”: A new compression device for the treatment of keloid scars of the ear. Br. J. Plast. Surg. 1983, 36, 75–78. [Google Scholar] [CrossRef]
- Mamalis, A.; Lev-Tov, H.; Nguyen, D.H.; Jagdeo, J. Laser and light-based treatment of Keloids—A review. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 689–699. [Google Scholar] [CrossRef]
- Ragoowansi, R.; Cornes, P.G.; Moss, A.L.; Glees, J.P. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast. Reconstr. Surg. 2003, 111, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, Y.; Xu, S.; Zhang, Y.; Han, B.; Liu, Z.; Liu, X.; Zhu, Z. Secondary data mining of GEO database for long non-coding RNA and Competing endogenous RNA network in keloid-prone individuals. Aging (Albany NY) 2020, 12, 25076. [Google Scholar] [CrossRef]
- Lee, Y.S.; Liang, Y.C.; Wu, P.; Kulber, D.A.; Tanabe, K.; Chuong, C.M.; Widelitz, R.; Tuan, T.L. STAT 3 signalling pathway is implicated in keloid pathogenesis by preliminary transcriptome and open chromatin analyses. Exp. Dermatol. 2019, 28, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Limandjaja, G.; van den Broek, L.; Waaijman, T.; van Veen, H.; Everts, V.; Monstrey, S.; Scheper, R.; Niessen, F.; Gibbs, S. Increased epidermal thickness and abnormal epidermal differentiation in keloid scars. Br. J. Dermatol. 2017, 176, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Jiao, H.; Zhang, T.; Fan, J.; Xiao, R. The superficial dermis may initiate keloid formation: Histological analysis of the keloid dermis at different depths. Front. Physiol. 2017, 8, 885. [Google Scholar] [CrossRef]
- Yuan, F.L.; Sun, Z.L.; Feng, Y.; Liu, S.Y.; Du, Y.; Yu, S.; Yang, M.L.; Lv, G.Z. Epithelial–mesenchymal transition in the formation of hypertrophic scars and keloids. J. Cell. Physiol. 2019, 234, 21662–21669. [Google Scholar] [CrossRef]
- Wu, J.; Del Duca, E.; Espino, M.; Gontzes, A.; Cueto, I.; Zhang, N.; Estrada, Y.D.; Pavel, A.B.; Krueger, J.G.; Guttman-Yassky, E. RNA Sequencing Keloid Transcriptome Associates Keloids With Th2, Th1, Th17/Th22, and JAK3-Skewing. Front. Immunol. 2020, 11, 2960. [Google Scholar] [CrossRef]
- Russell, S.B.; Russell, J.D.; Trupin, K.M.; Gayden, A.E.; Opalenik, S.R.; Nanney, L.B.; Broquist, A.H.; Raju, L.; Williams, S.M. Epigenetically altered wound healing in keloid fibroblasts. J. Investig. Dermatol. 2010, 130, 2489–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.; Diegelmann, R.; Oliver, N. Keloid fibroblasts exhibit an altered response to TGF-β. J. Investig. Dermatol. 1992, 99, 650–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Ishitsuka, Y.; Hayasaka, M.; Yamada, Y.; Miyata, K.; Endo, M.; Kondo, Y.; Moriuchi, H.; Irikura, M.; Tanaka, K.-i. The exacerbating roles of CCAAT/enhancer-binding protein homologous protein (CHOP) in the development of bleomycin-induced pulmonary fibrosis and the preventive effects of tauroursodeoxycholic acid (TUDCA) against pulmonary fibrosis in mice. Pharmacol. Res. 2015, 99, 52–62. [Google Scholar] [CrossRef]
- Heubi, J.E.; Wiechmann, D.A.; Creutzinger, V.; Setchell, K.D.; Squires, R., Jr.; Couser, R.; Rhodes, P. Tauroursodeoxycholic acid (TUDCA) in the prevention of total parenteral nutrition-associated liver disease. J. Pediatr. 2002, 141, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wang, B.; Zhang, M.; Gu, Y. Involvement of endoplasmic reticulum stress in uremic cardiomyopathy: Protective effects of tauroursodeoxycholic acid. Cell. Physiol. Biochem. 2016, 38, 141–152. [Google Scholar] [CrossRef]
- Ladin, D.A.; Garner, W.L.; Smith Jr, D.J. Excessive scarring as a consequence of healing. Wound Repair Regen. 1995, 3, 6–14. [Google Scholar] [CrossRef]
- Lee, J.Y.-Y.; Yang, C.-C.; Chao, S.-C.; Wong, T.-W. Histopathological differential diagnosis of keloid and hypertrophic scar. Am. J. Dermatopathol. 2004, 26, 379–384. [Google Scholar] [CrossRef]
- Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, V.; Kehrl, J.H. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 4167–4171. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-C.; Chen, M.-J.; Yu, Y.-M.; Ko, S.-Y.; Chang, C.-C. Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: Its potential therapeutic use in the chemoprevention of keloid. Arch. Dermatol. Res. 2010, 302, 717–724. [Google Scholar] [CrossRef]
- Phan, T.-T.; Lim, I.J.; Chan, S.-Y.; Tan, E.-K.; Lee, S.-T.; Longaker, M.T. Suppression of transforming growth factor beta/smad signaling in keloid-derived fibroblasts by quercetin: Implications for the treatment of excessive scars. J. Trauma Acute Care Surg. 2004, 57, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, W.; Zeng, Q.; Ma, B.; Li, Z.; Meng, T.; Chen, J.; Yu, N.; Zhou, Z.; Long, X. Single-cell RNA-seq reveals lineage-specific regulatory changes of fibroblasts and vascular endothelial cells in keloids. J. Investig. Dermatol. 2021. [Google Scholar] [CrossRef]
- Lu, S.; Wang, H.; Ren, R.; Shi, X.; Zhang, Y.; Ma, W. Reduced expression of Twist 1 is protective against insulin resistance of adipocytes and involves mitochondrial dysfunction. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.; Dong, K.; Ma, Y. TWIST1 Alleviates Hypoxia-induced Damage of Trophoblast Cells by inhibiting mitochondrial apoptosis pathway. Exp. Cell Res. 2019, 385, 111687. [Google Scholar] [CrossRef]
- Syed, F.; Sherris, D.; Paus, R.; Varmeh, S.; Pandolfi, P.P.; Bayat, A. Keloid disease can be inhibited by antagonizing excessive mTOR signaling with a novel dual TORC1/2 inhibitor. Am. J. Pathol. 2012, 181, 1642–1658. [Google Scholar] [CrossRef]
- Shi-Wen, X.; Chen, Y.; Denton, C.P.; Eastwood, M.; Renzoni, E.A.; Bou-Gharios, G.; Pearson, J.D.; Dashwood, M.; du Bois, R.M.; Black, C.M. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol. Biol. Cell 2004, 15, 2707–2719. [Google Scholar] [CrossRef] [Green Version]
- Jumper, N.; Hodgkinson, T.; Paus, R.; Bayat, A. A role for Neuregulin-1 in promoting keloid fibroblast migration via ErbB2-mediated signaling. Acta Derm.-Venereol. 2017, 97, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.Z.; Liu, J.Q.; Yang, L.L.; Fan, L.; He, T.; Su, L.L.; Shi, J.H.; Tang, C.W.; Zheng, Z.; Hu, D.H. Identification of sirtuin 1 as a promising therapeutic target for hypertrophic scars. Br. J. Pharmacol. 2016, 173, 1589–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cominacini, L.; Mozzini, C.; Garbin, U.; Pasini, A.; Stranieri, C.; Solani, E.; Vallerio, P.; Tinelli, I.A.; Pasini, A.F. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic. Biol. Med. 2015, 88, 233–242. [Google Scholar] [CrossRef]
- Kouroku, Y.; Fujita, E.; Tanida, I.; Ueno, T.; Isoai, A.; Kumagai, H.; Ogawa, S.; Kaufman, R.; Kominami, E.; Momoi, T. ER stress (PERK/eIF2 α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007, 14, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Tang, H.-B.; Kang, J.; Shan, L.; Song, H.; Zhu, K.; Wang, J.; Ju, G.; Wang, Y.-Z. Involvement of endoplasmic reticulum stress in the necroptosis of microglia/macrophages after spinal cord injury. Neuroscience 2015, 311, 362–373. [Google Scholar] [CrossRef]
- Tanaka, K.-i.; Kaji, H.; Yamaguchi, T.; Kanazawa, I.; Canaff, L.; Hendy, G.N.; Sugimoto, T. Involvement of the osteoinductive factors, Tmem119 and BMP-2, and the ER stress response PERK–eIF2α–ATF4 pathway in the commitment of myoblastic into osteoblastic cells. Calcif. Tissue Int. 2014, 94, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Kostović-Knežević, L.; Bradamante, Ž.; Švajger, A. Ultrastructure of elastic cartilage in the rat external ear. Cell Tissue Res. 1981, 218, 149–160. [Google Scholar] [CrossRef]
- Jeon, Y.R.; Roh, H.; Jung, J.H.; Ahn, H.M.; Lee, J.H.; Yun, C.-O.; Lee, W.J. Antifibrotic effects of high-mobility group box 1 protein inhibitor (Glycyrrhizin) on keloid fibroblasts and keloid spheroids through reduction of autophagy and induction of apoptosis. Int. J. Mol. Sci. 2019, 20, 4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Qin, Z.; Chen, B.; An, Y.; Nie, F.; Yang, X.; Pan, B.; Bi, H. Mitochondrial dysfunction and morphological abnormality in keloid fibroblasts. Adv. Wound Care 2020, 9, 539–552. [Google Scholar] [CrossRef]
- Hampton, R.Y. ER stress response: Getting the UPR hand on misfolded proteins. Curr. Biol. 2000, 10, R518–R521. [Google Scholar] [CrossRef] [Green Version]
- Burman, A.; Tanjore, H.; Blackwell, T.S. Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol. 2018, 68, 355–365. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef] [Green Version]
- Burman, A.; Kropski, J.A.; Calvi, C.L.; Serezani, A.P.; Pascoalino, B.D.; Han, W.; Sherrill, T.; Gleaves, L.; Lawson, W.E.; Young, L.R. Localized hypoxia links ER stress to lung fibrosis through induction of C/EBP homologous protein. JCI Insight 2018, 3, e99543. [Google Scholar] [CrossRef] [Green Version]
- Ono, M.; Ohkouchi, S.; Kanehira, M.; Tode, N.; Kobayashi, M.; Ebina, M.; Nukiwa, T.; Irokawa, T.; Ogawa, H.; Akaike, T. Mesenchymal stem cells correct inappropriate epithelial–mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol. Ther. 2015, 23, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Kassan, M.; Galán, M.; Partyka, M.; Saifudeen, Z.; Henrion, D.; Trebak, M.; Matrougui, K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1652–1661. [Google Scholar] [CrossRef] [Green Version]
- Lenna, S.; Trojanowska, M. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr. Opin. Rheumatol. 2012, 24. [Google Scholar] [CrossRef]
- Al-Attar, A.; Mess, S.; Thomassen, J.M.; Kauffman, C.L.; Davison, S.P. Keloid pathogenesis and treatment. Plast. Reconstr. Surg. 2006, 117, 286–300. [Google Scholar] [CrossRef]
- Jones, C.D.; Guiot, L.; Samy, M.; Gorman, M.; Tehrani, H. The use of chemotherapeutics for the treatment of keloid scars. Dermatol. Rep. 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Hietanen, K.; Järvinen, T.; Huhtala, H.; Tolonen, T.; Kuokkanen, H.; Kaartinen, I. Treatment of keloid scars with intralesional triamcinolone and 5-fluorouracil injections—A randomized controlled trial. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Bijlard, E.; Steltenpool, S.; Niessen, F.B. Intralesional 5-fluorouracil in keloid treatment: A systematic review. Acta Derm.-Venereol. 2015, 95, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Paumgartner, G.; Beuers, U. Ursodeoxycholic acid in cholestatic liver disease: Mechanisms of action and therapeutic use revisited. Hepatology 2002, 36, 525–531. [Google Scholar] [CrossRef]
- Kusaczuk, M. Tauroursodeoxycholate—Bile acid with chaperoning activity: Molecular and cellular effects and therapeutic perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef] [Green Version]
- Vang, S.; Longley, K.; Steer, C.J.; Low, W.C. The unexpected uses of urso-and tauroursodeoxycholic acid in the treatment of non-liver diseases. Glob. Adv. Health Med. 2014, 3, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cash, J.G.; Kuhel, D.G.; Basford, J.E.; Jaeschke, A.; Chatterjee, T.K.; Weintraub, N.L.; Hui, D.Y. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J. Biol. Chem. 2012, 287, 27876–27884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, J.M.; McFarland, K.L.; Combs, K.A.; Anness, M.C.; Supp, D.M. Analysis of HOX gene expression and the effects of HOXA9 overexpression in fibroblasts derived from keloid lesions and normal skin. Wound Repair Regen. 2021. [Google Scholar] [CrossRef] [PubMed]





| Patient | Group | Race | Age | Site | Etiology | Lesion Duration | Lesion Size | Prior Treatments | Keloid Family History |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | East Asian | 43/F | Abdomen | Normal | Normal | Normal | Normal | Normal |
| 2 | Control | East Asian | 28/F | Chest | Normal | Normal | Normal | Normal | Normal |
| 3 | Control | East Asian | 36/F | Flank | Normal | Normal | Normal | Normal | Normal |
| 4 | Keloid | East Asian | 31/F | Ear | Piercing | 7 years | 4x2cm | Excision | Keloid |
| 5 | Keloid | East Asian | 33/F | Ear | Piercing | 8 years | 3x1cm | Excision | Normal |
| 6 | Keloid | East Asian | 23/F | Ear | Piercing | 5 years | 2x2cm | None | Normal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, S.E.; Yi, S.; Jun, S.; Yi, Y.-S.; Nagar, H.; Kim, C.-S.; Shin, C.; Yeo, M.-K.; Kang, Y.E.; et al. Tauroursodeoxycholic Acid Decreases Keloid Formation by Reducing Endoplasmic Reticulum Stress as Implicated in the Pathogenesis of Keloid. Int. J. Mol. Sci. 2021, 22, 10765. https://doi.org/10.3390/ijms221910765
Kim S, Lee SE, Yi S, Jun S, Yi Y-S, Nagar H, Kim C-S, Shin C, Yeo M-K, Kang YE, et al. Tauroursodeoxycholic Acid Decreases Keloid Formation by Reducing Endoplasmic Reticulum Stress as Implicated in the Pathogenesis of Keloid. International Journal of Molecular Sciences. 2021; 22(19):10765. https://doi.org/10.3390/ijms221910765
Chicago/Turabian StyleKim, Sunje, Seong Eun Lee, Shinae Yi, Sangmi Jun, Yoon-Sun Yi, Harsha Nagar, Cuk-Seong Kim, Chungmin Shin, Min-Kyung Yeo, Yea Eun Kang, and et al. 2021. "Tauroursodeoxycholic Acid Decreases Keloid Formation by Reducing Endoplasmic Reticulum Stress as Implicated in the Pathogenesis of Keloid" International Journal of Molecular Sciences 22, no. 19: 10765. https://doi.org/10.3390/ijms221910765
APA StyleKim, S., Lee, S. E., Yi, S., Jun, S., Yi, Y.-S., Nagar, H., Kim, C.-S., Shin, C., Yeo, M.-K., Kang, Y. E., & Oh, S.-H. (2021). Tauroursodeoxycholic Acid Decreases Keloid Formation by Reducing Endoplasmic Reticulum Stress as Implicated in the Pathogenesis of Keloid. International Journal of Molecular Sciences, 22(19), 10765. https://doi.org/10.3390/ijms221910765








