Exosomes in Alzheimer’s Disease: From Being Pathological Players to Potential Diagnostics and Therapeutics
Abstract
:1. Introduction
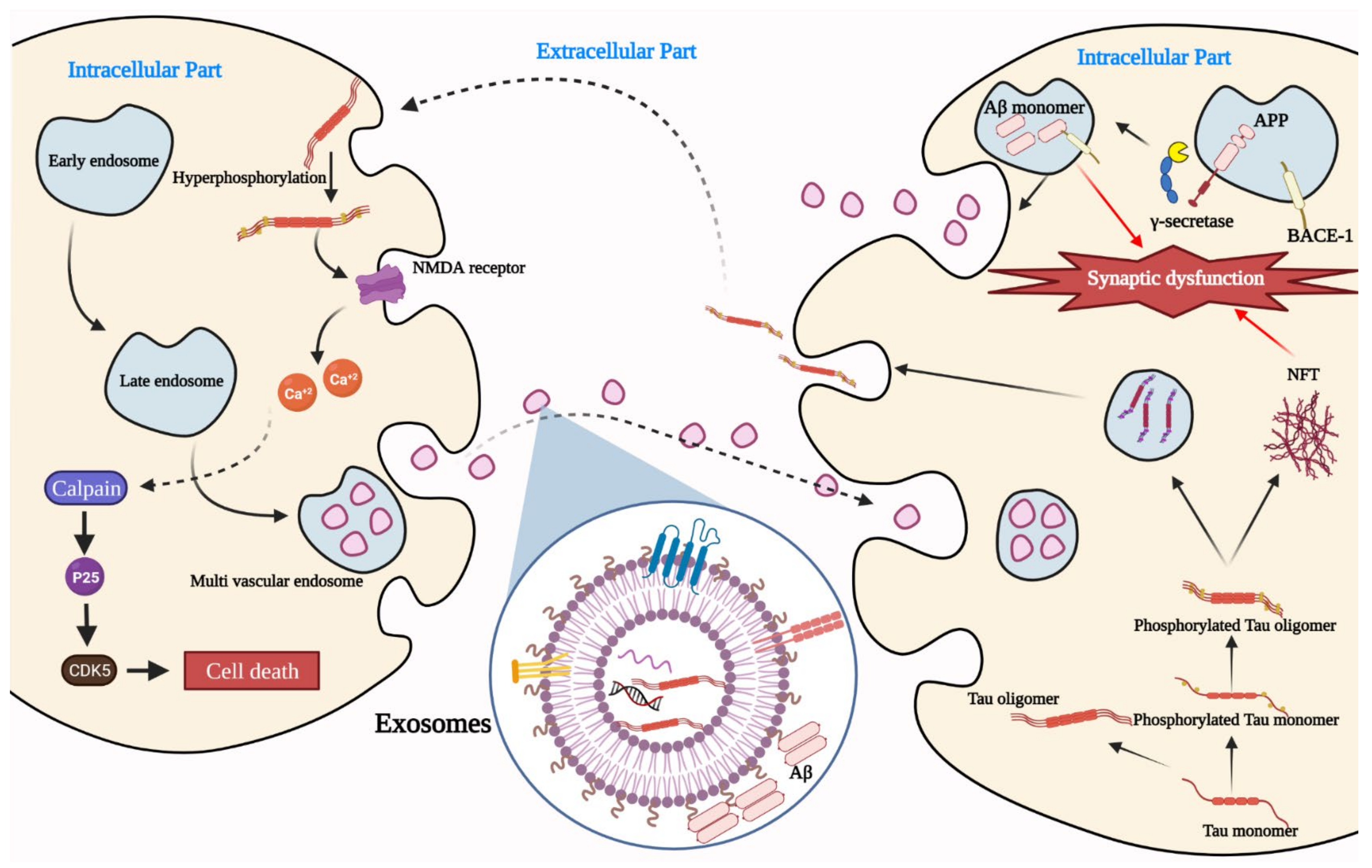
2. Exosomes as a Player in AD Pathogenesis
3. Exosomes and Blood–Brain Barrier
4. Exosomes as a Drug Carrier
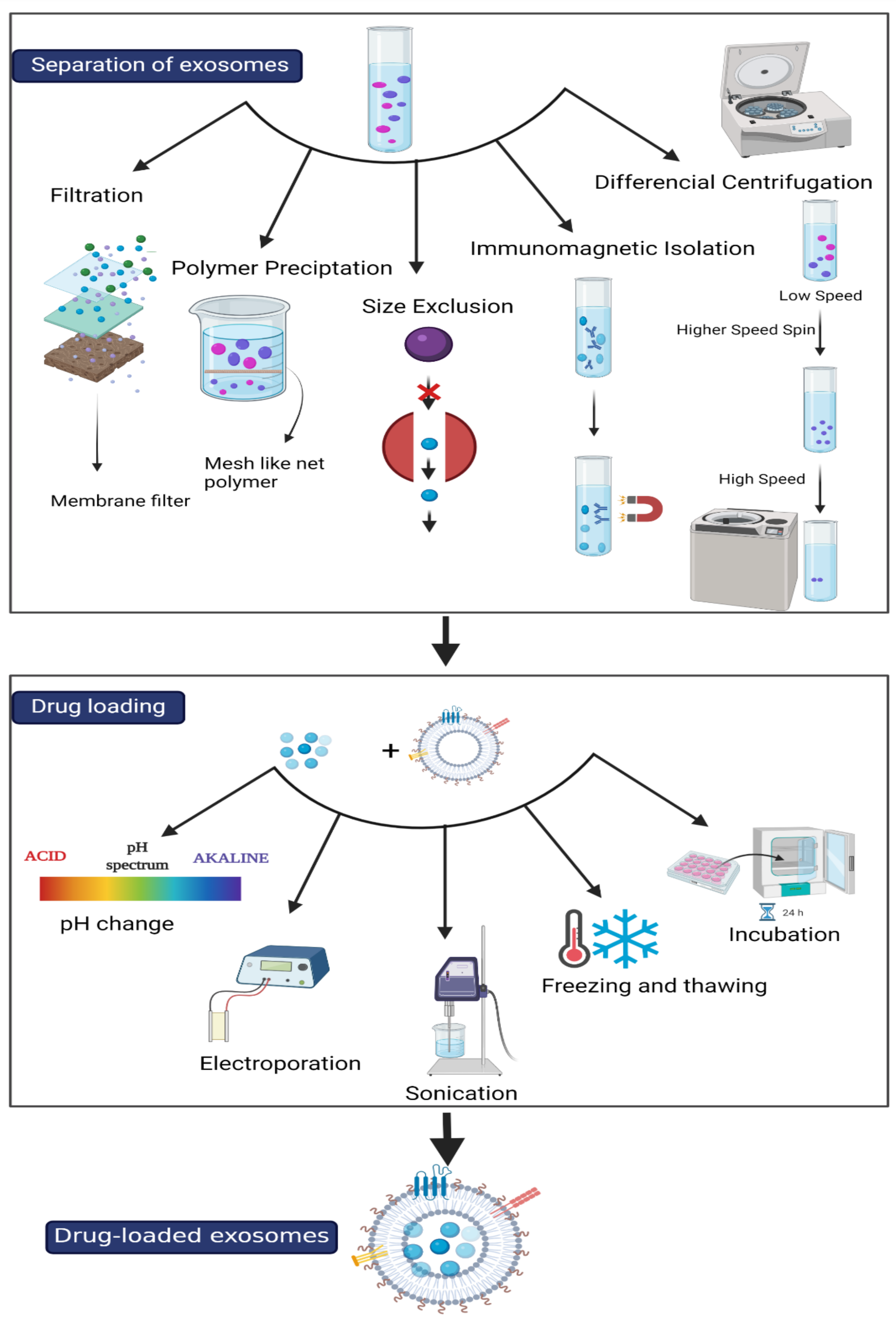
4.1. Isolation of Exosomes
4.1.1. Ultracentrifugation
4.1.2. Size-Exclusion Liquid Chromatography
4.1.3. Immuno-Isolation
4.1.4. Microfluidic Isolation
4.1.5. Paper-Based Immunoaffinity
| Isolation Method | Advantages | Disadvantages | Ref |
|---|---|---|---|
| UC |
|
| [56,57,58,60] |
| SEC |
|
| [59,60] |
| Microfluidic Isolation |
|
| [57,60] |
| Paper-Based Immunoaffinity |
|
| [60,62] |
| Immuno-Isolation |
|
| [56,61] |
4.2. Drug Loading to Exosomes
4.2.1. Passive Loading
4.2.2. Active Loading
4.3. Role of Exosomes in AD Treatment
4.3.1. As a Drug Delivery Vehicle
4.3.2. Enzyme Delivery
4.3.3. miRNA Delivery
4.3.4. Toxic Waste Scavenging
4.3.5. Neuroprotective Effects
5. Diagnostic Role of Exosomes
5.1. Role of Exosomes in AD Diagnosis
5.1.1. Blood Samples
5.1.2. Plasma Samples
5.1.3. Serum Samples
5.1.4. Cerebrospinal Fluid Samples
5.1.5. Saliva Samples
5.1.6. Urine Samples
| Biofluid | Increasing | Decreasing | Ref |
|---|---|---|---|
| Serum | miR-193b | [139] | |
| miR-193b * | [140] | ||
| miR-135a and miR-384 | [124,141] | ||
| miR-193b | [124] | ||
| miR-128 | [142] | ||
| Plasma | miR-193b | [139] | |
| miR-92a-3p, miR-181c-5p and miR-210-3p | [143] | ||
| CSF | miR-193b | [139] | |
| miR-135a | [141] | ||
| miR-193b * | [140] |
6. Future Outlook
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reisberg, B.; Borenstein, J.; Salob, S.P.; Ferris, S.H.; Franssen, E.; Georgotas, A. Behavioral symptoms in Alzheimer’s disease: Phenomenology and treatment. J. Clin. Psychiatry 1987, 48, 9–15. [Google Scholar]
- Merriam, A.E.; Aronson, M.K.; Gaston, P.; Wey, S.-L.; Katz, I. The Psychiatric Symptoms of Alzheimer’s Disease. J. Am. Geriatr. Soc. 1988, 36, 7–22. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Ahn, G.; Park, G.-Y.; Park, D.-Y.; Lee, S.-H.; Ahn, J.-Y.; Kim, Y.-H. The Role of Aptamer Loaded Exosome Complexes in the Neurodegenerative Diseases. Toxicol. Environ. Heal. Sci. 2019, 11, 85–93. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. Ekavali A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.A.M.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernández, J.; Campos-Peña, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Martins, T.S.; Trindade, D.; Vaz, M.; Campelo, I.; Almeida, M.; Trigo, G.; Silva, O.A.B.D.C.E.; Henriques, A.G. Diagnostic and therapeutic potential of exosomes in Alzheimer’s disease. J. Neurochem. 2020, 156, 162–181. [Google Scholar] [CrossRef]
- Farlow, M.R.; Miller, M.L.; Pejovic, V. Treatment Options in Alzheimer’s Disease: Maximizing Benefit, Managing Expectations. Dement. Geriatr. Cogn. Disord. 2008, 25, 408–422. [Google Scholar] [CrossRef]
- Braccioli, L.; van Velthoven, C.; Heijnen, C.J. Exosomes: A New Weapon to Treat the Central Nervous System. Mol. Neurobiol. 2013, 49, 113–119. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Watson, L.S.; Hamlett, E.D.; Stone, T.D.; Sims-Robinson, C. Neuronally derived extracellular vesicles: An emerging tool for understanding Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 22. [Google Scholar] [CrossRef]
- Lotvall, J.; Valadi, H. Cell to Cell Signalling via Exosomes Through esRNA. Cell Adhes. Migr. 2007, 1, 156–158. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, T.; Zhu, Y.; Ali, D.J.; Hu, F.; Qi, Y.; Sun, B.; Xiao, Z. Exosomes Transfer Among Different Species Cells and Mediating miRNAs Delivery. J. Cell. Biochem. 2017, 118, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Ramos, D.; Albuquerque, P.; Carmona, V.; Perfeito, R.; Nobre, R.J.; de Almeida, L.P. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J. Control. Release 2017, 262, 247–258. [Google Scholar] [CrossRef]
- Saeedi, S.; Israel, S.; Nagy, C.; Turecki, G. The emerging role of exosomes in mental disorders. Transl. Psychiatry 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mustapic, M.; Eitan, E.; Werner, K.; Berkowitz, S.; Lazaropoulos, M.P.; Tran, J.; Goetzl, E.J.; Kapogiannis, D. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front. Neurosci. 2017, 11, 278. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Pisitkun, T.; Aponte, A.; Yuen, P.; Hoffert, J.; Yasuda, H.; Hu, X.; Chawla, L.; Shen, R.-F.; Knepper, M.; et al. Exosomal Fetuin-A identified by proteomics: A novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006, 70, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Liu, J.; Huang, B.; Chen, X.; Chen, X.-M.; Xu, Y.-M.; Huang, L.-F.; Wang, X.-Z. Exosomes: Novel Biomarkers for Clinical Diagnosis. Sci. World J. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Zhou, H.; Cheruvanky, A.; Hu, X.; Matsumoto, T.; Hiramatsu, N.; Cho, M.E.; Berger, A.; Leelahavanichkul, A.; Doi, K.; Chawla, L.S.; et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008, 74, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Howitt, J.; Hill, A.F. Exosomes in the Pathology of Neurodegenerative Diseases. J. Biol. Chem. 2016, 291, 26589–26597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busche, M.A.; Staufenbiel, M.; Willem, M.; Haass, C.; Förstl, H. Mechanismen der Alzheimer-Krankheit. Der Nervenarzt 2016, 87, 1163–1174. [Google Scholar] [CrossRef]
- Lakshmi, S.; Essa, M.M.; Hartman, R.E.; Guillemin, G.; Sivan, S.; Elumalai, P. Exosomes in Alzheimer’s Disease: Potential Role as Pathological Mediators, Biomarkers and Therapeutic Targets. Neurochem. Res. 2020, 45, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-Beta: A Crucial Factor in Alzheimer’s Disease. Med. Princ. Pr. 2014, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Sagare, A.P.; Ma, Q.; Halliday, M.R.; Kong, P.; Kisler, K.; Winkler, E.A.; Ramanathan, A.; Kanekiyo, T.; Bu, G. Cen-tral Role for PICALM in Amyloid-β Blood-Brain Barrier Transcytosis and Clearance. Nature neuroscience 2015, 18, 978–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer’s Disease β-Amyloid Pep-tides Are Released in Association with Exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharples, R.A.; Vella, L.; Nisbet, R.; Naylor, R.; Perez, K.; Barnham, K.J.; Masters, C.L.; Hill, A. Inhibition of γ-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008, 22, 1469–1478. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated Exosome Secretion Promotes Clearance of Amyloid-β by Microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef] [Green Version]
- Sinha, M.S.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, K.; Liu, F.; Gong, C.-X.; Grundke-Iqbal, I. Tau in Alzheimer Disease and Related Tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Avila, J.; Lucas, J.J.; Pérez, M.; Hernandez, F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef]
- Drubin, D.G.; Kirschner, M.W. Tau protein function in living cells. J. Cell Biol. 1986, 103, 2739–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, A.D.C.; Zaidi, T.; Novak, M.; Grundke-Iqbal, I.; Iqbal, K. Hyperphosphorylation induces self-assembly of into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. USA 2001, 98, 6923–6928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perea, J.R.; Llorens-Martín, M.; Avila, J.; Bolós, M. The Role of Microglia in the Spread of Tau: Relevance for Tauopathies. Front. Cell. Neurosci. 2018, 12, 172. [Google Scholar] [CrossRef] [Green Version]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-Y.; Ke, Z.-Y.; Xiao, M.; Quazi, S.H. Exosomes: A novel therapeutic target for Alzheimer’s disease? Neural Regen. Res. 2018, 13, 930–935. [Google Scholar] [CrossRef]
- Engel, P.A. Does metabolic failure at the synapse cause Alzheimer’s disease? Med. Hypotheses 2014, 83, 802–808. [Google Scholar] [CrossRef]
- Chen, J.X.; Yan, S.S. Role of Mitochondrial Amyloid-β in Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 20, S569–S578. [Google Scholar] [CrossRef] [Green Version]
- Crews, L.; Masliah, E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010, 19, R12–R20. [Google Scholar] [CrossRef]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. In Handbook of Clinical Neurology; Elsevier, B.V.: Amsterdam, The Netherlands, 2019; Volume 167, pp. 231–255. [Google Scholar]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 1–24. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Serlin, Y.; Shelef, I.; Knyazer, B.; Friedman, A. Anatomy and physiology of the blood–brain barrier. Semin. Cell Dev. Biol. 2015, 38, 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 523–534. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug Targeting to the Brain. Pharmaceutical research 2007, 24, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef]
- Alvarez, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered An-ticancer Drugs across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharmaceutical research 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [Green Version]
- Lazo, P. Roles of tetraspanin proteins in cell and tumor biology. Atlas Genet. Cytogenet. Oncol. Haematol. 2011. [Google Scholar] [CrossRef]
- Hantak, M.P.; Qing, E.; Earnest, J.T.; Gallagher, T. Tetraspanins: Architects of Viral Entry and Exit Platforms. J. Virol. 2019, 93, e01429-17. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-P.; Lin, Z.-X.; Jiang, X.-Y.; Yu, X.-Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Li, Y.; Chopp, M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-A.; Lu, C.-H.; Ke, C.-C.; Chiu, S.-J.; Jeng, F.-S.; Chang, C.-W.; Yang, B.-H.; Liu, R.-S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Akuma, P.; Okagu, O.D.; Udenigwe, C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Lobb, R.; Möller, A. Size Exclusion Chromatography: A Simple and Reliable Method for Exosome Purification. In Extracellular Vesicles; Springer: Berlin/Heidelberg, Germany, 2017; pp. 105–110. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Sung, C.W.-H.; Chen, C.; Cheng, C.-M.; Lin, D.P.-C.; Huang, C.-T.; Hsu, M.-Y. Advances in exosomes technology. Clin. Chim. Acta 2019, 493, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Chiu, C.-C.; Wang, J.-Y.; Huang, C.-T.; Huang, Y.-F.; Liou, J.-C.; Chen, C.; Chen, H.-C.; Cheng, C.-M. Paper-Based Microfluidic Platforms for Understanding the Role of Exosomes in the Pathogenesis of Major Blindness-Threatening Diseases. Nanomaterials 2018, 8, 310. [Google Scholar] [CrossRef] [Green Version]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Yuyama, K.; Igarashi, Y. Exosomes as Carriers of Alzheimer’s Amyloid-ß. Front. Neurosci. 2017, 11, 229. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, P.; Yao, X.; Li, H.; Shen, H.; Li, X.; Wu, J.; Lu, X. Exosomes Derived From miR-133b-Modified Mesenchymal Stem Cells Promote Recovery After Spinal Cord Injury. Front. Neurosci. 2018, 12, 845. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O.; et al. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharmacol. 2018, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Chen, C.-L.; Rajesh, R. Optimized liposomes with transactivator of transcription peptide and anti-apoptotic drugs to target hippocampal neurons and prevent tau-hyperphosphorylated neurodegeneration. Acta Biomater. 2019, 87, 207–222. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, L.; Jiang, Y.; Shi, Y.; Sui, H.; Zhao, L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020, 27, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, M.; Maurya, A.K. Quercetin Loaded Nanoparticles in Targeting Cancer: Recent Development. Anti-Cancer Agents Med. Chem. 2019, 19, 1560–1576. [Google Scholar] [CrossRef]
- Fernandes, M.; Lopes, I.; Magalhães, L.; Sárria, M.P.; Machado, R.; Sousa, J.C.; Botelho, C.; Teixeira, J.; Gomes, A.C. Novel concept of exosome-like liposomes for the treatment of Alzheimer’s disease. J. Control. Release 2021, 336, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective Effects of Curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Sprigner: Berlin/Heidelberg, Germany, 2007; Volume 595, pp. 197–212. [Google Scholar] [CrossRef] [Green Version]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, srep01197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.E.-M.B.; Murakami, M.; Hirose, Y.; Nakashima, M. Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer’s Disease Treatment: An In Vitro Study. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Oki, K.; Ochiya, T. Potential Application of Extracellular Vesicles of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Alzheimer’s Disease Therapeutics. Stem Cell Renew. Cell-Cell Commun. 2014, 1212, 171–181. [Google Scholar] [CrossRef]
- Bulloj, A.; Leal, M.C.; Xu, H.; Castaño, E.M.; Morelli, L. Insulin-Degrading Enzyme Sorting in Exosomes: A Secretory Path-way for a Key Brain Amyloid-β Degrading Protease. J. Alzheimer’s Dis. 2010, 19, 79–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-L.; Miller, J.D.; Ying, S.-Y. Intronic MicroRNA (miRNA). J. Biomed. Biotechnol. 2006, 2006, 1–13. [Google Scholar] [CrossRef]
- Manna, I.; De Benedittis, S.; Quattrone, A.; Maisano, D.; Iaccino, E.; Quattrone, A. Exosomal miRNAs as Potential Diagnostic Biomarkers in Alzheimer’s Disease. Pharmaceuticals 2020, 13, 243. [Google Scholar] [CrossRef]
- Manczak, M.; Reddy, P.H. RNA silencing of genes involved in Alzheimer’s disease enhances mitochondrial function and synaptic activity. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 2368–2378. [Google Scholar] [CrossRef] [Green Version]
- Morel, L.; Regan, M.; Higashimori, H.; Ng, S.K.; Esau, C.; Vidensky, S.; Rothstein, J.; Yang, Y. Neuronal Exosomal MiR-NA-Dependent Translational Regulation of Astroglial Glutamate Transporter GLT1. J. Biol. Chem. 2013, 288, 7105–7116. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.R.; Binder, D.K. Post-translational Regulation of GLT-1 in Neurological Diseases and Its Potential as an Effective Therapeutic Target. Front. Mol. Neurosci. 2019, 12, 164. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Sakai, S.; Mitsutake, S.; Okada, M.; Tahara, H.; Furukawa, J.; Fujitani, N.; Shinohara, Y.; Igarashi, Y. De-creased Amyloid-β Pathologies by Intracerebral Loading of Glycosphingolipid-Enriched Exosomes in Alzheimer Model Mice. J. Biol. Chem. 2014, 289, 24488–24498. [Google Scholar] [CrossRef] [Green Version]
- An, K.; Klyubin, I.; Kim, Y.; Jung, J.H.; Mably, A.J.; O’Dowd, S.T.; Lynch, T.; Kanmert, D.; Lemere, C.A.; Finan, G.M.; et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Mol. Brain 2013, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Ji, X.; Lv, R.; Pei, J.-J.; Du, Y.; Shen, C.; Hou, X. Targetting Exosomes as a New Biomarker and Therapeutic Approach for Alzheimer’s Disease. Clin. Interv. Aging 2020, ume 15, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Hao, P.; Liang, Z.; Piao, H.; Ji, X.; Wang, Y.; Liu, Y.; Liu, R.; Liu, J. Conditioned Medium of Human Adipose-Derived Mesen-chymal Stem Cells Mediates Protection in Neurons Following Glutamate Excitotoxicity by Regulating Energy Metabolism and GAP-43 Expression. Metab. Brain Dis. 2014, 29, 193–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [Green Version]
- Meffert, M.K.; Chang, J.M.; Wiltgen, B.J.; Fanselow, M.S.; Baltimore, D. NF-κB functions in synaptic signaling and behavior. Nat. Neurosci. 2003, 6, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wu, J.; Mou, F.; Xie, W.; Wang, F.; Wang, Q.; Fang, J.; Xu, Y.; Dong, Y.; Liu, J. Exosomes Derived from Hypox-ia-preconditioned Mesenchymal Stromal Cells Ameliorate Cognitive Decline by Rescuing Synaptic Dysfunction and Regu-lating Inflammatory Responses in APP/PS1 Mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malm, T.; Loppi, S.; Kanninen, K.M. Exosomes in Alzheimer’s disease. Neurochem. Int. 2016, 97, 193–199. [Google Scholar] [CrossRef]
- Liu, W.; Bai, X.; Zhang, A.; Huang, J.; Xu, S.; Zhang, J. Role of Exosomes in Central Nervous System Diseases. Front. Mol. Neurosci. 2019, 12, 240. [Google Scholar] [CrossRef]
- Xiao, T.; Zhang, W.; Jiao, B.; Pan, C.-Z.; Liu, X.; Shen, L. The role of exosomes in the pathogenesis of Alzheimer’ disease. Transl. Neurodegener. 2017, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellu-lar Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteom. Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.; Wildman, D.E. Extracellular Vesicles and the Promise of Continuous Liquid Biopsies. J. Pathol. Transl. Med. 2018, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Li, Y.; Zhang, J.; Rong, J.; Ye, S. Epidermal Growth Factor Receptor-Containing Exosomes Induce Tumor-Specific Regulatory T Cells. Cancer Investig. 2013, 31, 330–335. [Google Scholar] [CrossRef]
- Clark, D.J.; Fondrie, W.; Yang, A.; Mao, L. Triple SILAC quantitative proteomic analysis reveals differential abundance of cell signaling proteins between normal and lung cancer-derived exosomes. J. Proteom. 2016, 133, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N.; Baek, R.; Jakobsen, K.R.; Meldgaard, P.; Folkersen, B.; Rasmussen, T.R.; Varming, K.; Jørgensen, M.M.; Sorensen, B. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 2016, 10, 1595–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, J.; Su, W.; Liu, L.; Rao, B.; Lin, M.; Feng, Y.; Qiu, F.; Chen, J.; Zhou, Q.; Zhao, Z.; et al. Identification of Three Circular RNA Cargoes in Serum Exosomes as Diagnostic Biomarkers of Non–Small-Cell Lung Cancer in the Chinese Population. J. Mol. Diagn. 2020, 22, 1096–1108. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef]
- Mitchell, P.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [Green Version]
- Brase, J.C.; Johannes, M.; Schlomm, T.; Fälth, M.; Haese, A.; Steuber, T.; Beißbarth, T.; Kuner, R.; Sültmann, H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer 2010, 128, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jutzy, J.; Valenzuela, M.M.A.; Turay, D.; Aspe, J.R.; Ashok, A.; Mirshahidi, S.; Mercola, D.; Lilly, M.B.; Wall, N.R. Plasma-Derived Exosomal Survivin, a Plausible Biomarker for Early Detection of Prostate Cancer. PLoS ONE 2012, 7, e46737. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.; Widmark, A. Prostate Can-cer-Derived Urine Exosomes: A Novel Approach to Biomarkers for Prostate Cancer. Br. J. Cancer 2009, 100, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kamohara, H.; Kinoshita, K.; Kurashige, J.; Ishimoto, T.; Iwatsuki, M.; Watanabe, M.; Baba, H. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer 2012, 119, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, N.; Hoshino, I.; Mori, M.; Akutsu, Y.; Hanari, N.; Yoneyama, Y.; Ikeda, N.; Isozaki, Y.; Maruyama, T.; Akanuma, N.; et al. Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br. J. Cancer 2013, 108, 644–652. [Google Scholar] [CrossRef]
- Corcoran, C.; Friel, A.; Duffy, M.J.; Crown, J.; O’Driscoll, L. Intracellular and Extracellular MicroRNAs in Breast Cancer. Clin. Chem. 2011, 57, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Hong, B.S.; Cho, J.-H.; Kim, H.; Choi, E.-J.; Rho, S.; Kim, J.; Kim, J.H.; Choi, D.-S.; Kim, Y.-K.; Hwang, D.; et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genom. 2009, 10, 556. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Gao, W.; Lv, X.; Xu, X.; Zhang, Z.; Yan, J.; Mao, G.; Bu, Z. The Diagnostic Value of Exosome-Derived Biomarkers in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis. Front. Aging Neurosci. 2021, 13. [Google Scholar] [CrossRef]
- Longobardi, A.; Benussi, L.; Nicsanu, R.; Bellini, S.; Ferrari, C.; Saraceno, C.; Zanardini, R.; Catania, M.; Di Fede, G.; Squitti, R. Plasma Extracellular Vesicle Size and Concentration Are Altered in Alzheimer’s Disease, Dementia With Lewy Bodies, and Frontotemporal Dementia. Front. Cell Dev. Biol. 2021, 9, 667369. [Google Scholar]
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Krüger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener. 2017, 12, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2014, 11, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Liu, F.; Meng, M.; Zhang, L.; Gordon, M.L.; Wang, Y.; Cai, L.; Zhang, N. Elevated matrix metalloproteinase-9 levels in neuronal extracellular vesicles in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2020, 7, 1681–1691. [Google Scholar] [CrossRef]
- Abner, E.L.; Jicha, G.A.; Shaw, L.M.; Trojanowski, J.Q.; Goetzl, E.J. Plasma neuronal exosomal levels of Alzheimer’s disease biomarkers in normal aging. Ann. Clin. Transl. Neurol. 2016, 3, 399–403. [Google Scholar] [CrossRef]
- Winston, C.N.; Goetzl, E.J.; Akers, J.C.; Carter, B.S.; Rockenstein, E.M.; Galasko, D.; Masliah, E.; Rissman, R.A. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2016, 3, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.; Li, Y.; Yan, Y.; Qiu, Y.; Li, B.; Xu, W.; Wang, Y.; Liu, J.; Deng, Y. Increased Prediction Value of Biomarker Combina-tions for the Conversion of Mild Cognitive Impairment to Alzheimer’s Dementia. Transl. Neurodegener. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Hamlett, E.D.; Ledreux, A.; Potter, H.; Chial, H.J.; Patterson, D.; Espinosa, J.M.; Bettcher, B.M.; Granholm, A.-C. Exosomal biomarkers in Down syndrome and Alzheimer’s disease. Free. Radic. Biol. Med. 2017, 114, 110–121. [Google Scholar] [CrossRef]
- Hamlett, E.D.; Goetzl, E.J.; Ledreux, A.; Vasilevko, V.; Boger, H.A.; LaRosa, A.; Clark, D.; Carroll, S.L.; Carmona-Iragui, M.; Fortea, J.; et al. Neuronal exosomes reveal Alzheimer’s disease biomarkers in Down syndrome. Alzheimer’s Dement. 2016, 13, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Fu, A.K.; Ip, N.Y. Synaptic dysfunction in Alzheimer’s disease: Mechanisms and therapeutic strategies. Pharmacol. Ther. 2018, 195, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, Q.; Liu, F.-F.; Hu, F.; Xie, A.; Zhu, L.-Q.; Liu, D. Synaptic Dysfunction in Alzheimer’s Disease: Aβ, Tau, and Epi-genetic Alterations. Mol. Neurobiol. 2018, 55, 3021–3032. [Google Scholar] [CrossRef]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y.; et al. Blood neuro-exosomal synaptic proteins predict Alzheimer’s disease at the asymptomatic stage. Alzheimer’s Dement. 2020, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Nogueras-Ortiz, C.; Mustapic, M.; Mullins, R.J.; Abner, E.L.; Schwartz, J.B.; Kapogiannis, D. Deficient neurotrophic factors of CSPG4-type neural cell exosomes in Alzheimer disease. FASEB J. 2018, 33, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Liu, C.G.; Gao, S.C.; Zhang, Y.; Wang, P.C. The Serum Exosome Derived MicroRNA− 135a,− 193b, And− 384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. 2018, 31, 87–96. [Google Scholar]
- Dong, H.; Li, J.; Huang, L.; Chen, X.; Li, D.; Wang, T.; Hu, C.; Xu, J.; Zhang, C.; Zen, K.; et al. Serum MicroRNA Profiles Serve as Novel Biomarkers for the Diagnosis of Alzheimer’s Disease. Dis. Markers 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, L.; Chen, J.; Ni, F.; Zhang, Y.; Liu, F. Isolation of Exosome Nanoparticles from Human Cerebrospinal Fluid for Proteomic Analysis. ACS Appl. Nano Mater. 2021, 4, 3351–3359. [Google Scholar] [CrossRef]
- Jia, L.; Qiu, Q.; Zhang, H.; Chu, L.; Du, Y.; Zhang, J.; Zhou, C.; Liang, F.; Shi, S.; Wang, S.; et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s Dement. 2019, 15, 1071–1080. [Google Scholar] [CrossRef]
- Blennow, K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRX 2004, 1, 213–225. [Google Scholar] [CrossRef]
- Andreasen, N.; Minthon, L.; Davidsson, P.; Vanmechelen, E.; Vanderstichele, H.; Winblad, B.; Blennow, K. Evaluation of CSF-tau and CSF-Aβ42 as Diagnostic Markers for Alzheimer Disease in Clinical Practice. Arch. Neurol. 2001, 58, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.; Perestelo-Pérez, L.; Westman, E.; Wahlund, L.O.; Sarrisa, A.; Serrano-Aguilar, P. Meta-Review of CSF Core Bi-omarkers in Alzheimer’s Disease: The State-of-the-Art after the New Revised Diagnostic Criteria. Front. Aging Neuro-Sci. 2014, 6, 47. [Google Scholar]
- Tarawneh, R.; D’Angelo, G.; Ba, E.M.; Xiong, C.; Carter, D.; Cairns, N.J.; Fagan, A.M.; Head, D.; Mintun, M.A.; Ladenson, J.H.; et al. Visinin-like protein-1: Diagnostic and prognostic biomarker in Alzheimer disease. Ann. Neurol. 2011, 70, 274–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagan, A.M.; Roe, C.M.; Xiong, C.; Mintun, M.A.; Morris, J.C.; Holtzman, D.M. Cerebrospinal Fluid tau/β-Amyloid42 Ratio as a Prediction of Cognitive Decline in Nondemented Older Adults. Arch. Neurol. 2007, 64, 343–349. [Google Scholar] [CrossRef]
- Janelidze, S.; Zetterberg, H.; Mattsson-Carlgren, N.; Palmqvist, S.; Vanderstichele, H.; Lindberg, O.; van Westen, D.; Stomrud, E.; Minthon, L.; Blennow, K.; et al. CSF A β 42/A β 40 and A β 42/A β 38 ratios: Better diagnostic markers of Alzheimer disease. Ann. Clin. Transl. Neurol. 2016, 3, 154–165. [Google Scholar] [CrossRef] [Green Version]
- Wiltfang, J.; Esselmann, H.; Bibl, M.; Hull, M.; Hampel, H.; Kessler, H.; Frölich, L.; Schröder, J.; Peters, O.; Jessen, F.; et al. Amyloid β peptide ratio 42/40 but not Aβ42 correlates with phospho-Tau in patients with low- and high-CSF Aβ40 load. J. Neurochem. 2006, 101, 1053–1059. [Google Scholar] [CrossRef]
- Gervaise-Henry, C.; Watfa, G.; Albuisson, E.; Kolodziej, A.; Dousset, B.; Olivier, J.-L.; Jonveaux, T.R.; Malaplate-Armand, C. Cerebrospinal Fluid Aβ42/Aβ40 as a Means to Limiting Tube- and Storage-Dependent Pre-Analytical Variability in Clinical Setting. J. Alzheimer’s Dis. 2017, 57, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.; Rastogi, S.; Vishwakarma, P.; Bharti, P.S.; Sharma, V.; Renu, K.; Modi, G.P.; Vishnu, V.Y.; Chatterjee, P.; Dey, A.B.; et al. A novel approach to correlate the salivary exosomes and their protein cargo in the progression of cognitive impairment into Alzheimer’s disease. J. Neurosci. Methods 2020, 347, 108980. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yang, H.C.; Rhee, W.J. Development and comparative analysis of human urine exosome isolation strategies. Process. Biochem. 2020, 88, 197–203. [Google Scholar] [CrossRef]
- Sun, R.; Wang, H.; Shi, Y.; Gao, D.; Sun, Z.; Chen, Z.; Jiang, H.; Zhang, J. A Pilot Study of Urinary Exosomes in Alzheimer’s Disease. Neurodegener. Dis. 2019, 19, 184–191. [Google Scholar] [CrossRef]
- Liu, C.-G.; Song, J.; Zhang, Y.-Q.; Wang, P.-C. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol. Med. Rep. 2014, 10, 2395–2400. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-G.; Zhao, Y.; Lu, Y.; Wang, P.-C. ABCA1-Labeled Exosomes in Serum Contain Higher MicroRNA-193b Levels in Alzheimer’s Disease. BioMed Res. Int. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Liu, C.G.; Shuang, M.; Ying, L.; Yao, L.; Yue, Z.; Wang, P.C. MicroRNA-135a in ABCA1-Labeled Exosome Is a Serum Bi-omarker Candidate for Alzheimer’s Disease. Biomed. Environ. Sci. 2021, 34, 19–28. [Google Scholar]
- Zhang, M.; Han, W.; Xu, Y.; Li, D.; Xue, Q. Serum MiR-128 Serves as a Potential Diagnostic Biomarker for Alzheimer’s Dis-ease. Neuropsychiatr. Dis. Treat. 2021, 17, 269. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki-Wullich, D.; Català-Solsona, J.; Fábregas, C.; Hernández, I.; Clarimon, J.; Lleó, A.; Boada, M.; Saura, C.A.; Rodríguez-Álvarez, J.; Miñano-Molina, A.J. Altered microRNAs related to synaptic function as potential plasma biomarkers for Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Ding, L.; Yang, X.; Gao, Z.; Effah, C.Y.; Zhang, X.; Wu, Y.; Qu, L. A Holistic Review of the State-of-the-Art Microfluidics for Exosome Separation: An Overview of the Current Status, Existing Obstacles, and Future Outlook. Small 2021, 17, 2007174. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal. Transduct. Target. Ther. 2020, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chiriacò, M.S.; Bianco, M.; Nigro, A.; Primiceri, E.; Ferrara, F.; Romano, A.; Quattrini, A.; Furlan, R.; Arima, V.; Maruccio, G. Lab-on-Chip for Exosomes and Microvesicles Detection and Characterization. Sensors 2018, 18, 3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCastro, J.; Littig, J.; Chou, P.; Mack-Onyeike, J.; Srinivasan, A.; Conboy, M.; Conboy, I.; Aran, K. The Microfluidic Toolbox for Analyzing Exosome Biomarkers of Aging. Molecules 2021, 26, 535. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab. a Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Asghari, M.; Aslan, M.K.; Yilmaz, A.; Mateescu, B.; Stavrakis, S.; Demello, A.J. Microfluidics for extracellular vesicle separation and mimetic synthesis: Recent advances and future perspectives. Chem. Eng. J. 2020, 404, 126110. [Google Scholar] [CrossRef]
- Poupardin, R.; Wolf, M.; Strunk, D. Adherence to Minimal Experimental Requirements for Defining Extracellular Vesicles and Their Functions: A Systematic Review. Adv. Drug Deliv. Rev. 2021, 176, 113872. [Google Scholar] [CrossRef] [PubMed]

| Diseases | Exosomal Biomarkers | Ref |
|---|---|---|
| Prostate cancer | Survivin, PCA-3, TMPRSS2:ERG, PSA, miR-141, and miR-375 | [102,103,104,105] |
| Esophageal squamous cell cancer (ESCC) | miR-21 and miR-1246 | [106,107] |
| Breast cancer | miR-21 | [108] |
| Colorectal cancer | mRNAs | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, H.M.; Ghonaim, G.A.; Gharib, S.M.; Chopra, H.; Farag, A.K.; Hassanin, M.H.; Nagah, A.; Emad-Eldin, M.; Hashem, N.E.; Yahya, G.; et al. Exosomes in Alzheimer’s Disease: From Being Pathological Players to Potential Diagnostics and Therapeutics. Int. J. Mol. Sci. 2021, 22, 10794. https://doi.org/10.3390/ijms221910794
Soliman HM, Ghonaim GA, Gharib SM, Chopra H, Farag AK, Hassanin MH, Nagah A, Emad-Eldin M, Hashem NE, Yahya G, et al. Exosomes in Alzheimer’s Disease: From Being Pathological Players to Potential Diagnostics and Therapeutics. International Journal of Molecular Sciences. 2021; 22(19):10794. https://doi.org/10.3390/ijms221910794
Chicago/Turabian StyleSoliman, Hagar M., Ghada A. Ghonaim, Shaza M. Gharib, Hitesh Chopra, Aya K. Farag, Mohamed H. Hassanin, Abdalrazeq Nagah, Mahmoud Emad-Eldin, Nevertary E. Hashem, Galal Yahya, and et al. 2021. "Exosomes in Alzheimer’s Disease: From Being Pathological Players to Potential Diagnostics and Therapeutics" International Journal of Molecular Sciences 22, no. 19: 10794. https://doi.org/10.3390/ijms221910794






