Contributing to Understand the Crosstalk between Brain and Periphery in Methylmercury Intoxication: Neurotoxicity and Extracellular Vesicles
Abstract
:1. Introduction
2. Results
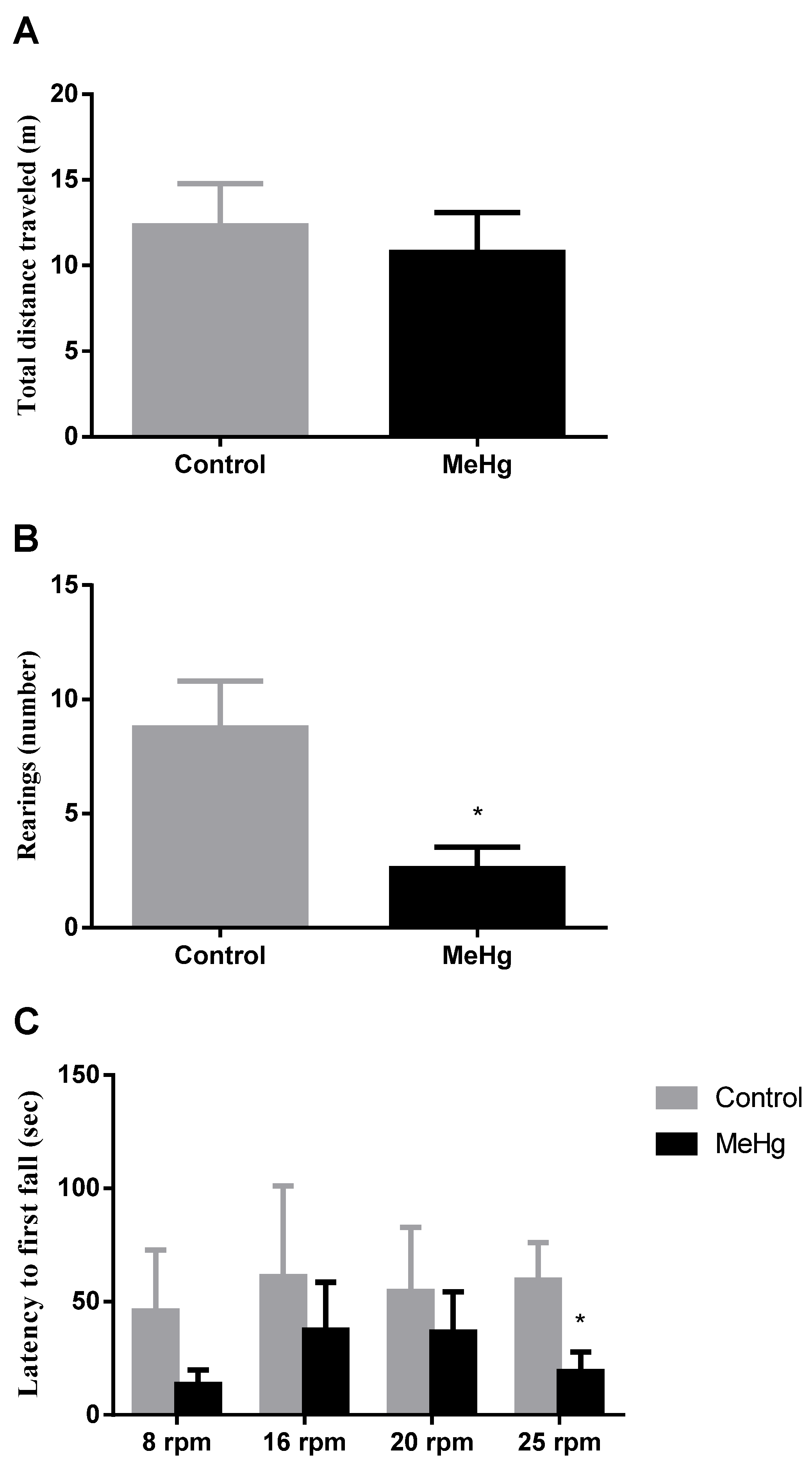
2.1. MeHg Induced Motor Impairment
2.2. MeHg Induced Histological Changes
2.3. MeHg Induced Changes in Neuronal and Astrocytic Reactivity Molecular Markers
2.4. Plasma Extracellular Vesicles
3. Discussion
4. Materials and Methods
4.1. Animals Treatment
4.2. Samples Collection
4.3. Behavioral Tests
4.3.1. Open Field Test
4.3.2. Rotarod Test
4.4. Platelet-Free Plasma Obtention
4.5. Immunohistochemistry
4.6. Relative Quantification of Gene Expression in Brain Areas
4.7. EV Isolation:
4.8. Nanoparticle Tracking Analysis (NTA):
4.9. Transmission Electron Microscopy (TEM)
4.10. Western Blotting
4.11. Relative Quantification of Gene Expression in Plasma EVs
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Mercury and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 20 August 2021).
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araujo, A.; Santos-Sacramento, L.; Yuki Takeda, P.; Macchi, B.M.; do Nascimento, J.L.M.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What can we learn from the Amazon? Environ. Int. 2021, 146, 106223. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Martin-Doimeadios, R.C.R.; Jimenez-Moreno, M.; Ramirez-Mateos, V.; da Silva, N.F.S.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.O.; Macchi, B.M.; do Nascimento, J.L.M.; et al. Large-scale projects in the amazon and human exposure to mercury: The case-study of the Tucurui Dam. Ecotoxicol. Environ. Saf. 2018, 147, 299–305. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Martin-Doimeadios, R.C.R.; Jimenez-Moreno, M.; Fernandez-Trujillo, S.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Macchi, B.M.; Alvarez-Leite, J.I.; do Nascimento, J.L.M.; Amador, M.T.; et al. Genetic Susceptibility to Neurodegeneration in Amazon: Apolipoprotein E Genotyping in Vulnerable Populations Exposed to Mercury. Front. Genet. 2018, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez Martin-Doimeadios, R.C.; Berzas Nevado, J.J.; Guzman Bernardo, F.J.; Jimenez Moreno, M.; Arrifano, G.P.; Herculano, A.M.; do Nascimento, J.L.; Crespo-Lopez, M.E. Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ. Sci. Pollut. Res. Int. 2014, 21, 7466–7479. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.L.R.; Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Arrifano, G.d.P.; Macchi, B.d.M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Souza-Monteiro, J.R.; Alvarez-Leite, J.I.; Souza, C.B.A.d. Eating in the Amazon: Nutritional Status of the Riverine Populations and Possible Nudge Interventions. Foods 2021, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Ekino, S.; Susa, M.; Ninomiya, T.; Imamura, K.; Kitamura, T. Minamata disease revisited: An update on the acute and chronic manifestations of methyl mercury poisoning. J. Neurol. Sci. 2007, 262, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sacramento, L.; Arrifano, G.P.; Lopes-Araujo, A.; Augusto-Oliveira, M.; Albuquerque-Santos, R.; Takeda, P.Y.; Souza-Monteiro, J.R.; Macchi, B.M.; do Nascimento, J.L.M.; Lima, R.R.; et al. Human neurotoxicity of mercury in the Amazon: A scoping review with insights and critical considerations. Ecotoxicol. Environ. Saf. 2021, 208, 111686. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Yamamoto, M.; Figeys, D.; Ning, Z.; Chan, H.M. Proteomic Analysis of Cerebellum in Common Marmoset Exposed to Methylmercury. Toxicol. Sci. 2015, 146, 43–51. [Google Scholar] [CrossRef]
- Korogi, Y.; Takahashi, M.; Shinzato, J.; Okajima, T. MR findings in seven patients with organic mercury poisoning (Minamata disease). AJNR. Am. J. Neuroradiol. 1994, 15, 1575–1578. [Google Scholar]
- Jackson, A.C. Chronic Neurological Disease Due to Methylmercury Poisoning. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2018, 45, 620–623. [Google Scholar] [CrossRef] [Green Version]
- Augusto-Oliveira, M.A.; Arrifano, G.P.; Lopes-Araujo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293. [Google Scholar] [CrossRef] [Green Version]
- Augusto-Oliveira, M.; Arrifano, G.P.; Delage, C.I.; Tremblay, M.-È.; Crespo-Lopez, M.E.; Verkhratsky, A. Plasticity of microglia. Biol. Rev. 2021. [Google Scholar] [CrossRef]
- Ni, M.; Li, X.; Rocha, J.B.; Farina, M.; Aschner, M. Glia and methylmercury neurotoxicity. J. Toxicol. Environ. Health Part A 2012, 75, 1091–1101. [Google Scholar] [CrossRef] [Green Version]
- Arrifano, G.P.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Macchi, B.M.; Lima, R.R.; Sunol, C.; do Nascimento, J.L.M.; Crespo-Lopez, M.E. Revisiting Astrocytic Roles in Methylmercury Intoxication. Mol. Neurobiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, F.M.; Yilmaz, H.; Tutkun, E.; Uysal, S.; Carman, K.B.; Dilber, C.; Ercan, M. Serum biochemical markers of central nerve system damage in children with acute elemental mercury intoxication. Clin. Toxicol. 2014, 52, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.; Del Carmen Rodriguez Martin-Doimeadios, R.; Jimenez-Moreno, M.; Augusto-Oliveira, M.; Rogerio Souza-Monteiro, J.; Paraense, R.; Rodrigues Machado, C.; Farina, M.; Macchi, B.; do Nascimento, J.; et al. Assessing mercury intoxication in isolated/remote populations: Increased S100B mRNA in blood in exposed riverine inhabitants of the Amazon. Neurotoxicology 2018, 68, 151–158. [Google Scholar] [CrossRef]
- Branco, V.; Caito, S.; Farina, M.; Teixeira da Rocha, J.; Aschner, M.; Carvalho, C. Biomarkers of mercury toxicity: Past, present, and future trends. J. Toxicol. Environ. Health Part B Crit. Rev. 2017, 20, 119–154. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; de Paula Arrifano, G.; Lopes-Araújo, A.; Santos-Sacramento, L.; Lima, R.R.; Lamers, M.L.; Le Blond, J.; Crespo-Lopez, M.E. Salivary biomarkers and neuropsychological outcomes: A non-invasive approach to investigate pollutants-associated neurotoxicity and its effects on cognition in vulnerable populations. Environ. Res. 2021. [Google Scholar] [CrossRef]
- Van Eldik, L.J.; Wainwright, M.S. The Janus face of glial-derived S100B: Beneficial and detrimental functions in the brain. Restor. Neurol. Neurosci. 2003, 21, 97–108. [Google Scholar]
- Marostica, G.; Gelibter, S.; Gironi, M.; Nigro, A.; Furlan, R. Extracellular Vesicles in Neuroinflammation. Front. Cell Dev. Biol. 2021, 8, 623039. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Zhao, S.; Sheng, S.; Wang, Y.; Ding, L.; Xu, X.; Xia, X.; Zheng, J.C. Astrocyte-derived extracellular vesicles: A double-edged sword in central nervous system disorders. Neurosci. Biobehav. Rev. 2021, 125, 148–159. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Huang, Y.; Zhang, H.; Lu, H.; Zheng, J.C. Exosomal miRNAs in central nervous system diseases: Biomarkers, pathological mediators, protective factors and therapeutic agents. Prog. Neurobiol. 2019, 183, 101694. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Mustapic, M.; Kapogiannis, D.; Eitan, E.; Lobach, I.V.; Goetzl, L.; Schwartz, J.B.; Miller, B.L. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 3853–3859. [Google Scholar] [CrossRef] [Green Version]
- Goetzl, E.J.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Miller, B.L.; Kapogiannis, D. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 2015, 85, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Bottino, C.; Vazquez, M.; Devesa, V.; Laforenza, U. Impaired aquaporins expression in the gastrointestinal tract of rat after mercury exposure. J. Appl. Toxicol. JAT 2016, 36, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.N.; Pinheiro, A.M.; Belém-Filho, I.J.A.; Fernandes, L.M.P.; Cartágenes, S.C.; Ribera, P.C.; Fontes-Júnior, E.A.; Crespo-Lopez, M.E.; Monteiro, M.C.; Lima, M.O.; et al. Unravelling motor behaviour hallmarks in intoxicated adolescents: Methylmercury subtoxic-dose exposure and binge ethanol intake paradigm in rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 21937–21948. [Google Scholar] [CrossRef]
- Bellum, S.; Thuett, K.A.; Bawa, B.; Abbott, L.C. The effect of methylmercury exposure on behavior and cerebellar granule cell physiology in aged mice. J. Appl. Toxicol. JAT 2013, 33, 959–969. [Google Scholar] [CrossRef]
- Bourdineaud, J.P.; Laclau, M.; Maury-Brachet, R.; Gonzalez, P.; Baudrimont, M.; Mesmer-Dudons, N.; Fujimura, M.; Marighetto, A.; Godefroy, D.; Rostene, W.; et al. Effects of methylmercury contained in a diet mimicking the Wayana Amerindians contamination through fish consumption: Mercury accumulation, metallothionein induction, gene expression variations, and role of the chemokine CCL2. Int. J. Mol. Sci. 2012, 13, 7710–7738. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Lopez, M.E.; Soares, E.S.; Macchi, B.M.; Santos-Sacramento, L.; Takeda, P.Y.; Lopes-Araujo, A.; Paraense, R.S.O.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Luz, D.A.; et al. Towards Therapeutic Alternatives for Mercury Neurotoxicity in the Amazon: Unraveling the Pre-Clinical Effects of the Superfruit Acai (Euterpe oleracea, Mart.) as Juice for Human Consumption. Nutrients 2019, 11, 2585. [Google Scholar] [CrossRef] [Green Version]
- Farina, M.; Rocha, J.B.; Aschner, M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011, 89, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Santana, L.; Bittencourt, L.; Nascimento, P.; Fernandes, R.; Teixeira, F.; Fernandes, L.; Freitas Silva, M.; Nogueira, L.; Amado, L.; Crespo-Lopez, M.; et al. Low doses of methylmercury exposure during adulthood in rats display oxidative stress, neurodegeneration in the motor cortex and lead to impairment of motor skills. J. Trace Elem. Med. Biol. 2019, 51, 19–27. [Google Scholar] [CrossRef]
- Takahashi, T.; Fujimura, M.; Koyama, M.; Kanazawa, M.; Usuki, F.; Nishizawa, M.; Shimohata, T. Methylmercury Causes Blood-Brain Barrier Damage in Rats via Upregulation of Vascular Endothelial Growth Factor Expression. PLoS ONE 2017, 12, e0170623. [Google Scholar] [CrossRef] [Green Version]
- Roda, E.; Coccini, T.; Acerbi, D.; Castoldi, A.; Bernocchi, G.; Manzo, L. Cerebellum cholinergic muscarinic receptor (subtype-2 and -3) and cytoarchitecture after developmental exposure to methylmercury: An immunohistochemical study in rat. J. Chem. Neuroanat. 2008, 35, 285–294. [Google Scholar] [CrossRef]
- Farina, M.; Aschner, M. Methylmercury-Induced Neurotoxicity: Focus on Pro-oxidative Events and Related Consequences. Adv. Neurobiol. 2017, 18, 267–286. [Google Scholar] [CrossRef]
- Aschner, M.; Yao, C.P.; Allen, J.W.; Tan, K.H. Methylmercury alters glutamate transport in astrocytes. Neurochem. Int. 2000, 37, 199–206. [Google Scholar] [CrossRef]
- Xu, F.; Farkas, S.; Kortbeek, S.; Zhang, F.X.; Chen, L.; Zamponi, G.W.; Syed, N.I. Mercury-induced toxicity of rat cortical neurons is mediated through N-Methyl-D-Aspartate receptors. Mol. Brain 2012, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Bosch, L.; Van Damme, P.; Bogaert, E.; Robberecht, W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2006, 1762, 1068–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chater, T.E.; Goda, Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Colón-Rodríguez, A.; Colón-Carrión, N.M.; Atchison, W.D. AMPA receptor contribution to methylmercury-mediated alteration of intracellular Ca2+ concentration in human induced pluripotent stem cell motor neurons. Neurotoxicology 2020, 81, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.M.; Colón-Rodríguez, A.; Atchison, W.D. Evaluating a Gene-Environment Interaction in Amyotrophic Lateral Sclerosis: Methylmercury Exposure and Mutated SOD1. Curr. Environ. Health Rep. 2017, 4, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, R.; Di Iorio, P.; Bruno, V.; Battaglia, G.; D’Alimonte, I.; D’Onofrio, M.; Nicoletti, F.; Caciagli, F. Activation of A(1) adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S-100beta protein from cultured astrocytes. Glia 1999, 27, 275–281. [Google Scholar] [CrossRef]
- Amorini, A.M.; Lazzarino, G.; Di Pietro, V.; Signoretti, S.; Lazzarino, G.; Belli, A.; Tavazzi, B. Metabolic, enzymatic and gene involvement in cerebral glucose dysmetabolism after traumatic brain injury. Biochim. Biophys. Acta 2016, 1862, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Isgrò, M.A.; Bottoni, P.; Scatena, R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Polcyn, R.; Matzelle, D.; Banik, N.L. New Insights into the Role of Neuron-Specific Enolase in Neuro-Inflammation, Neurodegeneration, and Neuroprotection. Brain Sci. 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Bylicky, M.A.; Mueller, G.P.; Day, R.M. Mechanisms of Endogenous Neuroprotective Effects of Astrocytes in Brain Injury. Oxidative Med. Cell. Longev. 2018, 2018, 6501031. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Augusto-Oliveira, M.; Arrifano, G.P.; Takeda, P.Y.; Lopes-Araujo, A.; Santos-Sacramento, L.; Anthony, D.C.; Verkhratsky, A.; Crespo-Lopez, M.E. Astroglia-specific contributions to the regulation of synapses, cognition and behaviour. Neurosci. Biobehav. Rev. 2020, 118, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Brozzi, F.; Arcuri, C.; Giambanco, I.; Donato, R. S100B Protein Regulates Astrocyte Shape and Migration via Interaction with Src Kinase: IMPLICATIONS FOR ASTROCYTE DEVELOPMENT, ACTIVATION, AND TUMOR GROWTH. J. Biol. Chem. 2009, 284, 8797–8811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschner, M.; Syversen, T.; Souza, D.O.; Rocha, J.B.; Farina, M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz. J. Med Biol. Res. Rev. Bras. 2007, 40, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couch, Y.; Akbar, N.; Roodselaar, J.; Evans, M.C.; Gardiner, C.; Sargent, I.; Romero, I.A.; Bristow, A.; Buchan, A.M.; Haughey, N.; et al. Circulating endothelial cell-derived extracellular vesicles mediate the acute phase response and sickness behaviour associated with CNS inflammation. Sci. Rep. 2017, 7, 9574. [Google Scholar] [CrossRef] [PubMed]
- Dickens, A.M.; Tovar-Y-Romo, L.B.; Yoo, S.W.; Trout, A.L.; Bae, M.; Kanmogne, M.; Megra, B.; Williams, D.W.; Witwer, K.W.; Gacias, M.; et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha, D.; Lorenz, D.R.; Misra, V.; Chettimada, S.; Morgello, S.; Gabuzda, D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. J. Neuroinflamm. 2019, 16, 254. [Google Scholar] [CrossRef] [Green Version]
- Yardan, T.; Erenler, A.K.; Baydin, A.; Aydin, K.; Cokluk, C. Usefulness of S100B protein in neurological disorders. JPMA J. Pak. Med. Assoc. 2011, 61, 276–281. [Google Scholar]
- Goncalves, C.A.; Leite, M.C.; Nardin, P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin. Biochem. 2008, 41, 755–763. [Google Scholar] [CrossRef]
- Streitburger, D.P.; Arelin, K.; Kratzsch, J.; Thiery, J.; Steiner, J.; Villringer, A.; Mueller, K.; Schroeter, M.L. Validating serum S100B and neuron-specific enolase as biomarkers for the human brain—A combined serum, gene expression and MRI study. PLoS ONE 2012, 7, e43284. [Google Scholar] [CrossRef]
- Yilmaz, S.; Karakayali, O.; Kale, E.; Akdogan, A. Could serum S100B be a predictor of neuronal damage and clinical poor outcomes associated with the use of synthetic cannabinoids? S100B to predict neuronal damage of SC in the ED. Am. J. Emerg. Med. 2017, 36, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Janas, A.M.; Sapoń, K.; Janas, T.; Stowell, M.H.; Janas, T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim. Biophys. Acta 2016, 1858, 1139–1151. [Google Scholar] [CrossRef]
- Fernandes, L.M.P.; Lopes, K.S.; Santana, L.N.S.; Fontes-Júnior, E.A.; Ribeiro, C.; Silva, M.C.F.; de Oliveira Paraense, R.S.; Crespo-López, M.E.; Gomes, A.R.Q.; Lima, R.R.; et al. Repeated Cycles of Binge-Like Ethanol Intake in Adolescent Female Rats Induce Motor Function Impairment and Oxidative Damage in Motor Cortex and Liver, but Not in Blood. Oxidative Med. Cell. Longev. 2018, 2018, 3467531. [Google Scholar] [CrossRef] [Green Version]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrifano, G.d.P.; Augusto-Oliveira, M.; Sealey-Bright, M.; Zainal, J.; Imbiriba, L.; Fernandes, L.M.P.; Maia, C.S.F.; Anthony, D.; Crespo-Lopez, M.E. Contributing to Understand the Crosstalk between Brain and Periphery in Methylmercury Intoxication: Neurotoxicity and Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 10855. https://doi.org/10.3390/ijms221910855
Arrifano GdP, Augusto-Oliveira M, Sealey-Bright M, Zainal J, Imbiriba L, Fernandes LMP, Maia CSF, Anthony D, Crespo-Lopez ME. Contributing to Understand the Crosstalk between Brain and Periphery in Methylmercury Intoxication: Neurotoxicity and Extracellular Vesicles. International Journal of Molecular Sciences. 2021; 22(19):10855. https://doi.org/10.3390/ijms221910855
Chicago/Turabian StyleArrifano, Gabriela de Paula, Marcus Augusto-Oliveira, Megan Sealey-Bright, Jaezah Zainal, Luciana Imbiriba, Luanna Melo Pereira Fernandes, Cristiane Socorro Ferraz Maia, Daniel Anthony, and Maria Elena Crespo-Lopez. 2021. "Contributing to Understand the Crosstalk between Brain and Periphery in Methylmercury Intoxication: Neurotoxicity and Extracellular Vesicles" International Journal of Molecular Sciences 22, no. 19: 10855. https://doi.org/10.3390/ijms221910855
APA StyleArrifano, G. d. P., Augusto-Oliveira, M., Sealey-Bright, M., Zainal, J., Imbiriba, L., Fernandes, L. M. P., Maia, C. S. F., Anthony, D., & Crespo-Lopez, M. E. (2021). Contributing to Understand the Crosstalk between Brain and Periphery in Methylmercury Intoxication: Neurotoxicity and Extracellular Vesicles. International Journal of Molecular Sciences, 22(19), 10855. https://doi.org/10.3390/ijms221910855








