Osteogenic Properties of 3D-Printed Silica-Carbon-Calcite Composite Scaffolds: Novel Approach for Personalized Bone Tissue Regeneration
Abstract
1. Introduction
2. Results
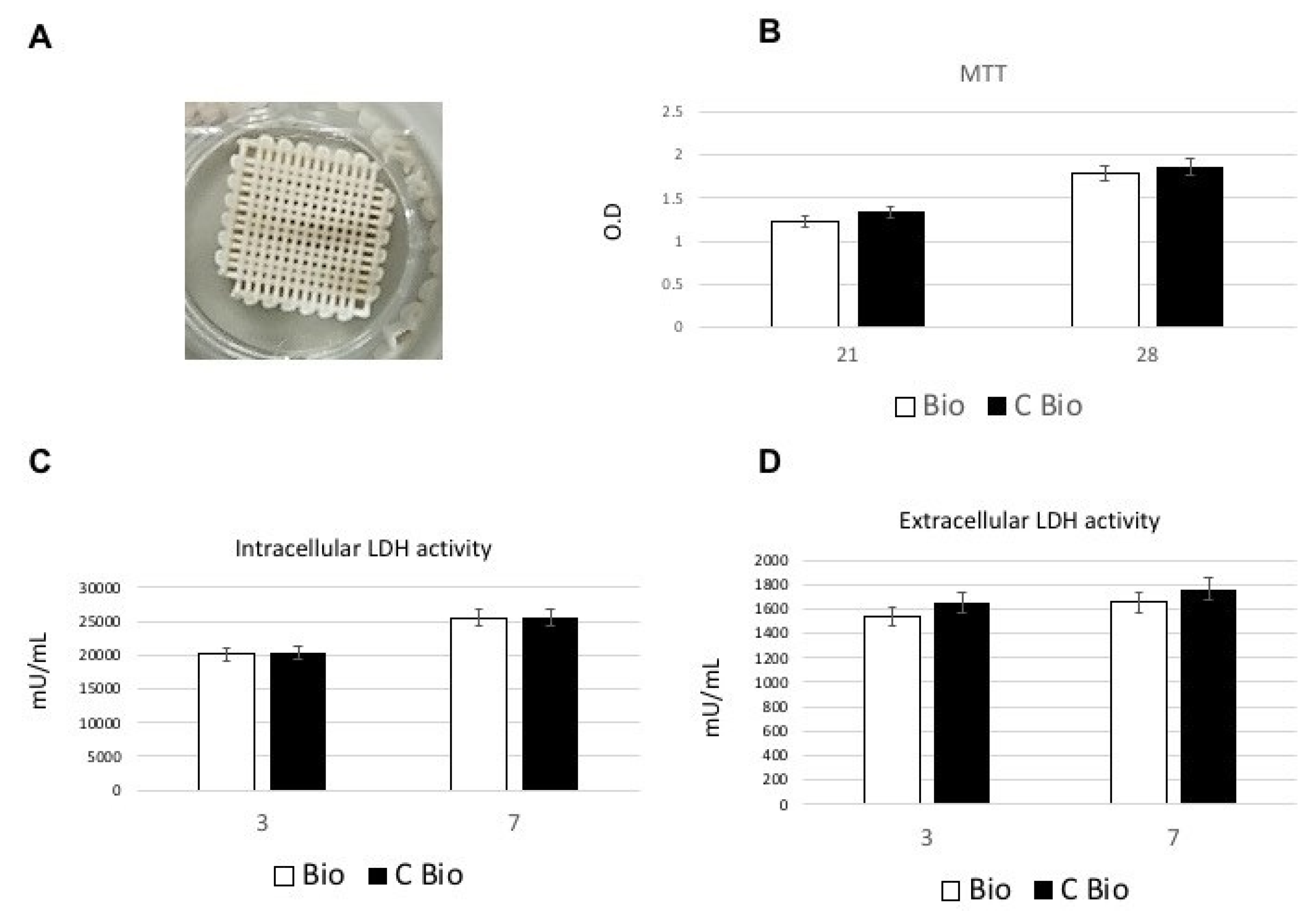
2.1. Biocompatibility and Cell Proliferation
2.1.1. Lactate Dehydrogenase (LDH) and 3-(4,5-dimethyl- thiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Assays
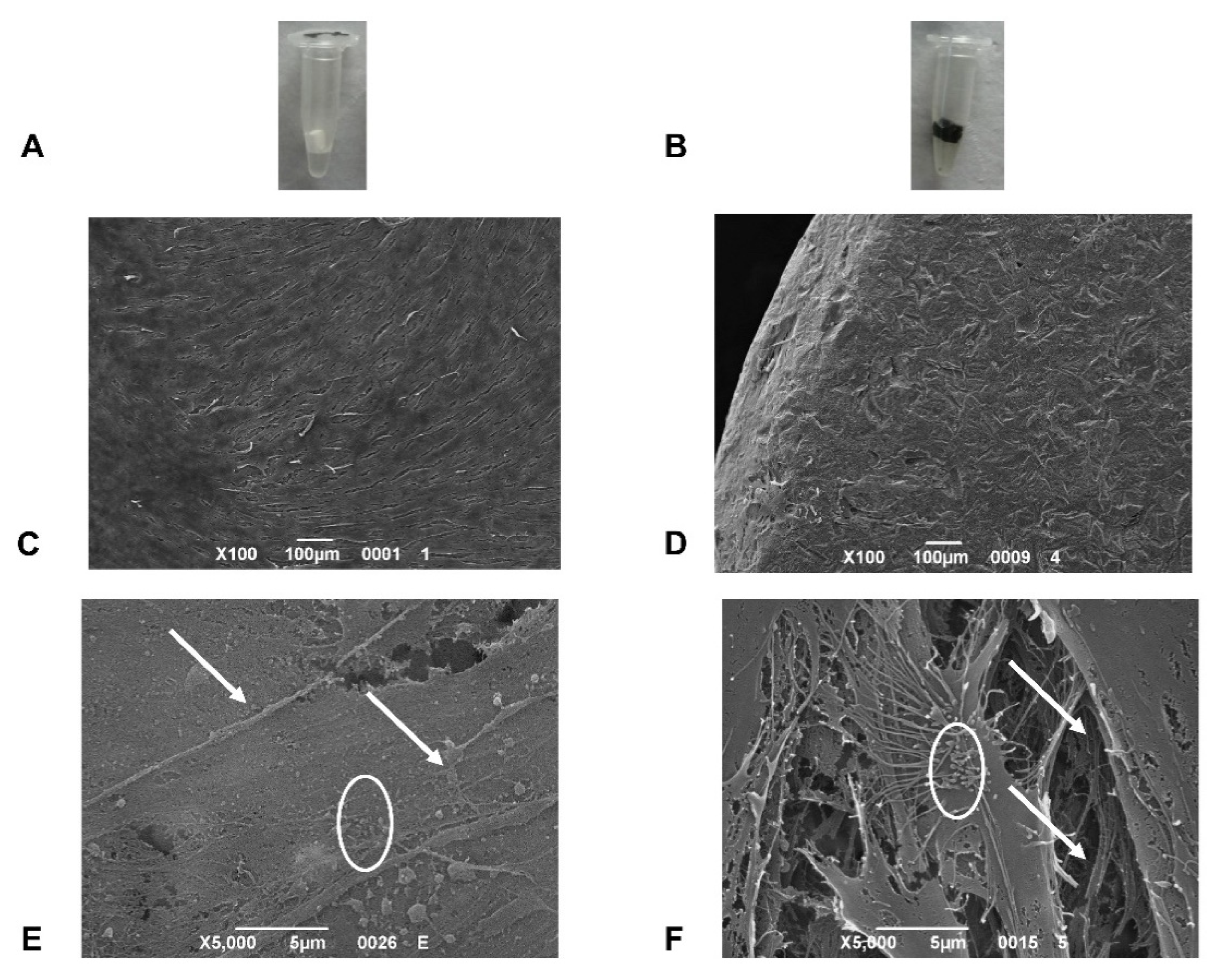
2.1.2. Hemolysis Assay
2.1.3. Ames Test
2.2. Osteogenic Commitment
2.3. Scanning Electron Microscopy (SEM) Analysis
3. Discussion
4. Materials and Methods
4.1. Scaffolds
4.2. Isolation and Culture of cAD-MSCs
4.3. MTT Assay
4.4. LDH Activity
4.5. ALP Activity Measurements
4.6. Ames Test
4.7. Hemolysis Assay
4.8. SEM Analysis
4.9. Gene Expression
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic potential of graphene in bone tissue engineering scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef]
- Lindsey, R.W.; Gugala, Z.; Milne, E.; Sun, M.; Gannon, F.H.; Latta, L.L. The efficacy of cylindrical titanium mesh cage for the reconstruction of a critical-size canine segmental femoral diaphyseal defect. J. Orthop. Res. 2006, 24, 1438–1453. [Google Scholar] [CrossRef]
- Roffi, A.; Krishnakumar, G.S.; Gostynska, N.; Kon, E.; Candrian, C.; Filardo, G.; Shankar Krishnakumar, G.; Gostynska, N.; Kon, E.; Candrian, C.; et al. The Role of Three-Dimensional Scaffolds in Treating Long Bone Defects: Evidence from Preclinical and Clinical Literature—A Systematic Review. Biomed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M.; Khalilzadeh, B. Graphene based scaffolds on bone tissue engineering. Bioengineered 2018, 9, 38–47. [Google Scholar] [CrossRef]
- Sitharaman, B.; Shi, X.; Walboomers, X.F.; Liao, H.; Cuijpers, V.; Wilson, L.J.; Mikos, A.G.; Jansen, J.A. In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering. Bone 2008, 43, 362–370. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 1–27. [Google Scholar] [CrossRef]
- Brunello, G.; Sivolella, S.; Meneghello, R.; Ferroni, L.; Gardin, C.; Piattelli, A.; Zavan, B.; Bressan, E. Powder-based 3D printing for bone tissue engineering. Biotechnol. Adv. 2016, 34, 740–753. [Google Scholar] [CrossRef]
- Yoon, H.H.; Bhang, S.H.; Kim, T.; Yu, T.; Hyeon, T.; Kim, B.-S.S. Dual roles of graphene oxide in chondrogenic differentiation of adult stem cells: Cell-adhesion substrate and growth factor-delivery carrier. Adv. Funct. Mater. 2014, 24, 6455–6464. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, U.J.; Kim, H.S.; Li, C.; Wada, M.; Leisk, G.G.; Kaplan, D.L. Bone tissue engineering with premineralized silk scaffolds. Bone 2008, 42, 1226–1234. [Google Scholar] [CrossRef]
- Tatavarty, R.; Ding, H.; Lu, G.; Taylor, R.J.; Bi, X. Synergistic acceleration in the osteogenesis of human mesenchymal stem cells by graphene oxide-calcium phosphate nanocomposites. Chem. Commun. 2014, 50, 8484–8487. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, L.; Elsayed, H.; Badocco, D.; Pastore, P.; Bellucci, D.; Cannillo, V.; Detsch, R.; Boccaccini, A.R.; Bernardo, E. Direct ink writing of silica-bonded calcite scaffolds from preceramic polymers and fillers. Biofabrication 2017, 9, 025012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Q.; Zhao, W.; Qiao, B.; Cui, H.; Fan, J.; Li, H.; Tu, X.; Jiang, D. In vitro and in vivo biocompatibility and osteogenesis of graphene-reinforced nanohydroxyapatite polyamide66 ternary biocomposite as orthopedic implant material. Int. J. Nanomed. 2016, 11, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Ettorre, V.; De Marco, P.; Zara, S.; Perrotti, V.; Scarano, A.; Di Crescenzo, A.; Petrini, M.; Hadad, C.; Bosco, D.; Zavan, B.; et al. In vitro and in vivo characterization of graphene oxide coated porcine bone granules. Carbon N.Y. 2016, 103, 291–298. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Gao, C.; Liu, T.; Shuai, C.; Peng, S. Enhancement mechanisms of graphene in nano-58S bioactive glass scaffold: Mechanical and biological performance. Sci. Rep. 2014, 4, 4712. [Google Scholar] [CrossRef]
- Elkhenany, H.; Amelse, L.; Lafont, A.; Bourdo, S.; Caldwell, M.; Neilsen, N.; Dervishi, E.; Derek, O.; Biris, A.S.; Anderson, D.; et al. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: Potential for bone tissue engineering. J. Appl. Toxicol. 2015, 35, 367–374. [Google Scholar] [CrossRef]
- Elsayed, H.; Carraro, F.; Agnoli, S.; Bellucci, D.; Cannillo, V.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Direct ink writing of silica-carbon-calcite composite scaffolds from a silicone resin and fillers. J. Eur. Ceram. Soc. 2018, 38, 5200–5207. [Google Scholar] [CrossRef]
- Sun, H.; Yang, H.L. Calcium phosphate scaffolds combined with bone morphogenetic proteins or mesenchymal stem cells in bone tissue engineering. Chin. Med. J. 2015, 128, 1121–1127. [Google Scholar] [CrossRef]
- Sokolova, V.; Ludwig, A.K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, J.; Liu, F.; Nie, Y.; Ren, L.; Liu, B. A new composite scaffold of bioactive glass nanoparticles/graphene: Synchronous improvements of cytocompatibility and mechanical property. Colloids Surf. B Biointerfaces 2016, 145, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Sbricoli, L.; Gobbato, L.; Ludovichetti, F.S.; Tocco, I.; Carraro, A.; Piattelli, A.; Zavan, B. Graphene based scaffolds effects on stem cells commitment. J. Transl. Med. 2014, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Briso, A.L.; Aachmann, F.L.; Moreno-Manzano, V.; Serrano-Aroca, Á. Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 2020, 143, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Turk, M.; Deliormanll, A.M. Electrically conductive borate-based bioactive glass scaffolds for bone tissue engineering applications. J. Biomater. Appl. 2017, 32, 28–39. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.R.; Ahn, J.H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [PubMed]

| Sample | OD | HI | Results |
|---|---|---|---|
| PC | 0.91 ± 0.008 | 100% | Hemolytic |
| Bio | 0.0126 ± 0.003 | 0% | Nonhemolytic |
| C-Bio | 0.0125 ± 0.002 | 0% | Nonhemolytic |
| Sample | STDisc™ TA1535 | STDisc™ TA1537 | STDisc™ TA98 | STDisc™ TA100 | ||||
|---|---|---|---|---|---|---|---|---|
| Rev/Plate a | Result | Rev/Plate a | Result | Rev/Plate a | Result | Rev/Plate a | Result | |
| blank | 4 ± 3 | not mutagenic | 5 ± 3 | not mutagenic | 5 ± 3 | not mutagenic | 3 ± 3 | not mutagenic |
| NC b | 3 ± 2 | not mutagenic | 4 ± 2 | not mutagenic | 2 ± 2 | not mutagenic | 4 ± 2 | not mutagenic |
| PC1 c | 945 ± 64 | mutagenic | 956 ± 94 | mutagenic | 932 ± 69 | mutagenic | 933 ± 81 | mutagenic |
| PC2 d | 858 ± 54 | mutagenic | 874 ± 41 | mutagenic | 859 ± 43 | mutagenic | 862 ± 63 | mutagenic |
| Bio e | 3 ± 2 | not mutagenic | 4 ± 2 | not mutagenic | 2 ± 2 | not mutagenic | 4 ± 2 | not mutagenic |
| C-Bio f | 3 ± 2 | not mutagenic | 4 ± 2 | not mutagenic | 2 ± 2 | not mutagenic | 4 ± 2 | not mutagenic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memarian, P.; Sartor, F.; Bernardo, E.; Elsayed, H.; Ercan, B.; Delogu, L.G.; Zavan, B.; Isola, M. Osteogenic Properties of 3D-Printed Silica-Carbon-Calcite Composite Scaffolds: Novel Approach for Personalized Bone Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 475. https://doi.org/10.3390/ijms22020475
Memarian P, Sartor F, Bernardo E, Elsayed H, Ercan B, Delogu LG, Zavan B, Isola M. Osteogenic Properties of 3D-Printed Silica-Carbon-Calcite Composite Scaffolds: Novel Approach for Personalized Bone Tissue Regeneration. International Journal of Molecular Sciences. 2021; 22(2):475. https://doi.org/10.3390/ijms22020475
Chicago/Turabian StyleMemarian, Parastoo, Francesco Sartor, Enrico Bernardo, Hamada Elsayed, Batur Ercan, Lucia Gemma Delogu, Barbara Zavan, and Maurizio Isola. 2021. "Osteogenic Properties of 3D-Printed Silica-Carbon-Calcite Composite Scaffolds: Novel Approach for Personalized Bone Tissue Regeneration" International Journal of Molecular Sciences 22, no. 2: 475. https://doi.org/10.3390/ijms22020475
APA StyleMemarian, P., Sartor, F., Bernardo, E., Elsayed, H., Ercan, B., Delogu, L. G., Zavan, B., & Isola, M. (2021). Osteogenic Properties of 3D-Printed Silica-Carbon-Calcite Composite Scaffolds: Novel Approach for Personalized Bone Tissue Regeneration. International Journal of Molecular Sciences, 22(2), 475. https://doi.org/10.3390/ijms22020475








