Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)—New Aspects
Abstract
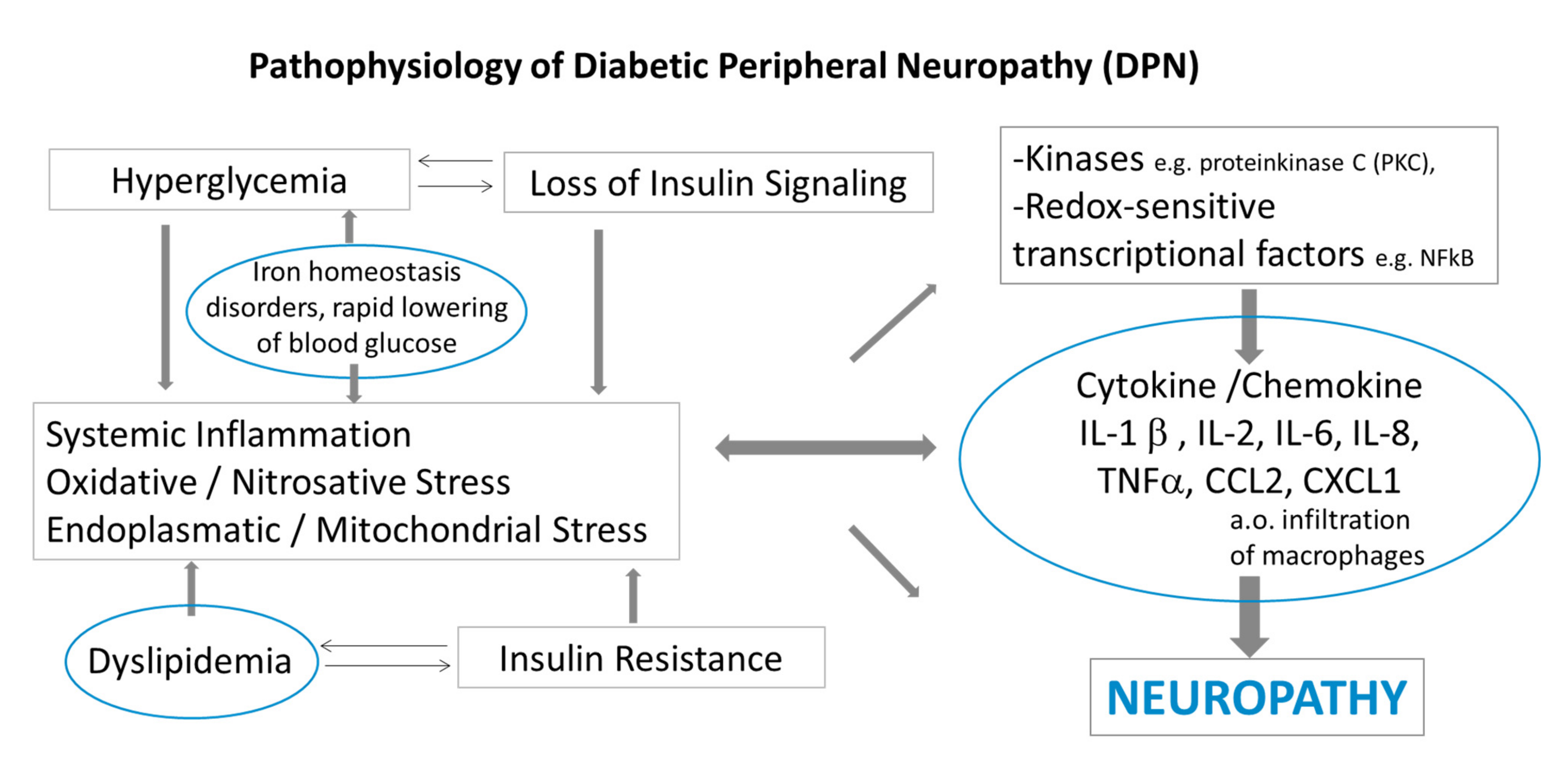
:1. Introduction
2. The Neuropathogenic Role of Inflammation
3. Neuropathogenic Role of Iron Intake
4. Neuropathogenetic Role of Rapid Blood Glucose Lowering in Patients with Diabetes
5. Neuropathogenic Role of Dyslipidemia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020; Centers for Disease Control and Prevention: Atlanta, GA, USA; U.S. Department of Health and Human Services: Washington, DC, USA, 2020.
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvin, E.; Parrinello, C.M.; Sacks, D.B.; Coresh, J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann. Intern. Med. 2014, 160, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Hanewinckel, R.; van Oijen, M.; Ikram, M.A.; van Doorn, P.A. The epidemiology and risk factors of chronic polyneuropathy. Eur. J. Epidemiol. 2016, 31, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, S.; Selvarajah, D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab. Res. Rev. 2012, 28, 8–14. [Google Scholar] [CrossRef]
- Dyck, P.J.; Albers, J.W.; Andersen, H.; Arezzo, J.C.; Biessels, G.-J.; Bril, V.; Feldman, E.L.; Litchy, W.J.; O’Brien, P.C.; Russell, J.W.; et al. Diabetic polyneuropathies: Update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab. Res. Rev. 2011, 27, 620–628. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.T.; Witte, D.; Dalsgaard, E.-M.; Andersen, H.; Nawroth, P.; Fleming, T.; Jensen, T.M.; Finnerup, N.; Lauritzen, T.; Feldman, E.; et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care 2018, 41, 1068–1075. [Google Scholar] [CrossRef] [Green Version]
- Calcutt, N.; Fernyhough, P. An introduction to the history and controversies of animal models of diabetic neuropathy. Int. Rev. Neurobiol. 2016, 127, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Gao, L.; Li, Y.; Zhou, X.; Reynolds, E.; Banerjee, M.; Pop-Busui, R.; Feldman, E.; Ji, L. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann. Clin. Transl. Neurol. 2018, 5, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Rajabally, Y.A. Neuropathy and impaired glucose tolerance: An updated review of the evidence. Acta Neurol. Scand. 2010, 124, 1–8. [Google Scholar] [CrossRef]
- Diaz, L.A.; Gupta, V. Diabetic Amyotrophy; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dyck, P.J.B.; Norell, J.E. Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology 1999, 53, 2113. [Google Scholar] [CrossRef] [PubMed]
- Massie, R.; Mauermann, M.L.; Staff, N.; Amrami, K.K.; Mandrekar, J.N.; Dyck, P.J.B.; Klein, C. Diabetic cervical radiculoplexus neuropathy: A distinct syndrome expanding the spectrum of diabetic radiculoplexus neuropathies. Brain 2012, 135, 3074–3088. [Google Scholar] [CrossRef] [Green Version]
- Baum, P.; Kosacka, J.; Estrela-Lopis, I.; Woidt, K.; Serke, H.; Paeschke, S.; Stockinger, M.; Klöting, N.; Blüher, M.; Dorn, M.; et al. The role of nerve inflammation and exogenous iron load in experimental peripheral diabetic neuropathy (PDN). Metabolism 2016, 65, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Kosacka, J.; Woidt, K.; Toyka, K.V.; Paeschke, S.; Klöting, N.; Bechmann, I.; Blüher, M.; Thiery, J.; Ossmann, S.; Baum, P.; et al. The role of dietary non-heme iron load and peripheral nerve inflammation in the development of peripheral neuropathy (PN) in obese non-diabetic leptin-deficient ob/ob mice. Neurol. Res. 2019, 41, 341–353. [Google Scholar] [CrossRef]
- Paeschke, S.; Baum, P.; Toyka, K.V.; Blüher, M.; Koj, S.; Klöting, N.; Bechmann, I.; Thiery, J.; Kosacka, J.; Nowicki, M. The role of iron and nerve inflammation in diabetes mellitus type 2-induced peripheral neuropathy. Neuroscience 2019, 406, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Nukada, H.; McMorran, P.D.; Baba, M.; Ogasawara, S.; Yagihashi, S. Increased susceptibility to ischemia and macrophage activation in STZ-diabetic rat nerve. Brain Res. 2011, 1373, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Kosacka, J.; Nowicki, M.; Klöting, N.; Kern, M.; Stumvoll, M.; Bechmann, I.; Serke, H.; Blüher, M. COMP-angiopoietin-1 recovers molecular biomarkers of neuropathy and improves vascularisation in sciatic nerve of ob/ob mice. PLoS ONE 2012, 7, e32881. [Google Scholar] [CrossRef] [PubMed]
- Kosacka, J.; Nowicki, M.; Blüher, M.; Baum, P.; Stockinger, M.; Toyka, K.; Klöting, I.; Stumvoll, M.; Serke, H.; Bechmann, I. Increased autophagy in peripheral nerves may protect Wistar Ottawa Karlsburg W rats against neuropathy. Exp. Neurol. 2013, 250, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Koj, S.; Klöting, N.; Blüher, M.; Classen, J.; Paeschke, S.; Gericke, M.; Toyka, K.V.; Nowicki, M.; Kosacka, J. Treatment-induced neuropathy in diabetes (TIND)—Developing a disease model in type 1 diabetic rats. Int. J. Mol. Sci. 2021, 22, 1571. [Google Scholar] [CrossRef]
- Cruz, N.G.; Sousa, L.; Sousa, M.O.; Pietrani, N.T.; Fernandes, A.P.; Gomes, K.B. The linkage between inflammation and type 2 diabetes mellitus. Diabetes Res. Clin. Pr. 2013, 99, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G. Low-grade systemic inflammation and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes 2003, 52, 1799–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Bhati, P.; Alam, R.; Moiz, J.A.; Hussain, M.E. Subclinical inflammation and endothelial dysfunction are linked to cardiac autonomic neuropathy in type 2 diabetes. J. Diabetes Metab. Disord. 2019, 18, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Schamarek, I.; Nowotny, B.; Carstensen-Kirberg, M.; Straßburger, K.; Nowotny, P.; Kannenberg, J.M.; Strom, A.; Püttgen, S.; Müssig, K.; et al. Inflammatory markers are associated with cardiac autonomic dysfunction in recent-onset type 2 diabetes. Heart 2016, 103, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Kannenberg, J.M.; Huth, C.; Carstensen-Kirberg, M.; Rathmann, W.; Koenig, W.; Heier, M.; Püttgen, S.; Thorand, B.; Peters, A.; et al. Proinflammatory cytokines predict the incidence and progression of distal sensorimotor polyneuropathy: KORA F4/FF4 study. Diabetes Care 2017, 40, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Hall, B.E.; Macdonald, E.; Cassidy, M.; Yun, S.; Sapio, M.R.; Ray, P.; Doty, M.; Nara, P.; Burton, M.D.; Shiers, S.; et al. Transcriptomic analysis of human sensory neurons in painful diabetic neuropathy reveals inflammation and neuronal loss. bioRxiv 2021. bioRxiv 2021.07.23.453576. [Google Scholar] [CrossRef]
- Martini, R.; Toyka, K.V. Immune-mediated components of hereditary demyelinating neuropathies: Lessons from animal models and patients. Lancet Neurol. 2004, 3, 457–465. [Google Scholar] [CrossRef]
- Carenini, S.; Mäurer, M.; Werner, A.; Blazyca, H.; Toyka, K.V.; Schmid, C.D.; Raivich, G.; Martini, R. The role of macrophages in demyelinating peripheral nervous system of mice heterozygously deficient in p0. J. Cell Biol. 2001, 152, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Kohl, B.; Fischer, S.; Groh, J.; Wessig, C.; Martini, R. MCP-1/CCL2 modifies axon properties in a PMP22-overexpressing mouse model for charcot-marie-tooth 1A neuropathy. Am. J. Pathol. 2010, 176, 1390–1399. [Google Scholar] [CrossRef] [Green Version]
- Martini, R.; Fischer, S.; López-Vales, R.; David, S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia 2008, 56, 1566–1577. [Google Scholar] [CrossRef]
- Lindenlaub, T.; Sommer, C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol. 2003, 105, 593–602. [Google Scholar] [CrossRef]
- Üçeyler, N.; Braunsdorf, S.; Kunze, E.; Riediger, N.; Scheytt, S.; Divisova, Š.; Bekircan-Kurt, C.E.; Toyka, K.V.; Sommer, C. Cellular infiltrates in skin and sural nerve of patients with polyneuropathies. Muscle Nerve 2017, 55, 884–893. [Google Scholar] [CrossRef]
- Schmidt, B.; Toyka, K.V.; Kiefer, R.; Full, J.; Hartung, H.-P.; Pollard, J. Inflammatory infiltrates in sural nerve biopsies in Guillain-Barré syndrome and chronic inflammatory demyelinating neuropathy. Muscle Nerve 1996, 19, 474–487. [Google Scholar] [CrossRef]
- Sommer, C.; Koch, S.; Lammens, M.; Gabreels-Festen, A.; Stoll, G.; Toyka, K.V. Macrophage clustering as a diagnostic marker in sural nerve biopsies of patients with CIDP. Neurology 2005, 65, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Üçeyler, N.; Geng, A.; Reiners, K.; Toyka, K.V.; Sommer, C. Non-systemic vasculitic neuropathy: Single-center follow-up of 60 patients. J. Neurol. 2015, 262, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Willison, H.; Stoll, G.; Toyka, K.V.; Berger, T.; Hartung, H.-P. Autoimmunity and inflammation in the peripheral nervous system. Trends Neurosci. 2002, 25, 127–129. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Ang, L.; Holmes, C.; Gallagher, K.; Feldman, E.L. Inflammation as a therapeutic target for diabetic neuropathies. Curr. Diabetes Rep. 2016, 16, 29. [Google Scholar] [CrossRef] [Green Version]
- Hartung, H.-P.; Schäfer, B.; Heininger, K.; Toyka, K.V. Suppression of experimental autoimmune neuritis by the oxygen radical scavengers superoxide dismutase and catalase. Ann. Neurol. 1988, 23, 453–460. [Google Scholar] [CrossRef]
- Hartung, H.-P.; Schäfer, B.; Heininger, K.; Stoll, G.; Toyka, K.V. The role of macrophages and eicosanoids in the pathogenesis of experimental allergic neuritis. Brain 1988, 111, 1039–1059. [Google Scholar] [CrossRef]
- Mallet, M.-L.; Hadjivassiliou, M.; Sarrigiannis, P.G.; Zis, P. The role of oxidative stress in peripheral neuropathy. J. Mol. Neurosci. 2020, 70, 1009–1017. [Google Scholar] [CrossRef]
- Vincent, A.M.; Callaghan, B.C.; Smith, A.L.; Feldman, E. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 2011, 7, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Uzar, E.; Tamam, Y.; Evliya, O.; Tuzcu, A.; Beyaz, C.; Acar, A.; Aydın, B.; Tasdemir, N. Serum prolidase activity and oxidative status in patients with diabetic neuropathy. Neurol. Sci. 2011, 33, 875–880. [Google Scholar] [CrossRef]
- Sofic, E.; Rustembegovic, A.; Kroyer, G.; Cao, G. Serum antioxidant capacity in neurological, psychiatric, renal diseases and cardiomyopathy. J. Neural Transm. 2002, 109, 711–719. [Google Scholar] [CrossRef]
- Strom, A.; Kaul, K.; Brüggemann, J.; Ziegler, I.; Rokitta, I.; Püttgen, S.; Szendroedi, J.; Müssig, K.; Roden, M.; Ziegler, D. Lower serum extracellular superoxide dismutase levels are associated with polyneuropathy in recent-onset diabetes. Exp. Mol. Med. 2017, 49, e394. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.A.; Lawson, H.A. Ironing out the details: Untangling dietary iron and genetic background in diabetes. Nutrients 2018, 10, 1437. [Google Scholar] [CrossRef] [Green Version]
- Aregbesola, A.; Voutilainen, S.; Virtanen, J.K.; Mursu, J.; Tuomainen, T.-P. Body iron stores and the risk of type 2 diabetes in middle-aged men. Eur. J. Endocrinol. 2013, 169, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.-L.; Cheung, T.T.; Lam, K.S.; Cheung, B.M. High ferritin and low transferrin saturation are associated with pre-diabetes among a national representative sample of U.S. adults. Clin. Nutr. 2013, 32, 1055–1060. [Google Scholar] [CrossRef]
- Rawal, S.; Hinkle, S.; Bao, W.; Zhu, Y.; Grewal, J.; Albert, P.S.; Weir, N.L.; Tsai, M.; Zhang, C. A longitudinal study of iron status during pregnancy and the risk of gestational diabetes: Findings from a prospective, multiracial cohort. Diabetologia 2017, 60, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Y.; Bao, W.; Rong, S.; Fang, M.; Wang, D.; Yao, P.; Hu, F.B.; Liu, L. Hemochromatosis gene (HFE) polymorphisms and risk of type 2 diabetes mellitus: A meta-analysis. Am. J. Epidemiol. 2012, 176, 461–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Real, J.M.; López-Bermejo, A.; Ricart, W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin. Chem. 2005, 51, 1201–1205. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, X.-K.; Wang, Y.; Cai, L. The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: Therapeutic effects by chelators. Hemoglobin 2008, 32, 135–145. [Google Scholar] [CrossRef]
- Salis, C.; Davio, C.; Usach, V.; Urtasun, N.; Goitia, B.; Martinez-Vivot, R.; Pasquini, J.; Setton-Avruj, C. Iron and holotransferrin induce cAMP-dependent differentiation of Schwann cells. Neurochem. Int. 2012, 61, 798–806. [Google Scholar] [CrossRef]
- Kabakus, N.; Ayar, A.; Yoldas, T.K.; Ulvi, H.; Dogan, Y.; Yilmaz, B.; Kilice, N. Reversal of iron deficiency anemia-induced peripheral neuropathy by iron treatment in children with iron deficiency anemia. J. Trop. Pediatrics 2002, 48, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altamura, S.; Muckenthaler, M.U. Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. J. Alzheimer’s Dis. 2009, 16, 879–895. [Google Scholar] [CrossRef]
- Thompson, K.J.; Shoham, S.; Connor, J.R. Iron and neurodegenerative disorders. Brain Res. Bull. 2001, 55, 155–164. [Google Scholar] [CrossRef]
- Caravati, C.M. Insulin neuritis: A case report. VA Med. Mon. 1933, 59, 745–746. [Google Scholar]
- Gibbons, C.H.; Freeman, R. Treatment-induced diabetic neuropathy: A reversible painful autonomic neuropathy. Ann. Neurol. 2009, 67, 534–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.T.; Davies, G. ‘Insulin neuritis’ to ‘treatment-induced neuropathy of diabetes’: New name, same mystery. Pr. Neurol. 2015, 16, 53–55. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, C.H. Treatment-induced neuropathy of diabetes. Curr. Diabetes Rep. 2017, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.H.; Freeman, R. Treatment-induced neuropathy of diabetes: An acute, iatrogenic complication of diabetes. Brain 2015, 138, 43–52. [Google Scholar] [CrossRef]
- Low, P.A.; Singer, W. Treatment-induced neuropathy of diabetes: An energy crisis? Brain 2014, 138, 2–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storz, M.A. Treatment-induced neuropathy of diabetes: A call for acknowledgement. Diabet. Med. 2020, 37, 369–370. [Google Scholar] [CrossRef]
- Tuck, R.R.; Schmelzer, J.D.; Low, P.A. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain 1984, 107, 935–950. [Google Scholar] [CrossRef]
- Miscio, G.; Guastamacchia, G.; Brunani, A.; Priano, L.; Baudo, S.; Mauro, A. Obesity and peripheral neuropathy risk: A dangerous liaison. J. Peripher. Nerv. Syst. 2005, 10, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Petroff, D.; Classen, J.; Kiess, W.; Blüher, S. Dysfunction of autonomic nervous system in childhood obesity: A cross-sectional study. PLoS ONE 2013, 8, e54546. [Google Scholar] [CrossRef] [Green Version]
- Verrotti, A.; LoIacono, G.; Mohn, A.; Chiarelli, F. New insights in diabetic autonomic neuropathy in children and adolescents. Eur. J. Endocrinol. 2009, 161, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Haluzik, M.; Colombo, C.; Gavrilova, O.; Chua, S.; Wolf, N.; Chen, M.; Stannard, B.; Dietz, K.; le Roith, D.; Reitman, M. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology 2004, 145, 3258–3264. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.; Kosacka, J.; Serke, H.; Blüher, M.; Spanel-Borowski, K. Altered sciatic nerve fiber morphology and endoneural microvessels in mouse models relevant for obesity, peripheral diabetic polyneuropathy, and the metabolic syndrome. J. Neurosci. Res. 2012, 90, 122–131. [Google Scholar] [CrossRef]
- Blüher, S.; Petroff, D.; Keller, A.; Wagner, A.; Classen, J.; Baum, P. Effect of a 1-year obesity intervention (KLAKS program) on preexisting autonomic nervous dysfunction in childhood obesity. J. Child Neurol. 2015, 30, 1174–1181. [Google Scholar] [CrossRef]
- Hayashino, Y.; Jackson, J.L.; Hirata, T.; Fukumori, N.; Nakamura, F.; Fukuhara, S.; Tsujii, S.; Ishii, H. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Metabolism 2014, 63, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Hopps, E.; Canino, B.; Caimi, G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol. 2011, 48, 183–189. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baum, P.; Toyka, K.V.; Blüher, M.; Kosacka, J.; Nowicki, M. Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)—New Aspects. Int. J. Mol. Sci. 2021, 22, 10835. https://doi.org/10.3390/ijms221910835
Baum P, Toyka KV, Blüher M, Kosacka J, Nowicki M. Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)—New Aspects. International Journal of Molecular Sciences. 2021; 22(19):10835. https://doi.org/10.3390/ijms221910835
Chicago/Turabian StyleBaum, Petra, Klaus V. Toyka, Matthias Blüher, Joanna Kosacka, and Marcin Nowicki. 2021. "Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)—New Aspects" International Journal of Molecular Sciences 22, no. 19: 10835. https://doi.org/10.3390/ijms221910835





