Annexin 1 Is a Component of eATP-Induced Cytosolic Calcium Elevation in Arabidopsis thaliana Roots
Abstract
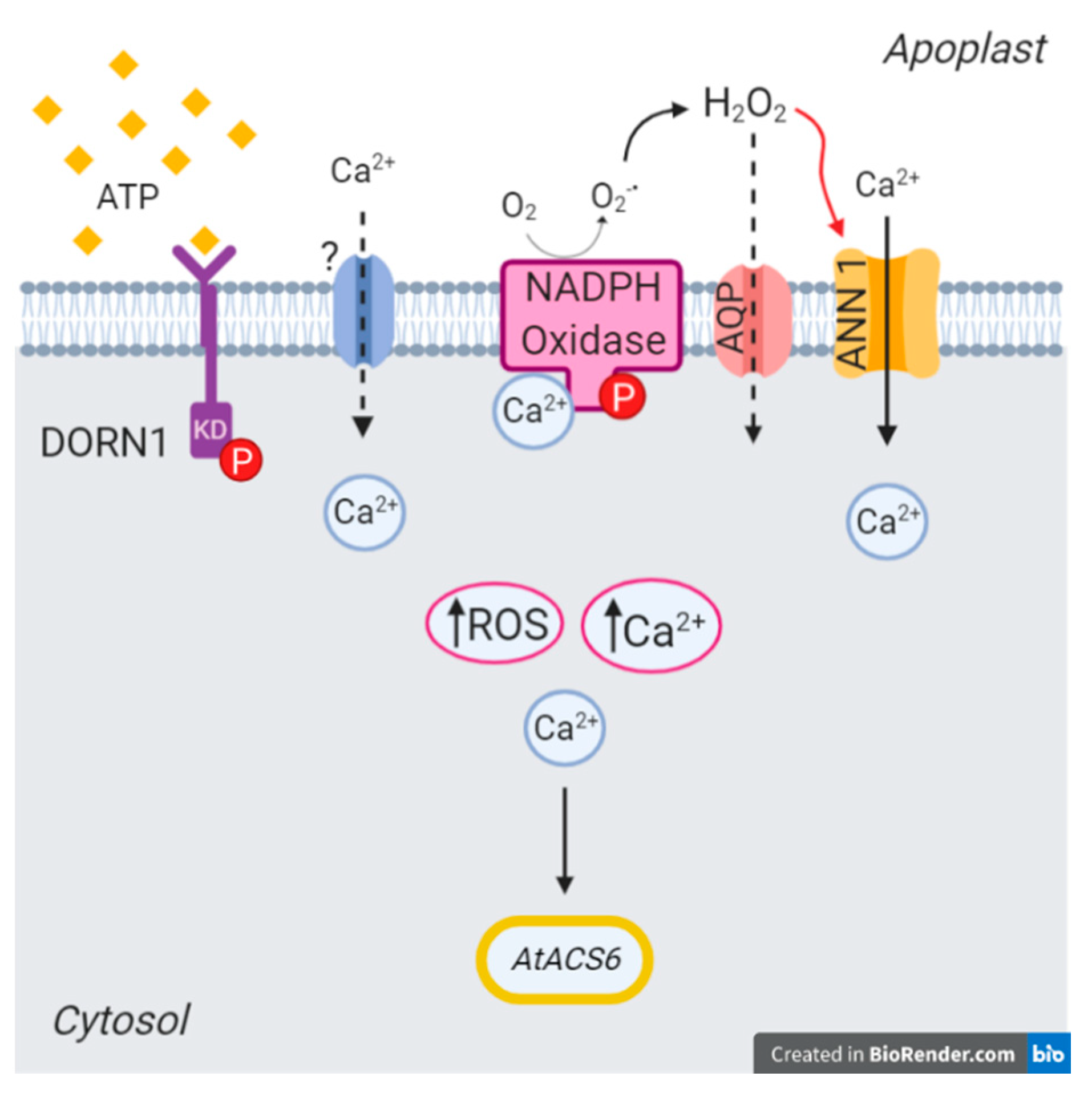
:1. Introduction
2. Results
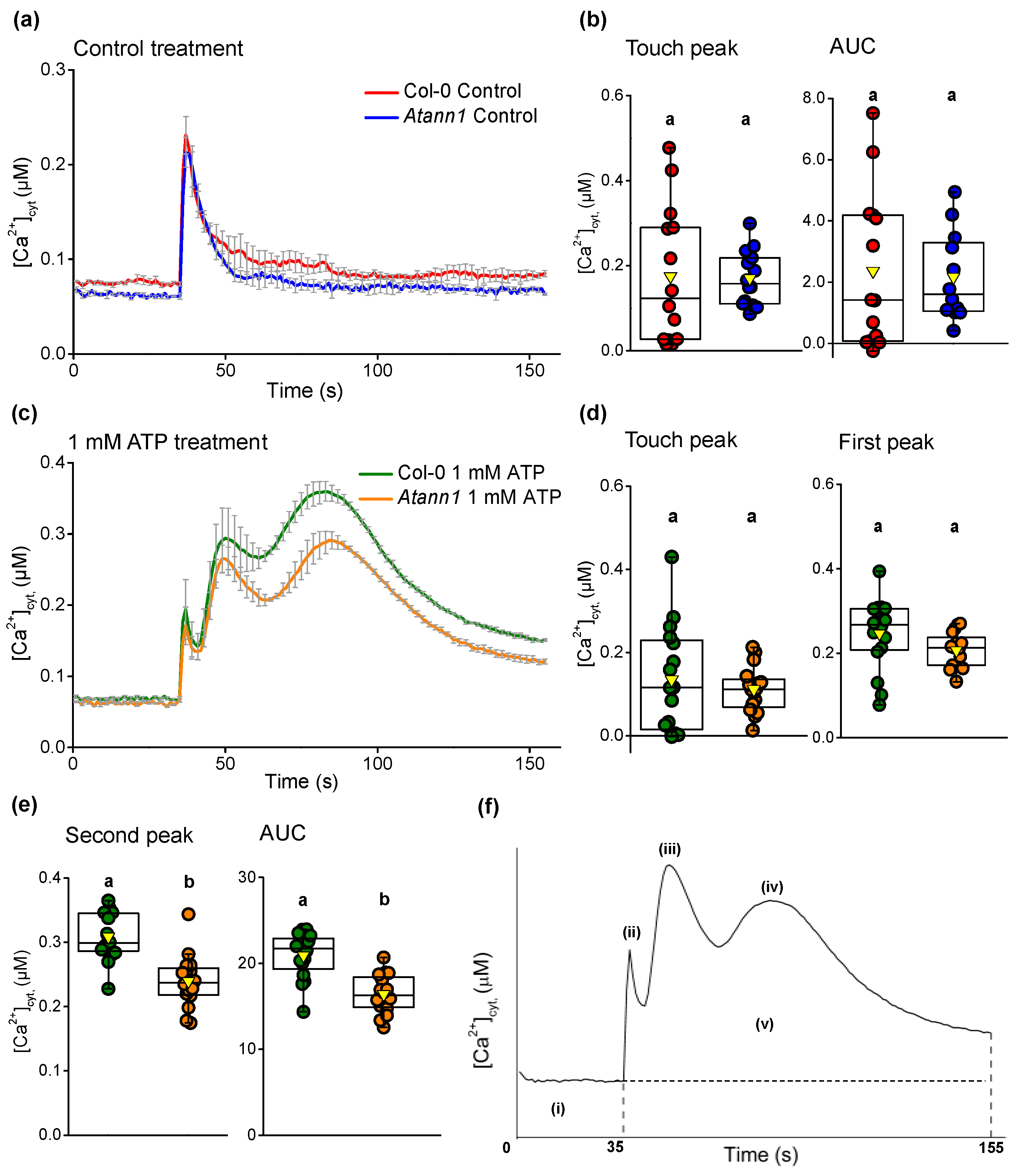
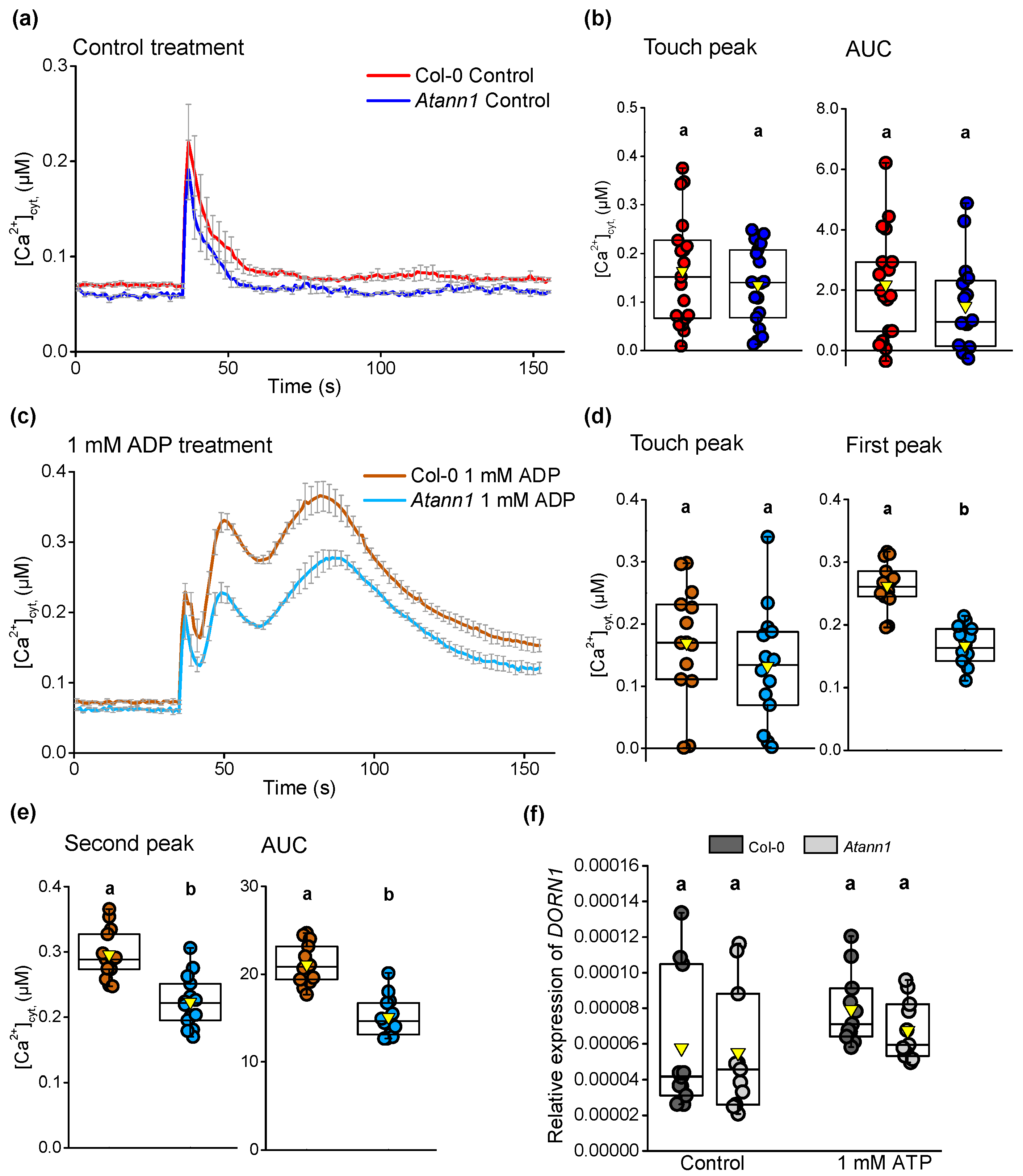
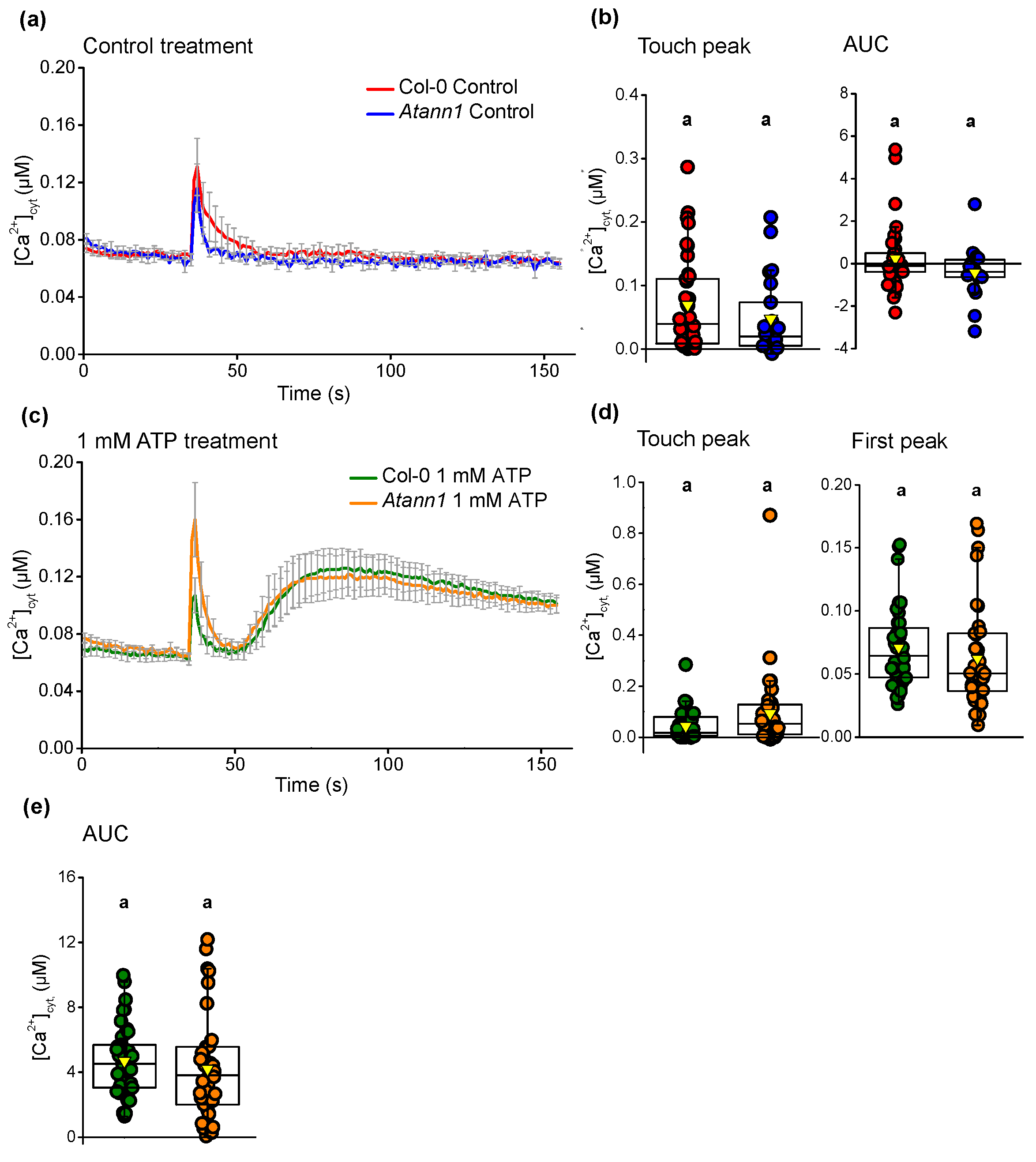
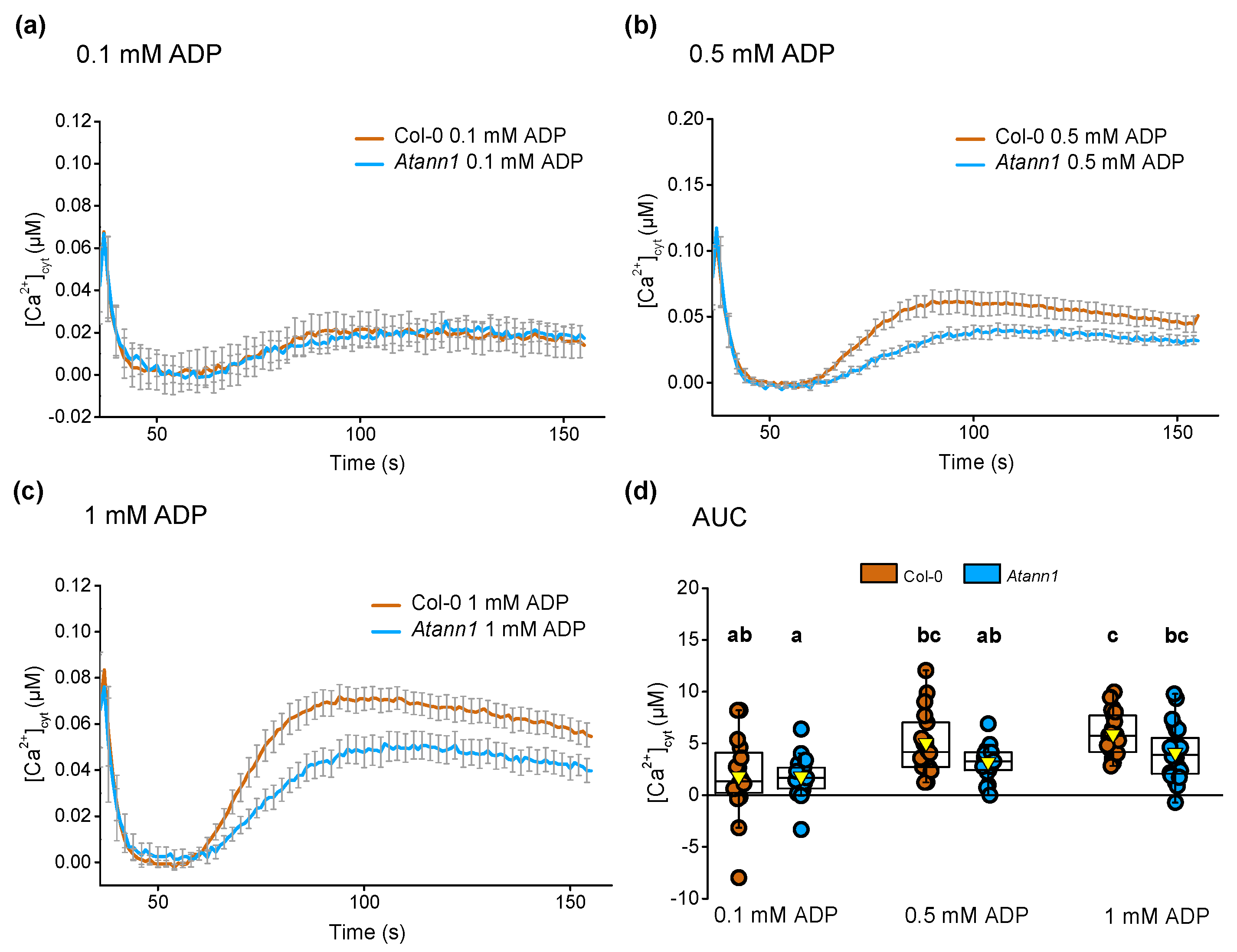
2.1. AtANN1 Mediates Root [Ca2+]cyt Elevation In Response To eATP and eADP
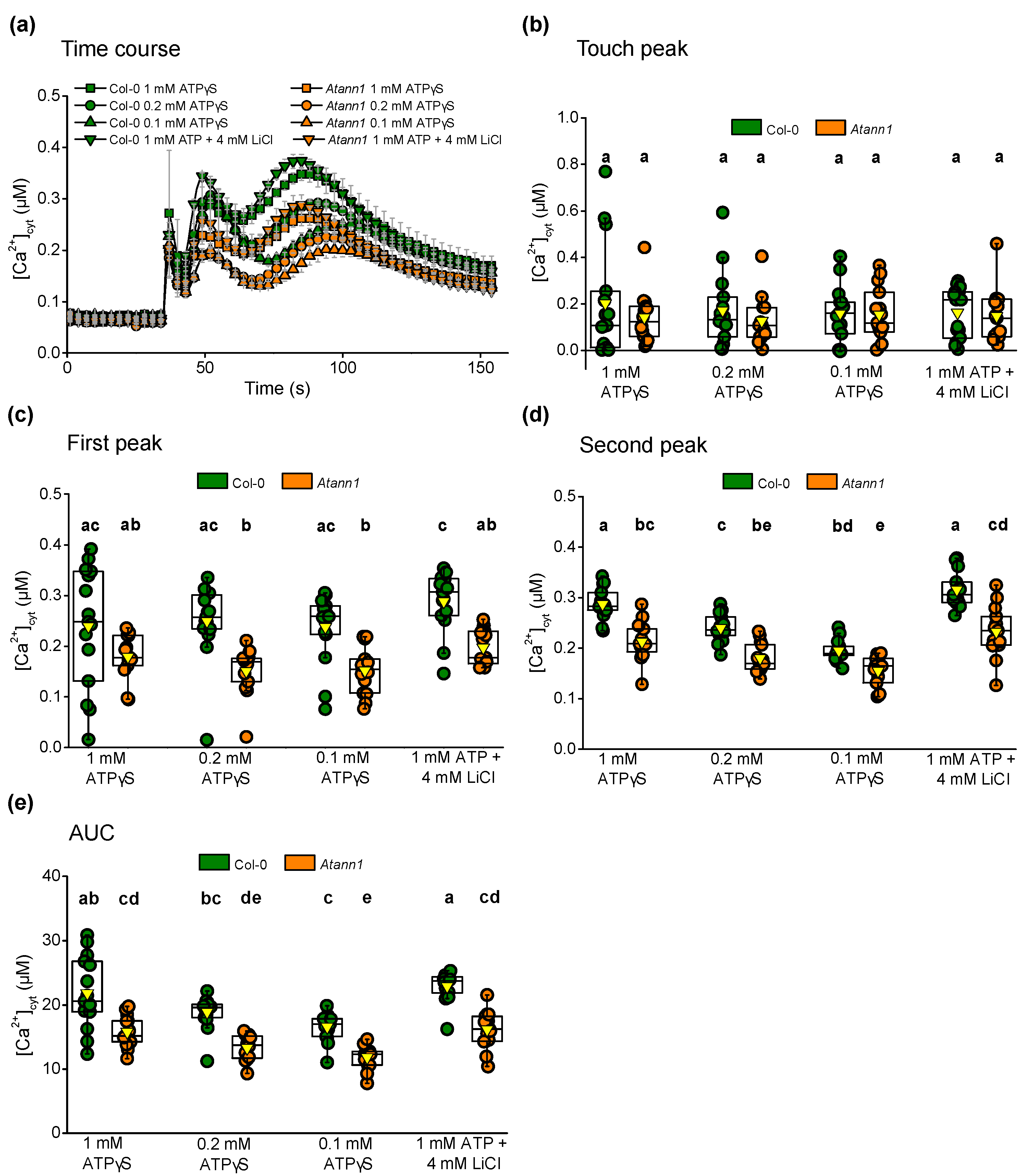
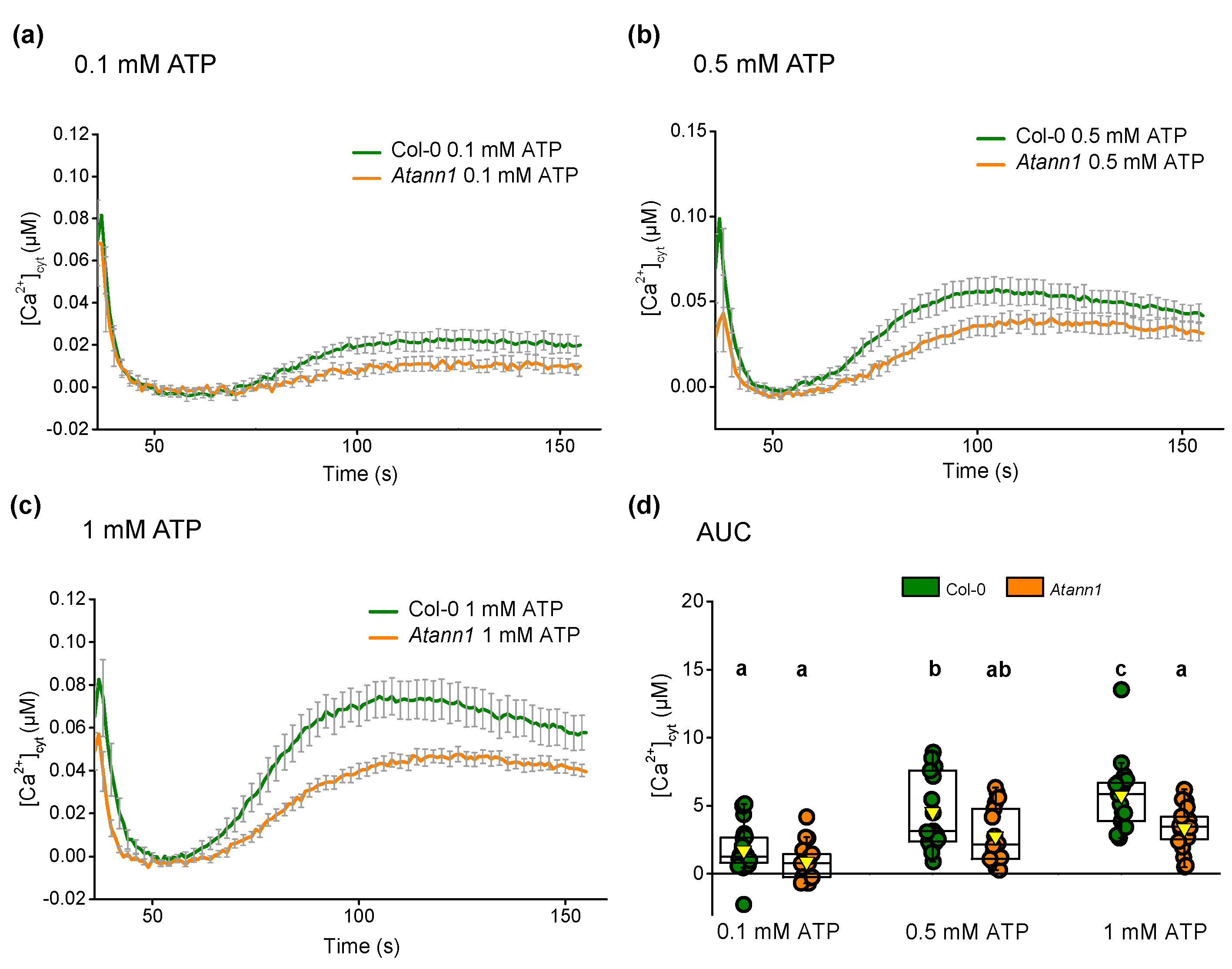
2.2. AtANN1’s Involvement in the eATP-Generated First Peak Response Is Concentration-Dependent
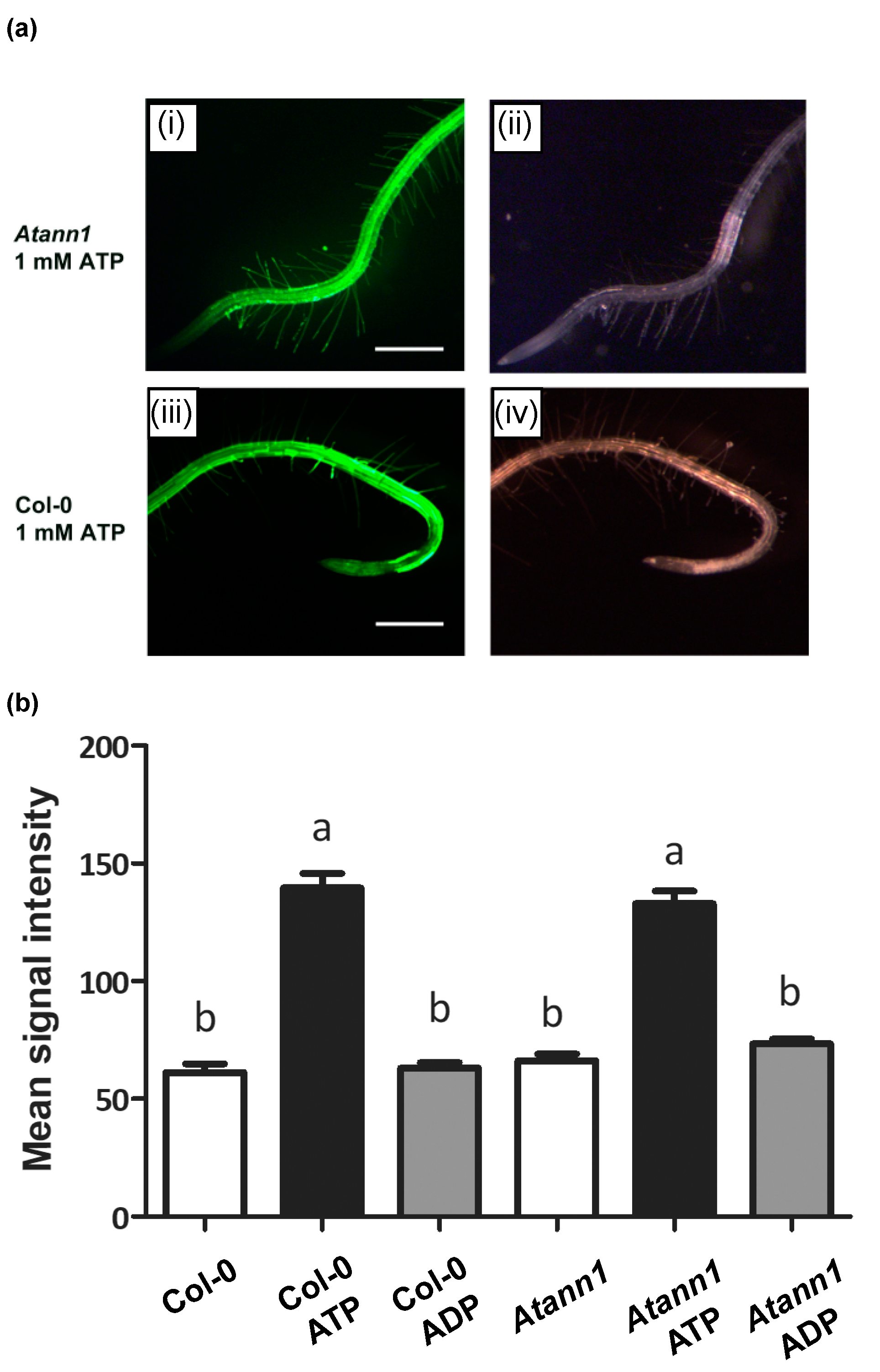
2.3. AtANN1 Sets the Spatial Extent of Intracellular ROS in Roots in Response to eATP
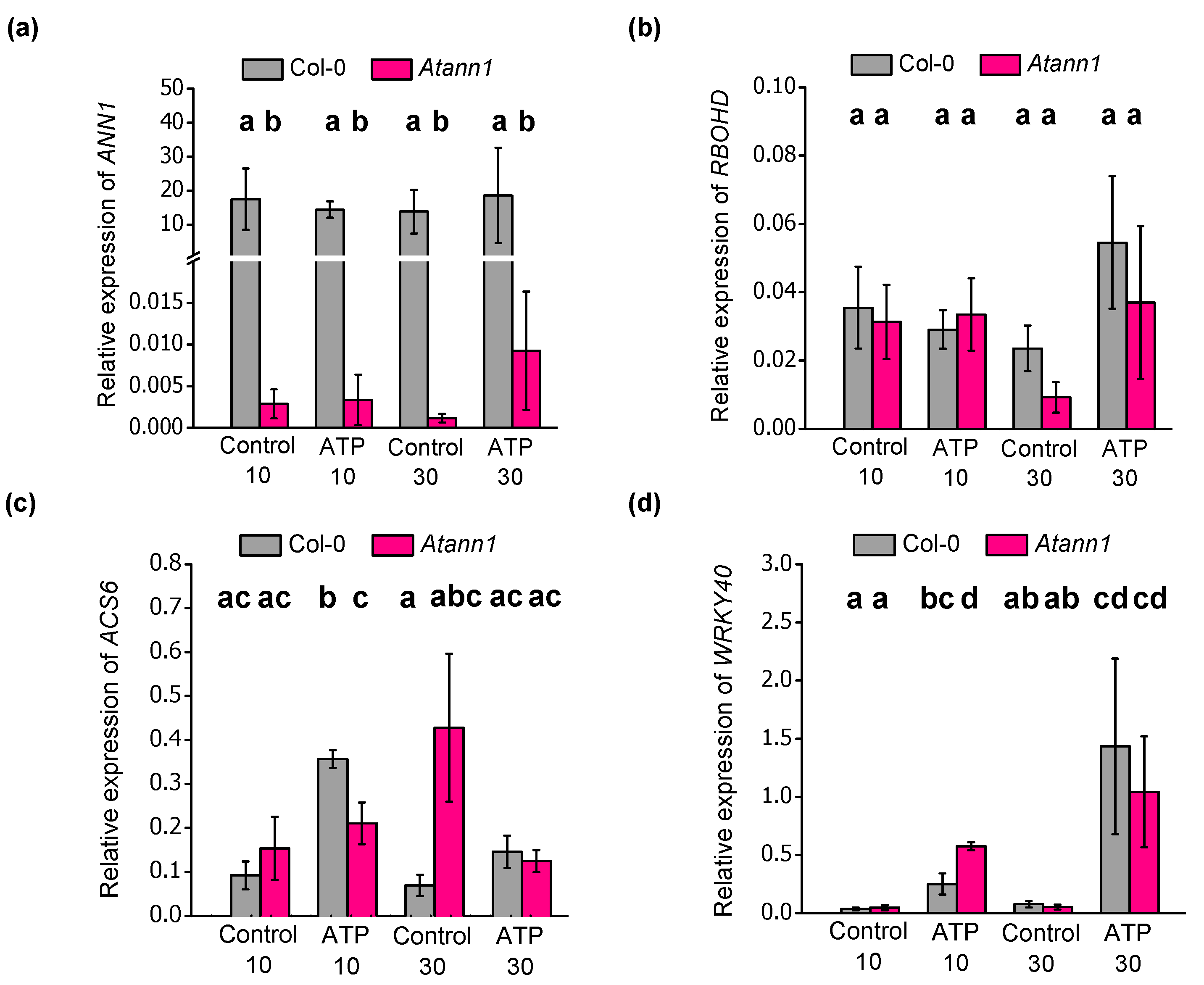
2.4. AtANN1 Is Required For eATP-Induced Changes in the Expression of ACS6 and WRKY40
2.5. eATP-Induced [Ca2+]cyt Elevation Is Not Mediated by AtANN1 in Cotyledons
2.6. AtANN1 Is Less Important in the [Ca2+]cyt Response of True Leaves to Extracellular ATP or ADP
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Measurement of [Ca2+]cyt
4.3. Determination of Intracellular ROS
4.4. Quantification of eATP-Induced Gene Expression
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | 1-Aminocyclopropane-1-carboxylate synthase |
| ADPβS | Adenosine 5′-[β-thio] diphosphate |
| ANN1 | Annexin1 |
| ANOVA | Analysis of Variance |
| AQP | Aquaporins |
| ATPγS | Adenosine 5′-[γ-thio] triphosphate |
| AUC | Area Under the Curve |
| [Ca2+]cyt | Cytosolic free calcium ion |
| CAM | Calmodulin |
| CAMTA | Calmodulin-binding Transcription Activators |
| CAMV | Cauliflower Mosaic Virus |
| cDNA | Complementary DNA |
| CM-H2DCFDA | 5-(and-6-)-Chloromethyl-2′,7′-dichlorodihydrofluorscein diacetate |
| DAMP | Damage Associated Molecular Pattern |
| DORN1 | Does not Respond to Nucleotides1 |
| eATP | Extracellular ATP |
| eADP | Extracellular ADP |
| H2O2 | Hydrogen peroxide |
| MES | 2-(N-morpholino) ethanesulfonic |
| MS | Murashige and Skoog nutrient medium |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NO | Nitric oxide |
| PA | Phosphatidic Acid |
| qRT-PCR | Quantitative reverse transcription-polymerase chain reaction |
| RBOH | Respiratory burst homologue |
| ROS | Reactive oxygen species |
| RNAseq | RNA sequencing |
| SEM | Standard error of mean |
| T-DNA | Transfer DNA |
| Tris | Tris base, 2-amino-2-(hydroxymethyl)-1,3-propanediol |
| TUB4 | Tubulin Beta Chain4 |
| UBQ10 | Polyubiquitin10 |
| YC3.6 | Yellow Cameleon 3.6 |
Appendix A
| Gene | Forward (5′–3′) | Reverse (3′–5′) |
|---|---|---|
| AtANN1 (AT1G35720.1) | TGTTCTTCGTTCAGCAATCAAC | GTACTCCTCTCCAATGACCTTC |
| AtDORN1 (AT5G60300) | ATGGTCACATTGCCTGCAGAAG | TCCCTCTTTACAGGCTGGACTCTC |
| AtRBOHD (AT5G47910) | ATGATCAAGGTGGCTGTTTACCC | ATCCTTGTGGCTTCGTCATGTG |
| AtACS6 (AT4G11280) | TATCCAGGGTTTGATAGAGA | TCCACCGTAATCTTGAACC |
| AtWRKY40 (AT1G80840) | AGCTTCTGACACTACCCTCGTTG | TTGACAGAACAGCTTGGAGCAC |
| AtUBQ10 (AT4G05320) | CCGACTACAACATTCAGAAGGA | TCAGAACTCTCCACCTCCAAA |
| AtTUB4 (AT5G44340) | AGGGAAACGAAGACAGCAAG | GCTCGCTAATCCTACCTTTGG |
References
- Chivasa, S.; Ndimba, B.K.; Simon, W.J.; Lindsey, K.; Slabas, A.R. Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell 2005, 17, 3019–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, G.; Torres, J.; Herz, N.; Wat, N.; Ogoti, J.; Aranda, G.; Blizard, M.; Wu, M.; Onyirimba, J.; Canales, A.A.; et al. Both the stimulation and inhibition of root hair growth induced by extracellular nucleotides in Arabidopsis are mediated by nitric oxide and reactive oxygen species. Plant Mol. Biol. 2010, 74, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, C.L.; Deng, S.R.; Lu, C.F.; Shen, X.; Zhou, X.Y.; Zheng, X.J.; Hu, Z.M.; Chen, S.L. An ATP signalling pathway in plant cells: Extracellular ATP triggers programmed cell death in Populus euphratica. Plant Cell Environ. 2012, 35, 893–916. [Google Scholar] [CrossRef] [PubMed]
- Nizam, S.; Qiang, X.; Wawra, S.; Nostadt, R.; Getzke, F.; Schwanke, F.; Dreyer, I.; Langen, G.; Zuccaro, A. Serendipita indica E5′NT modulates extracellular nucleotide levels in the plant apoplast and affects fungal colonization. EMBO Rep. 2019, 20, e47430. [Google Scholar] [CrossRef] [PubMed]
- Matthus, E.; Sun, J.; Wang, L.; Bhat, M.G.; Sidik, A.B.M.; Davies, J.M. DORN1/P2K1 and purino—Calcium signalling in plants; making waves with extracellular ATP. Ann. Bot. 2019, 124, 1227–1242. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Wang, Y.; Fan, B.; Sun, K.; Liang, J.; Feng, H.; Jia, L. Extracellular ATP affects cell viability, respiratory O2 uptake, and intracellular ATP production of tobacco cell suspension culture in response to hydrogen peroxide-induced oxidative stress. Biologia 2020, 75, 1437–1443. [Google Scholar] [CrossRef]
- Choi, J.; Tanaka, K.; Cao, Y.; Qi, Y.; Qiu, J.; Liang, Y.; Lee, S.Y.; Stacey, G. Identification of a plant receptor for extracellular ATP. Science 2014, 343, 290–294. [Google Scholar] [CrossRef]
- Pham, A.Q.; Cho, S.-H.; Nguyen, C.T.; Stacey, G. Arabidopsis lectin receptor kinase P2K2 is a second plant receptor for extracellular ATP and contributes to innate immunity. Plant Physiol. 2020, 183, 1364–1375. [Google Scholar] [CrossRef]
- Zhu, R.; Dong, X.; Hao, W.; Gao, W.; Zhang, W.; Xia, S.; Liu, T.; Shang, Z. Heterotrimeric G protein-regulated Ca2+ influx and PIN2 asymmetric distribution are involved in Arabidopsis thaliana roots’ avoidance response to extracellular ATP. Front. Plant Sci. 2017, 8, 1522. [Google Scholar] [CrossRef] [Green Version]
- Zhu, R.; Dong, X.; Xue, Y.; Xu, J.; Zhang, A.; Feng, M.; Zhao, Q.; Xia, S.; Yin, Y.; He, S.; et al. Redox-Responsive Transcription Factor 1 (RRFT1) is involved in extracellular ATP-regulated Arabidopsis thaliana seedling growth. Plant Cell Physiol. 2020, 61, 685–698. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.N.; Coutts, K.B.; Tester, M.A.; Davies, J.M. Free oxygen radicals regulate plasma membrane Ca2+-and K+-permeable channels in plant root cells. J. Cell Sci. 2003, 116, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeter, C.R.; Tang, W.; Henaff, E.; Butterfield, T.; Roux, S.J. Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 2004, 16, 2652–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-Y.; Sivaguru, M.; Stacey, G. Extracellular ATP in plants. Visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol. 2006, 142, 984–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidchik, V.; Shang, Z.; Shin, R.; Thompson, E.; Rubio, L.; Laohavisit, A.; Mortimer, J.C.; Chivasa, S.; Slabas, A.R.; Glover, B.J.; et al. Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J. 2009, 58, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Foresi, N.P.; Laxalt, A.M.; Tonón, C.V.; Casalongué, C.A.; Lamattina, L. Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol. 2007, 145, 589–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.J.; Wu, J.Y. Extracellular ATP-induced NO production and its dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. J. Exp. Bot. 2008, 59, 4007–4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, D.; Zhang, T.; Koo, A.J.; Stacey, G.; Tanaka, K. Extracellular ATP acts on jasmonate signaling to reinforce plant defense. Plant Physiol. 2017, 176, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Jewell, J.B.; Sowders, J.M.; He, R.; Willis, M.A.; Gang, D.R.; Tanaka, K. Extracellular ATP shapes a defense-related transcriptome both independently and along with other defense signaling pathways. Plant Physiol. 2019, 179, 1144–1158. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Zhu, R.; Kang, E.; Shang, Z. RRFT1 (Redox Responsive Transcription Factor 1) is involved in extracellular ATP-regulated gene expression in Arabidopsis thaliana seedlings. Plant Signal. Behav. 2020, 15, 1748282. [Google Scholar] [CrossRef]
- Loro, G.; Drago, I.; Pozzan, T.; Lo Schiavo, F.; Costa, A. Targeting of Cameleons to various subcellular compartments reveals a strict cytoplasmic/mitochondrial Ca2+ handling relationships in plant cells. Plant J. 2012, 71, 1–13. [Google Scholar] [CrossRef]
- Demidchik, V.; Shang, Z.; Shin, R.; Colaço, R.; Laohavisit, A.; Shabala, S.; Davies, J.M. Receptor-like activity evoked by extracellular ADP in Arabidopsis root epidermal plasma membrane. Plant Physiol. 2011, 156, 1375–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wilkins, K.A.; Davies, J.M. Arabidopsis DORN1 extracellular ATP receptor; activation of plasma membrane K+-and Ca2+-permeable conductances. New Phytol. 2018, 218, 1301–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Stacey, G.; Leblanc-Fournier, N.; Legué, V.; Moulia, B.; Davies, J.M. Early extracellular ATP signaling in Arabidopsis root epidermis: A multi-conductance process. Front. Plant Sci. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.; Roux, S.J. Role of Ca2+ in mediating plant responses to extracellular ATP and ADP. Int. J. Mol. Sci. 2018, 19, 3590. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Ye, J.; Yang, Y.; Lin, H.; Yue, L.; Luo, J.; Long, Y.; Fu, H.; Liu, X.; Zhang, Y.; et al. The SOS2-SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 2019, 48, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Laohavisit, A.; Shang, Z.; Rubio, L.; Cuin, T.A.; Véry, A.A.; Wang, A.; Mortimer, J.C.; Macpherson, N.; Coxon, K.M.; Battey, N.H.; et al. Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+-and K+-permeable conductance in root cells. Plant Cell 2012, 24, 1522–1533. [Google Scholar] [CrossRef] [Green Version]
- Laohavisit, A.; Richards, S.L.; Shabala, L.; Chen, C.; Colaco, R.D.D.R.; Swarbreck, S.M.; Shaw, E.; Dark, A.; Shabala, S.; Shang, Z.; et al. Salinity-induced calcium signaling and root adaptation in Arabidopsis require the calcium regulatory protein Annexin1. Plant Physiol. 2013, 163, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Richards, S.L.; Laohavisit, A.; Mortimer, J.C.; Shabala, L.; Swarbreck, S.M.; Shabala, S.; Davies, J.M. Annexin 1 regulates the H2O2-induced calcium signature in Arabidopsis thaliana roots. Plant J. 2014, 77, 136–145. [Google Scholar] [CrossRef]
- Chivasa, S.; Murphy, A.M.; Hamilton, J.M.; Lindsey, K.; Carr, J.P.; Slabas, A.R. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009, 60, 436–448. [Google Scholar] [CrossRef]
- Tanaka, K.; Choi, J.; Cao, Y.; Stacey, G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front. Plant Sci. 2014, 5, 446. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Tanaka, K.; Liang, Y.; Cao, Y.; Lee, S.Y.; Stacey, G. Extracellular ATP, a danger signal, is recognized by DORN1 in Arabidopsis. Biochem. J. 2014, 463, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.B.; Sessions, A.; Eastburn, D.J.; Roux, S.J. Differential expression of members of the annexin multigene family in Arabidopsis. Plant Physiol. 2001, 126, 1072–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantero, A.; Barthakur, S.; Bushart, T.J.; Chou, S.; Morgan, R.O.; Fernandez, M.P.; Clark, G.B.; Roux, S.J. Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol. Biochem. 2006, 44, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tichá, M.; Richter, H.; Ovečka, M.; Maghelli, N.; Hrbáčková, M.; Dvořák, P.; Šamaj, J.; Šamajová, O. Advanced microscopy reveals complex developmental and subcellular localization patterns of ANNEXIN 1 in Arabidopsis. Front. Plant Sci. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Weerasinghe, R.R.; Swanson, S.J.; Okada, S.F.; Garrett, M.B.; Kim, S.Y.; Stacey, G.; Boucher, R.C.; Gilroy, S.; Jones, A.M. Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett. 2009, 583, 2521–2526. [Google Scholar] [CrossRef] [Green Version]
- Matthus, E.; Wilkins, K.A.; Swarbreck, S.M.; Doddrell, N.H.; Doccula, H.G.; Costa, A.; Davies, J.M. Phosphate starvation alters root calcium signatures. Plant Physiol. 2019, 179, 1754–1767. [Google Scholar] [CrossRef] [Green Version]
- Rincón-Zachary, M.; Teaster, N.D.; Sparks, J.A.; Valster, A.H.; Motes, C.M.; Blancaflor, E.B. Fluorescence resonance energy transfer-sensitized emission of yellow cameleon 3.60 reveals root zone-specific calcium signatures in Arabidopsis in response to aluminum and other trivalent cations. Plant Physiol. 2010, 152, 1442–1458. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.; Candeo, A.; Fieramonti, L.; Valentini, G.; Bassi, A. Calcium dynamics in root cells of Arabidopsis thaliana visualized with selective plane illumination microscopy. PLoS ONE 2013, 8, e75646. [Google Scholar] [CrossRef]
- Krogman, W.; Sparks, J.A.; Blancaflor, E.B. Cell type-specific imaging of calcium signaling in Arabidopsis thaliana seedling roots using GCaMP3. Int. J. Mol. Sci. 2020, 21, 6385. [Google Scholar] [CrossRef]
- Chen, D.; Cao, Y.; Li, H.; Kim, D.; Ahsan, N.; Thelen, J.; Stacey, G. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat. Commun. 2017, 8, 2265. [Google Scholar] [CrossRef] [Green Version]
- Konopka-Postupolska, D.; Clark, G.; Goch, G.; Debski, J.; Floras, K.; Cantero, A.; Fijolek, B.; Roux, S.; Hennig, J. The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol. 2009, 150, 1394–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalal, A.; Kumar, A.; Yadav, D.; Gudla, T.; Viehhauser, A.; Dietz, J.-K.; Kirti, P.B. Alleviation of methyl viologen-mediated oxidative stress by Brassica juncea annexin-3 in transgenic Arabidopsis. Plant Sci. 2014, 219, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Zhang, Q.; Liu, D.; Wang, H.; Yin, J.; Wang, R.; He, M.; Cui, M.; Shang, Z.; Wang, D.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Expt. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Li, S.F.; Yang, S.M.; Wang, L.K.; Guo, W.Z. Overexpression of a cotton annexin gene, GhAnn1, enhances drought and salt stress tolerance in transgenic cotton. Plant Mol. Biol. 2015, 87, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.H.; Wu, J.; Yao, J.; Gallardo, I.F.; Dugger, J.W.; Webb, L.J.; Huang, J.; Salmi, M.L.; Song, J.; Clark, G.; et al. Apyrase suppression raises extracellular ATP levels and induces gene expression and cell wall changes characteristic of stress responses. Plant Physiol. 2014, 164, 2054–2067. [Google Scholar] [CrossRef] [Green Version]
- Song, C.J.; Steinebrunner, I.; Wang, X.; Stout, S.C.; Roux, S.J. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006, 140, 1222–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, T.; Deng, C.; Yao, J.; Zhang, H.; Wang, Y.; Deng, S. A salt-signaling network involving ethylene, extracellular ATP, hydrogen peroxide, and calcium mediates K+/Na+ homeostasis in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 8683. [Google Scholar] [CrossRef]
- Dark, A.; Demidchik, V.; Richards, S.L.; Shabala, S.; Davies, J.M. Release of extracellular purines from plant roots and effect on ion fluxes. Plant Signal. Behav. 2011, 6, 1855–1857. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Lai, Z.; Shi, J.; Xiao, Y.; Chen, Z.; Xu, X. Roles of Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol. 2010, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ma, X.L.; Wang, H.; Li, B.; Clark, G.; Guo, Y.; Roux, S.; Sun, D.; Tang, W. Proteomic study of microsomal proteins reveals a key role for Arabidopsis Annexin1 in mediating heat stress-induced increase in intracellular calcium levels. Mol. Cell Prot. 2015, 14, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, C.; Liang, Y.; Stacey, G. Chitin receptor CERK1 links salt stress and chitin-triggered innate immunity in Arabidopsis. Plant J. 2017, 98, 984–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.C.; Zheng, Y.; Guo, Y. MYB30 transcription factor regulates oxidative and heat stress responses through annexin-mediated cytosolic calcium signalling in Arabidopsis. New Phytol. 2017, 216, 163–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Li, L.; Liu, Q.; Liu, P.; Li, S.; Yang, D.; Chen, Y.; Pagnotta, S.; Favery, B.; Abad, P.; et al. A MIF-like effector suppresses plant immunity and facilitates nematode parasitism by interacting with plant annexins. J. Exp. Bot. 2019, 70, 5943–5958. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Nat. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997, 12, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.; Takeshi, Y.; Kondu, Y.; Matsui, M.; Shin, R. Identification and characterization of transcription factors regulating HAK5. Plant Cell Physiol. 2013, 54, 1478–1490. [Google Scholar] [CrossRef] [Green Version]
- Andrej-Nikolai Spiess. Package ‘qpcR’. 2018. Available online: https://cran.r-project.org/web/packages/qpcR/qpcR.pdf (accessed on 5 June 2020).
- Swarbreck, S.M.; Guerringue, Y.; Matthus, E.; Jamieson, F.J.C.; Davies, J.M. Impairment in karrikin but not strigolactone sensing enhances root skewing in Arabidopsis thaliana. Plant J. 2019, 98, 607–621. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad-Sidik, A.; Sun, J.; Shin, R.; Song, Z.; Ning, Y.; Matthus, E.; Wilkins, K.A.; Davies, J.M. Annexin 1 Is a Component of eATP-Induced Cytosolic Calcium Elevation in Arabidopsis thaliana Roots. Int. J. Mol. Sci. 2021, 22, 494. https://doi.org/10.3390/ijms22020494
Mohammad-Sidik A, Sun J, Shin R, Song Z, Ning Y, Matthus E, Wilkins KA, Davies JM. Annexin 1 Is a Component of eATP-Induced Cytosolic Calcium Elevation in Arabidopsis thaliana Roots. International Journal of Molecular Sciences. 2021; 22(2):494. https://doi.org/10.3390/ijms22020494
Chicago/Turabian StyleMohammad-Sidik, Amirah, Jian Sun, Ryoung Shin, Zhizhong Song, Youzheng Ning, Elsa Matthus, Katie A. Wilkins, and Julia M. Davies. 2021. "Annexin 1 Is a Component of eATP-Induced Cytosolic Calcium Elevation in Arabidopsis thaliana Roots" International Journal of Molecular Sciences 22, no. 2: 494. https://doi.org/10.3390/ijms22020494






