Neuroendocrine Effects of Carnitines on Reproductive Impairments
Abstract
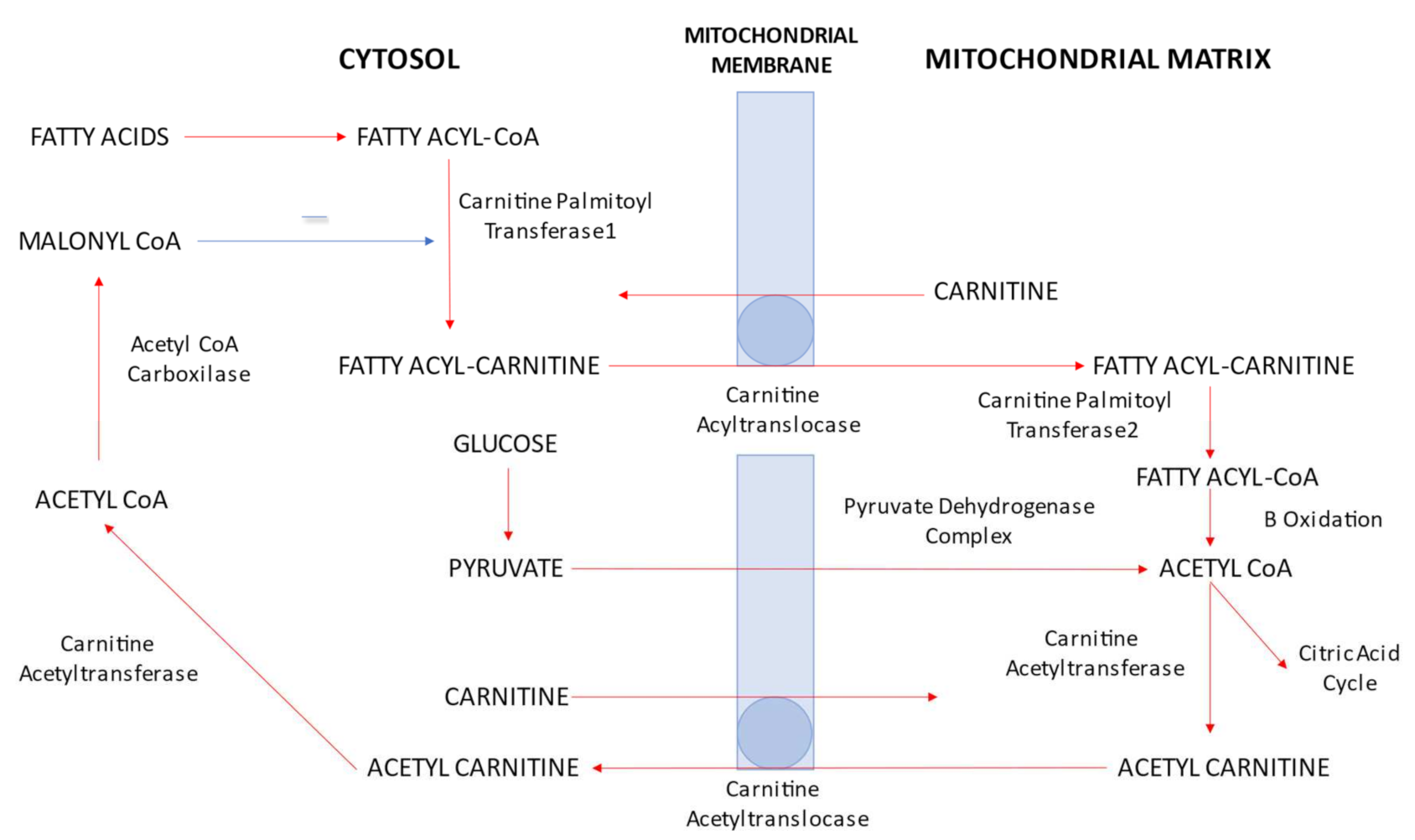
:1. What Are Carnitines?
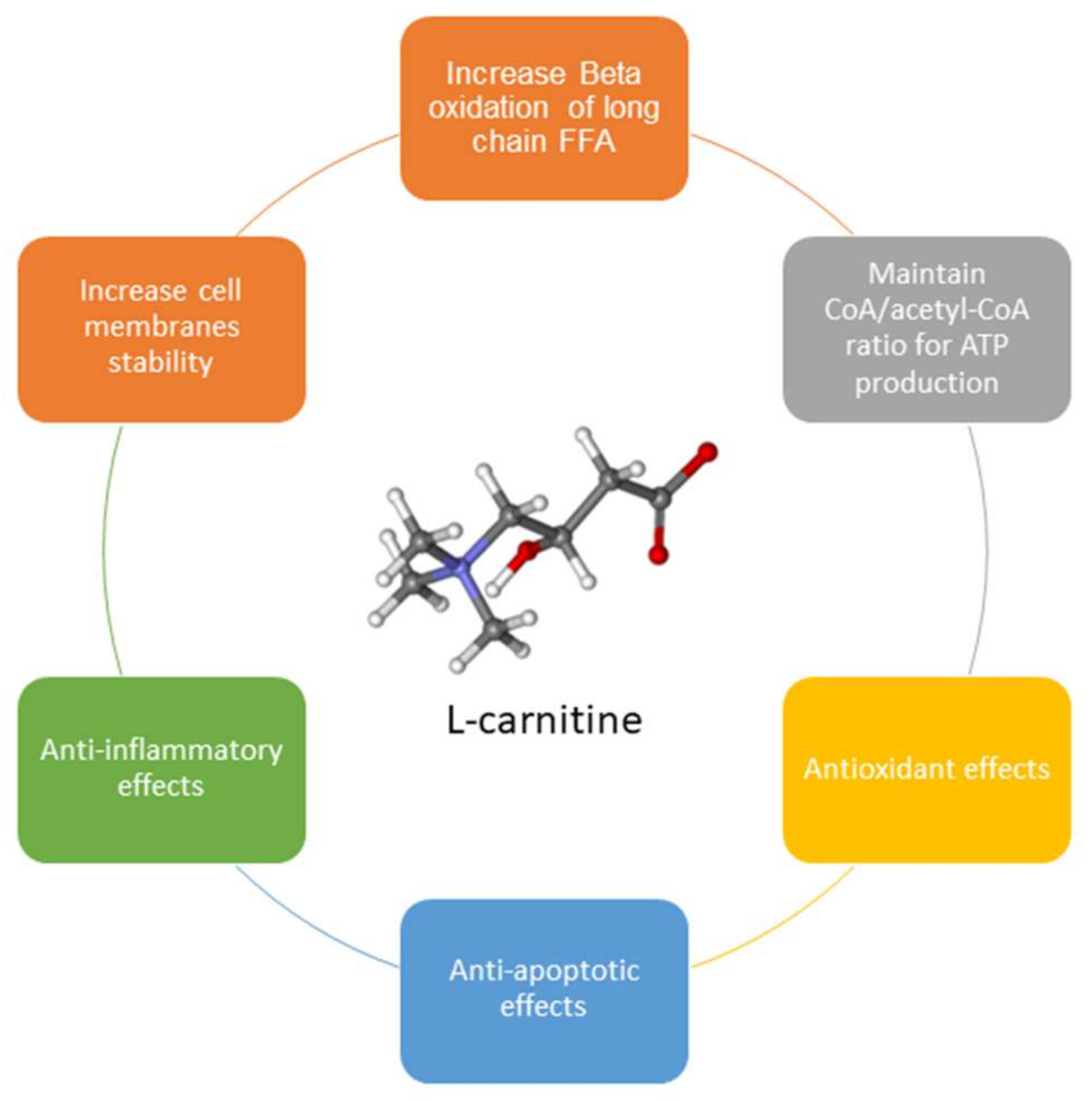
2. Carnitine Functions
3. Clinical Effects of Integration with Carnitines
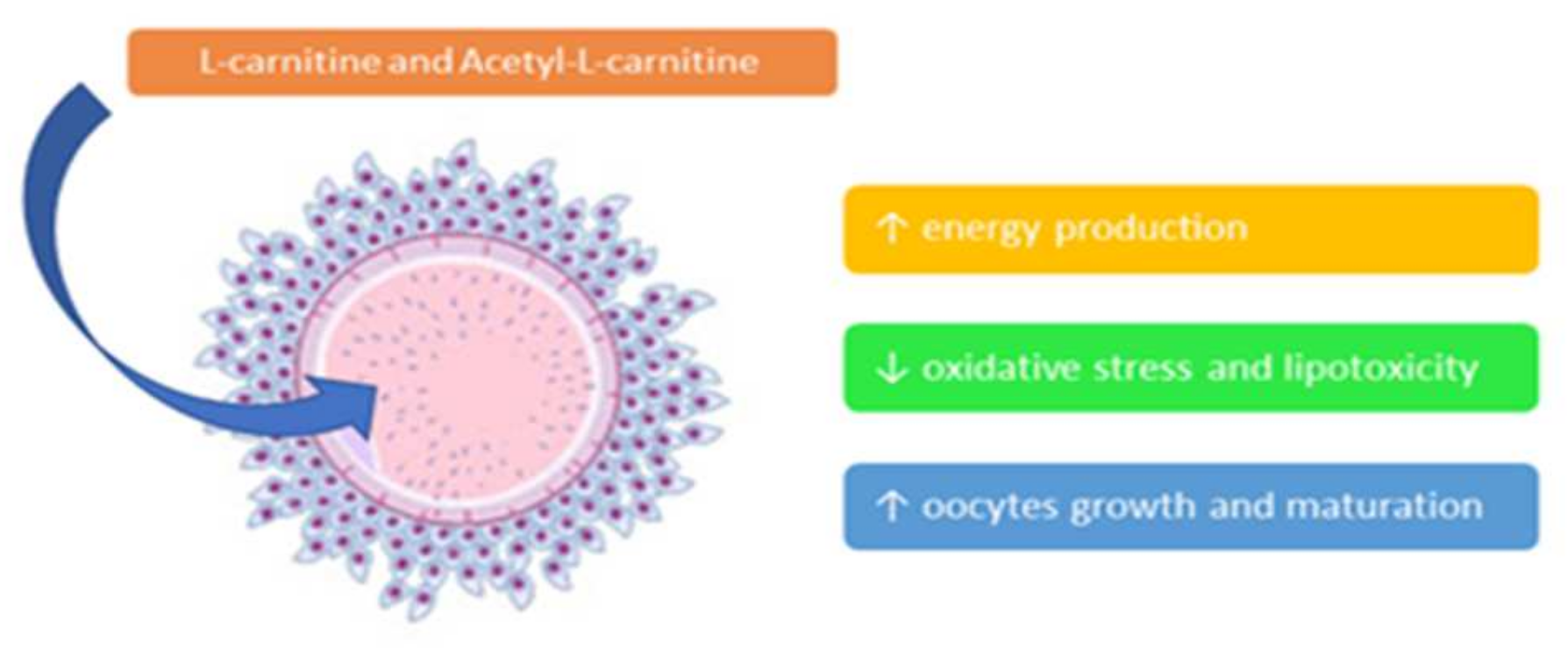
4. Polycystic Ovary Syndrome
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fraenkel, G.; Friedman, S. Carnitine. Vitam. Horm. 1957, 15, 73–118. [Google Scholar] [CrossRef]
- Pękala, J.W.; Patkowska-Sokola, B.; Bodkowski, R.; Jamroz, D.; Nowakowski, P.; Lochynski, S.; Librowski, T. L-Carnitine—Metabolic Functions and Meaning in Humans Life. Curr. Drug Metab. 2011, 12, 667–678. [Google Scholar] [CrossRef]
- Vogt, C.; Georgi, A.; Werner, G. Enantiomeric separation of D/L-carnitine using HPLC and CZE after derivatization. Chromatographia 1995, 40, 287–295. [Google Scholar] [CrossRef]
- Vaz, F.M.; Wanders, R.J.A. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, A.R.; Roe, C.R.; Stacey, E.T.; Hoppel, C.L. Urinary Excretion of l-Carnitine and Acylcarnitines by Patients with Disorders of Organic Acid Metabolism: Evidence for Secondary Insufficiency of l-Carnitine. Pediatr. Res. 1984, 18, 1325–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seline, K.; Johein, H. The determination of l-carnitine in several food samples. Food Chem. 2007, 105, 793–804. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Engel, A.G. Kinetic compartmental analysis of carnitine metabolism in the human carnitine deficiency syndromes. Evidence for alterations in tissue carnitine transport. J. Clin. Investig. 1984, 73, 857–867. [Google Scholar] [CrossRef] [Green Version]
- Lopaschuk, G.D.; De Simone, C.; Famularo, G. Carnitine and Myocardial Glucose Metabolism. In Carnitine Today; Springer: Boston, MA, USA, 1997; pp. 71–93. [Google Scholar] [CrossRef]
- Kerbey, A.L.; Vary, T.C.; Randle, P.J. Molecular mechanisms regulating myocardial glucose oxidation. Basic Res. Cardiol. 1985, 80, 93–96. [Google Scholar]
- Mingorance, C.; Rodriguez-Rodriguez, R.; Justo, M.L.; Herrera, M.D.; De Sotomayor, M.A. Pharmacological effects and clinical applications of propionyl-L-carnitine. Nutr. Rev. 2011, 69, 279–290. [Google Scholar] [CrossRef]
- Arduini, A.; Denisova, N.; Virmani, A.; Avrova, N.; Federici, G.; Arrigoni-Martelli, E. Evidence for the Involvement of Carnitine-Dependent Long-Chain Acyltransferases in Neuronal Triglyceride and Phospholipid Fatty Acid Turnover. J. Neurochem. 2008, 62, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sengupta, P.; Durairajanayagam, D. Role of L-carnitine in female infertility. Reprod. Biol. Endocrinol. 2018, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Dionyssopoulou, E.; Vassiliadis, S.; Evangeliou, A.; Koumantakis, E.; Athanassakis, I. Constitutive or induced elevated levels of l-carnitine correlate with the cytokine and cellular profile of endometriosis. J. Reprod. Immunol. 2005, 65, 159–170. [Google Scholar] [CrossRef]
- Pillich, R.T.; Scarsella, G.; Risuleo, G. Reduction of apoptosis through the mitochondrial pathway by the administration of acetyl-l-carnitine to mouse fibroblasts in culture. Exp. Cell Res. 2005, 306, 1–8. [Google Scholar] [CrossRef]
- Abdelrazik, H.; Sharma, R.; Mahfouz, R.Z.; Agarwal, A. L-Carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil. Steril. 2009, 91, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.; Wu, Y.; Sekar, M.; Chitranshi, N.; Malviya, R.; et al. Comprehensive Review of Methodology to Detect Reactive Oxygen Species (ROS) in Mammalian Species and Establish Its Relationship with Antioxidants and Cancer. Antioxidants 2021, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Williams, E.; Cadenas, E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001, 353, 411–416. [Google Scholar] [CrossRef]
- Aitken, R.; De Iuliis, G.; Gibb, Z.; Baker, M. The Simmet Lecture: New Horizons on an Old Landscape—Oxidative Stress, DNA Damage and Apoptosis in the Male Germ Line. Reprod. Domest. Anim. 2012, 47, 7–14. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [Green Version]
- Barhwal, K.; Hota, S.; Jain, V.; Prasad, D.; Singh, S.; Ilavazhagan, G. Acetyl-l-carnitine (ALCAR) prevents hypobaric hypoxia–induced spatial memory impairment through extracellular related kinase–mediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience 2009, 161, 501–514. [Google Scholar] [CrossRef]
- Aliabadi, E.; Mehranjani, M.S.; Borzoei, Z.; Talaei-Khozani, T.; Mirkhani, H.; Tabesh, H. Effects of L-carnitine and L-acetyl-carnitine on testicular sperm motility and chromatin quality. Iran. J. Reprod. Med. 2012, 10, 77–82. [Google Scholar]
- Sheweita, S.; Tilmisany, A.; Al-Sawaf, H. Mechanisms of Male Infertility: Role of Antioxidants. Curr. Drug Metab. 2005, 6, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Infante, J.P.; Tschanz, C.L.; Shaw, N.; Michaud, A.L.; Lawrence, P.; Brenna, J. Straight-Chain Acyl-CoA Oxidase Knockout Mouse Accumulates Extremely Long Chain Fatty Acids from α-Linolenic Acid: Evidence for Runaway Carousel-Type Enzyme Kinetics in Peroxisomal β-Oxidation Diseases. Mol. Genet. Metab. 2002, 75, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Falcone, T.; Mohamed, M.S.; Aleem, A.A.N.; Sharma, R.K.; Worley, S.E.; Thornton, J.; Agarwal, A. Differential growth of human embryos in vitro: Role of reactive oxygen species. Fertil. Steril. 2004, 82, 593–600. [Google Scholar] [CrossRef]
- Di Emidio, G.; Rea, F.; Placidi, M.; Rossi, G.; Cocciolone, D.; Virmani, A.; Macchiarelli, G.; Palmerini, M.G.; D’Alessandro, A.M.; Artini, P.G.; et al. Regulatory Functions of L-Carnitine, Acetyl, and Propionyl L-Carnitine in a PCOS Mouse Model: Focus on Antioxidant/Antiglycative Molecular Pathways in the Ovarian Microenvironment. Antioxidants 2020, 9, 867. [Google Scholar] [CrossRef]
- Várnagy, A.; Bene, J.; Sulyok, E.; Kovács, G.L.; Bódis, J.; Melegh, B. Acylcarnitine esters profiling of serum and follicular fluid in patients undergoing in vitro fertilization. Reprod. Biol. Endocrinol. 2013, 11, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krsmanović, L.Z.; Virmani, M.A.; Stojilković, S.S.; Catt, K.J. Actions of acetyl-L-carnitine on the hypothalamo-pituitary-gonadal system in female rats. J. Steroid Biochem. Mol. Biol. 1992, 43, 351–358. [Google Scholar] [CrossRef]
- Stark, R.; Reichenbach, A.; Andrews, Z.B. Hypothalamic carnitine metabolism integrates nutrient and hormonal feedback to regulate energy homeostasis. Mol. Cell. Endocrinol. 2015, 418, 9–16. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Tomatis, V.; Manzo, A.; Ressa, F.; Caroli, M.; Piccinini, M.; Ambrosetti, F.; Arnesano, M.; Despini, G.; Meczekalski, B. Treatment with carnitines, L-arginine and N-acetyl cysteine in patients affected by functional hypothalamic amenorrhea (FHA) induces hormonal and metabolic changes. Eur. Gynecol. Obstet. 2020, 2, 239–245. [Google Scholar]
- Genazzani, A.D.; Lanzoni, C.; Ricchieri, F.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Monteleone, P.; Jasonni, V.M. Acetyl-L-carnitine (ALC) administration positively affects reproductive axis in hypogonadotropic women with functional hypothalamic amenorrhea. J. Endocrinol. Investig. 2011, 34, 287–291. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Despini, G.; Prati, A.; Manzo, A.; Petrillo, T.; Tomatis, V.; Giannini, A.; Simoncini, T. Administration of Very Low Doses of Estradiol Modulates the LH Response to a GnRH Bolus and the LH and Cortisol Responses to Naloxone Infusion in Patients with Functional Hypothalamic Amenorrhea (FHA): A Pilot Study. Endocrines 2020, 1, 35–45. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Gastaldi, M.; Petraglia, F.; Battaglia, C.; Surico, N.; Volpe, A.; Genazzani, A.R. Naltrexone administration modulates the neuroendocrine control of luteinizing hormone secretion in hypothalamic amenorrhoea. Hum. Reprod. 1995, 10, 2868–2871. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Stomati, M.; Bersi, C.; Luisi, S.; Fedalti, M.; Santuz, M.; Esposito, G.; Petraglia, F.; Genazzani, A.R. Pivagabine decreases stress-related hormone secretion in women with hypothalamic amenorrhea. J. Endocrinol. Investig. 2000, 23, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Meczekalski, B.; Podfigurna-Stopa, A.; Santagni, S.; Rattighieri, E.; Ricchieri, F.; Chierchia, E.; Simoncini, T. Estriol administration modulates luteinizing hormone secretion in women with functional hypothalamic amenorrhea. Fertil. Steril. 2012, 97, 483–488. [Google Scholar] [CrossRef]
- Meczekalski, B.; Podfigurna-Stopa, A.; Warenik-Szymankiewicz, A.; Genazzani, A.R. Functional hypothalamic amenorrhea: Current view on neuroendocrine aberrations. Gynecol. Endocrinol. 2008, 24, 4–11. [Google Scholar] [CrossRef]
- Petraglia, F.; Vale, W.; Rivier, C. Opioids Act Centrally to Modulate Stress-Induced Decrease in Luteinizing Hormone in the Rat*. Endocrinology 1986, 119, 2445–2450. [Google Scholar] [CrossRef]
- Nappi, E.R.; Petraglia, F.; Genazzani, A.D.; D’Ambrogio, G.; Zara, C. Hypothalamic amenorrhea: Evidence for a central derangement of hypothalamic-pituitary-adrenal cortex axis activity. Fertil. Steril. 1993, 59, 571–576. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Petraglia, F.; Gastaldi, M.; Volpogni, C.; Gamba, O.; Genazzani, A.R. Naltrexone treatment restores menstrual cycles in patients with weight loss-related amenorrhea. Fertil. Steril. 1995, 64, 951–956. [Google Scholar] [CrossRef]
- Humphries, L.L.; Adams, L.J.; Eckfeldt, J.H.; Levitt, M.D.; McClain, C.J. Hyperamylasemia in Patients with Eating Disorders. Ann. Intern. Med. 1987, 106, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.R.; De Ramundo, B.M.; Criscuolo, M.; De Gaetani, C.; Ficarra, G.; Genazzani, A.; Petraglia, F.; Trentini, G.P. Acetyl-l-carnitine restores the daily pattern of hypothalamic β-endorphin in rats exposed to continuous light. Eur. J. Pharmacol. 1990, 186, 177–180. [Google Scholar] [CrossRef]
- Bidzinska, B.; Petraglia, F.; Angioni, S.; Genazzani, A.; Criscuoio, M.; Ficarra, G.; Gallinelli, A.; Trentini, G.P.; Genazzani, A.R. Acetyl-L-Carnitine Effect on Pituitary and Plasma?-Endorphin Responsiveness to Different Chronic Intermittent Stressors. J. Neuroendocr. 1993, 5, 151–155. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Petraglia, F.; Algeri, I.; Gastaldi, M.; Calvani, M.; Botticelli, G.; Genazzani, A.R. Acetyl-1-carnitine as possible drug in the treatment of hypothalamic amenorrhea. Acta Obstet. Gynecol. Scand. 1991, 70, 487–492. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Despini, G.; Czyzyk, A.; Podfigurna, A.; Simoncini, T.; Meczekalski, B. Modulatory effects of l-carnitine plus l-acetyl-carnitine on neuroendocrine control of hypothalamic functions in functional hypothalamic amenorrhea (FHA). Gynecol. Endocrinol. 2017, 33, 963–967. [Google Scholar] [CrossRef]
- Pocai, A.; Obici, S.; Schwartz, G.J.; Rossetti, L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005, 1, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genazzani, A.D.; Prati, A.; Genazzani, A.R.; Battipaglia, C.; Simoncini, T.; Szeliga, A.; Podfigurna, A.; Meczekalski, B. Synergistic effects of the integrative administration of acetyl-L-carnitine, L-carnitine, L-arginine and N-acetyl-cysteine on metabolic dynamics and on hepatic insulin extraction in overweight/obese patients with PCOS. Gynecol. Reprod. Endocrinol. Metab. 2020, 1, 56–63. [Google Scholar]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Chierchia, E.; Rattighieri, E.; Santagni, S.; Casarosa, E.; Luisi, M.; Genazzani, A.R. Metformin administration restores allopregnanolone response to adrenocorticotropic hormone (ACTH) stimulation in overweight hyperinsulinemic patients with PCOS. Gynecol. Endocrinol. 2010, 26, 684–689. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Despini, G.; Marini, G.; Prati, A.; Simoncini, T. Modulatory role of D-chiro-inositol (DCI) on LH and insulin secretion in obese PCOS patients. Gynecol. Endocrinol. 2014, 30, 438–443. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef] [Green Version]
- Malaguarnera, M.; Vacante, M.; Avitabile, T.; Malaguarnera, M.; Cammalleri, L.; Motta, M. l-Carnitine supplementation reduces oxidized LDL cholesterol in patients with diabetes. Am. J. Clin. Nutr. 2008, 89, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, H.; Jamilian, M.; Samimi, M.; Ebrahimi, F.A.; Rahimi, M.; Bahmani, F.; Aghababayan, S.; Kouhi, M.; Shahabbaspour, S.; Asemi, Z. Oral carnitine supplementation influences mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Gynecol. Endocrinol. 2017, 33, 442–447. [Google Scholar] [CrossRef]
- Fenkci, S.M.; Fenkci, V.; Oztekin, O.; Rota, S.; Karagenc, N. Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum. Reprod. 2008, 23, 1602–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molfino, A.; Cascino, A.; Conte, C.; Ramaccini, C.; Fanelli, F.R.; Laviano, A. Caloric Restriction and L-Carnitine Administration Improves Insulin Sensitivity in Patients With Impaired Glucose Metabolism. J. Parenter. Enter. Nutr. 2010, 34, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Samimi, M.; Jamilian, M.; Ebrahimi, F.A.; Rahimi, M.; Tajbakhsh, B.; Asemi, Z. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2016, 84, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Center, S.A.; Warner, K.L.; Randolph, J.F.; Sunvold, G.D.; Vickers, J.R. Influence of dietary supplementation withl-carnitine on metabolic rate, fatty acid oxidation, body condition, and weight loss in overweight cats. Am. J. Veter. Res. 2012, 73, 1002–1015. [Google Scholar] [CrossRef]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial Dysfunction in the Elderly: Possible Role in Insulin Resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef] [Green Version]
- Manco, M.; Calvani, M.; Mingrone, G. Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes. Metab. 2004, 6, 402–413. [Google Scholar] [CrossRef]

| Reference | Dose and Duration | Study Design/Subjects | Outcomes | |
|---|---|---|---|---|
| Stress in animal model | Bidzinska B et al. J Neuroendocrinol. 1993 [41] | ALC per os 10 mg/day/rat for 10 days at night. | Male Wistar rats were housed in hanging cages in groups of 6 in a light-controlled room (14 h light/ 10 h dark); temperature 20 °C. Food and water were available ad libitum. | ALC treatment was able to reverse the pituitary β-EP changes induced by stress. |
| Infertility in animal model | Krsmanović LZ et al. J Steroid Biochem Mol Biol. 1992 [27] | 50 mg pro kg/die of ALC for 2 consecutive estrous cycles | Female Sprague-Dawley rats of 3 months of age | Improved hormonal secretions through HPG axis: increased GnRH, LH, Estradiol and progesterone levels |
| Functional hypothalamic amenorrhea | Genazzani AD et al. Acta Obstet Gynecol Scand. 1991 [42] | ALC (2 g/day, per os) for 6 months | Twenty patients with hypothalamic amenorrhea subdivided in two groups according to their LH plasma levels: hypogonadotropic with plasma LH less than 3 mIU/mL and normogonadotropic with plasma LH greater than 3 mIU/mL. | The hypogonadotropic subjects showed an increase in baseline plasma LH levels, an increase in LH pulse amplitude with no changes in LH pulse frequency, and an increased response of LH to the latter GnRH bolus during the GnRH test. Hypogonadotropic patients also showed a significant increase in both estradiol and PRL. No significant differences were observed in the hormonal parameters of normogonadotropic patients. |
| Genazzani AD et al. J Endocrinol Invest. 2011 [30] | ALC (1 g/day, per os) for 16 weeks. | Twenty-four patients affected by stress-induced HA were divided into two groups according to LH plasma levels: hypo-LH (LH ≤ 3 mIU/mL; no. = 16), and normo-LH (LH > 3 mIU/mL; no. = 8). | Hypo-LH patients showed a significant increase in LH plasma levels and in LH pulse amplitude. No changes were observed in the normo-LH group. LH response to naloxone was restored under ALC therapy. Maximal LH response and area under the curve under naloxone were significantly increased.No changes were observed in the normo-LH patients. | |
| Genazzani AD et al. Gynecol Endocrinol. 2017 [43] | Combined integrative treatment for 12 weeks of ALC (250 mg/die) and L-carnitine (500 mg/die) | Twenty-seven patients with FHA. | Significant increase of LH plasma levels and the significant decrease of both cortisol and amylase plasma levels. The increased 17OHP/cortisol ratio, as index of the adrenal activity, demonstrated the reduced stress-induced adrenal activity. | |
| Genazzani AD et al. European Gynecology and Obstetrics. 2020 [29] | L-carnitine (500 mg) and acetyl-L-carnitine (250 mg) combined with LArg (500 mg), NAC (50 mg), and vitamins E and C as antioxidants, were administered daily for 12 weeks. | Twenty-nine patients with FHA. | LH and insulin increased, while amylase and cortisol decreased. | |
| Polycystic Ovary Syndrome | Malaguarnera M et al. Am J Clin Nutr. 2009 [51] | The 2 groups received either 2 g L-carnitine once daily or placebo. | Eighty-one patients with diabetes were randomly assigned to 1 of 2 treatment groups for 3 months. | L-carnitine-treated patients showed significant improvements compared with the placebo group in the following markers: LDL levels, triglycerides, apolipoprotein A1 and apolipoprotein B-100 concentrations decreased. |
| Molfino A. et al. J Parenter Enteral Nutr. 2010 [54] | Hypocaloric diet or the same dietetic regimen in addition to oral L-carnitine (2 g twice daily) supplementation for 10 days. | Sixteen Patients were randomly assigned to two groups | OGTT at 2 h improved in both groups. Only in the L-carnitine-supplemented group did plasma insulin levels and HOMA-IR significantly decrease when compared to baseline values. | |
| Samimi M et al. Clin Endocrinol (Oxf). 2016 [55] | 250 mg carnitine supplements or placebo for 12 weeks. | Sixty overweight patients diagnosed with PCOS; randomized. | Taking carnitine supplements resulted in a significant reduction in weight, BMI, waist circumference and hip circumference compared with placebo. In addition, carnitine administration in women with PCOS led to a significant reduction in fasting plasma glucose, serum insulin levels, homoeostasis model of assessment-insulin resistance and dehydroepiandrosterone sulphate. | |
| Jamilian H et al. Gynecological Endocrinology. 2017 [52] | 250 mg carnitine supplements or placebo for 12 weeks. | Sixty patients diagnosed with PCOS were randomized to take either carnitine supplements (n = 30) or placebo (n = 30). | Carnitine supplementation resulted in a significant improvement in Beck Depression Inventory total score, General Health Questionnaire scores and Depression Anxiety and Stress Scale scores. | |
| Genazzani AD et al. Gynecol Reprod Endocrinol Metab 2020 [45] | ALC (250 mg), L-carnitine (500 mg), L-arginine (500 mg) and N-acetyl cysteine (50 mg) were administered daily for 24 weeks | Fortyfive overweight/obese PCOS patients underwent daily integrative administration and were evaluated before and after 12 and 24 weeks of treatment. | After 12 and 24 weeks of treatment, all the subjects showed significant reduction of plasma insulin levels. Trygliceride, total cholesterol and HOMA index decreased while HDL increased significantly. On oral glucose tolerance testing, 39 out of the 45 PCOS patients showed a hyperinsulinemic response. This latter group showed the greatest significant reduction of all metabolic parameters and of the hepatic insulin extraction index (HIE). No changes were observed in normoinsulinemic PCOS patients (6 out of 45). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrillo, T.; Battipaglia, C.; Virmani, M.A.; Genazzani, A.R.; Genazzani, A.D. Neuroendocrine Effects of Carnitines on Reproductive Impairments. Int. J. Mol. Sci. 2021, 22, 10781. https://doi.org/10.3390/ijms221910781
Petrillo T, Battipaglia C, Virmani MA, Genazzani AR, Genazzani AD. Neuroendocrine Effects of Carnitines on Reproductive Impairments. International Journal of Molecular Sciences. 2021; 22(19):10781. https://doi.org/10.3390/ijms221910781
Chicago/Turabian StylePetrillo, Tabatha, Christian Battipaglia, Mohamed Ashraf Virmani, Andrea R. Genazzani, and Alessandro D. Genazzani. 2021. "Neuroendocrine Effects of Carnitines on Reproductive Impairments" International Journal of Molecular Sciences 22, no. 19: 10781. https://doi.org/10.3390/ijms221910781






