Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review
Abstract
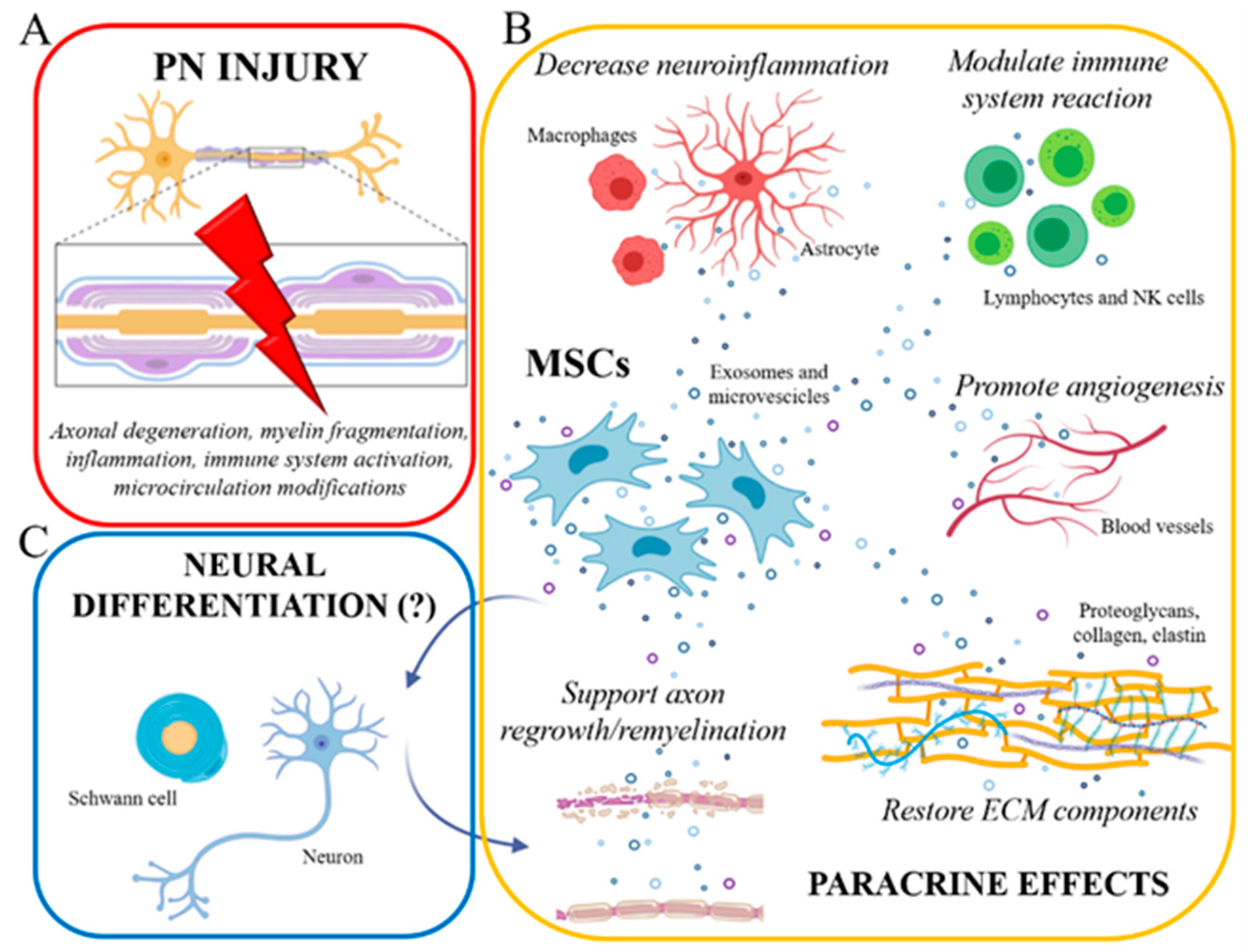
:1. Introduction
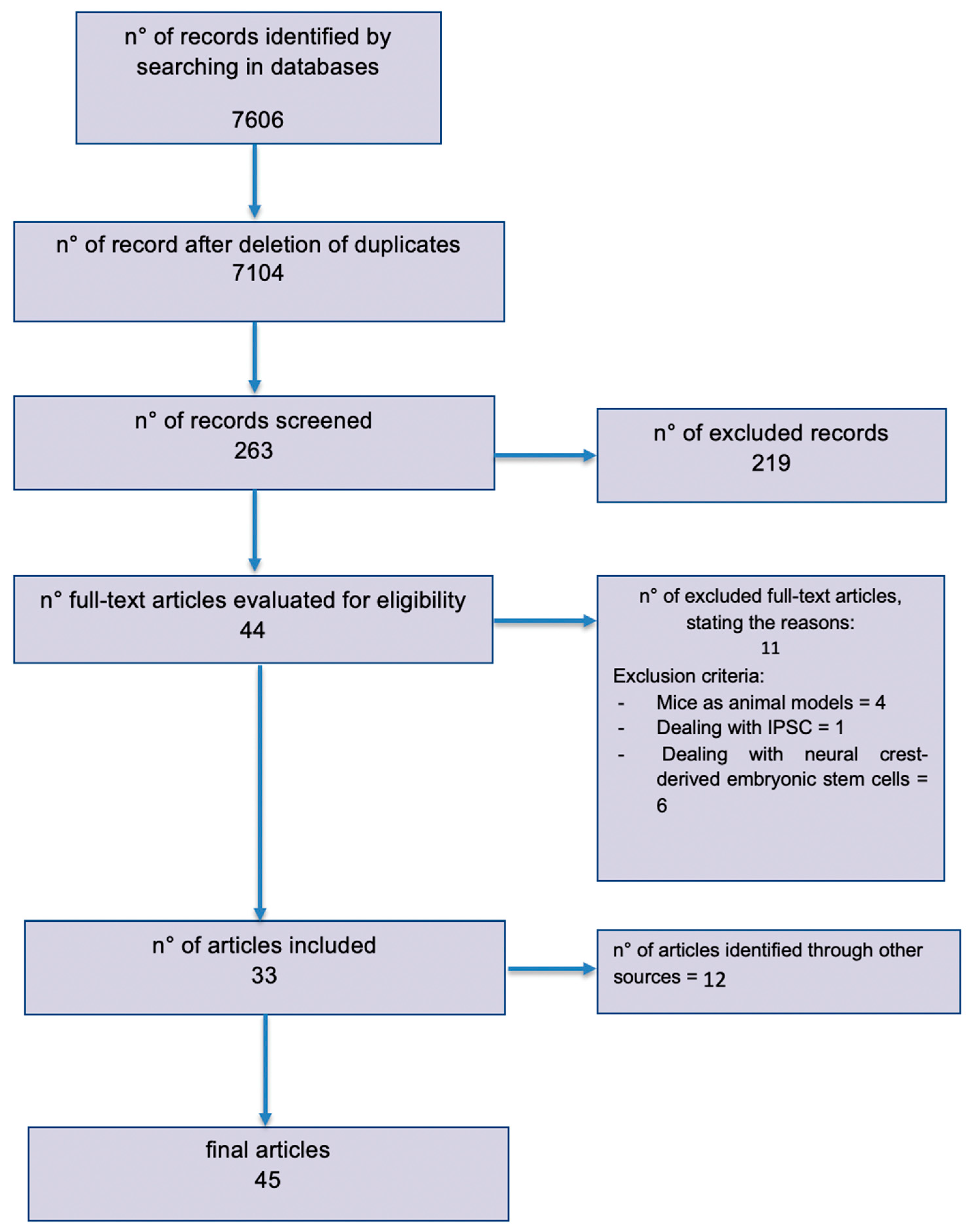
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
3. Results
3.1. Mesenchymal Stem Cells
3.1.1. Bone Marrow-Derived Mesenchymal Stem Cell
3.1.2. Adipose Tissue-Derived Mesenchymal Stem Cell
3.1.3. Fetal Tissue-Derived Mesenchymal Stem Cell
3.1.4. Dental Pulp-Derived Mesenchymal Stem Cell
3.1.5. Skeletal Muscle-Derived Mesenchymal Stem Cell
3.2. Stem Cell Delivery
3.2.1. Micro-Injection
3.2.2. Natural Nerve Conduits and Artificial Nerve Conduits
Natural Nerve Conduits
Artificial Nerve Conduits
3.2.3. Delivery Modalities
3.3. MSC Differentiation
3.3.1. Paracrine Role of MSCs
3.3.2. Targeted Stimulation of MSCs to Achieve Differentiation in Schwann-Like Cells
3.3.3. Targeted Differentiation of MSCs to Neuronal-Like Phenotype
4. Clinical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PNS | Peripheral Nervous System |
| MSCs | Mesenchymal Stem Cells |
| IPSCs | Induced Pluripotent Stem Cells |
| BMSCs | Bone Marrow Stem Cells |
| ADSCs | Adipose-Derived Stem Cells |
| DPSCs | Dental Pulp Stem Cells |
| FetalSCs | Fetal Stem Cells |
| SkSCs | Skeletal Muscle Stem Cells |
| GMSCs | gingiva-derived Stem Cells |
| CMAP | Compound muscle action potential |
| PFI | Peroneal function indices |
| EMG | Electromyography |
| SHAM | Sham-operated group |
| SNTG | Sciatic nerve transection group |
| SFI | Sciatic functional index |
| SSI | Static sciatic index |
| ANA | Acellular nerve allografts |
| DMEM | Dulbecco’s modified Eagle’s medium |
| APC | Adipose precursor cells |
| FSK | Forskolin |
| FGF | Fibroblast growth factor |
| GGF | Glial growth factor (neuregulin-1b1) |
| PDGF | Platelet-derived growth factor |
| SC | Stem cell |
| NGF | Nerve growth factor |
| FBS | Fetal bovine serum |
| GFAP | Anti-glial fibrillary acidic protein |
| GFP | Green Fluorescent Protein |
| EGF | Epidermal growth factor |
| LPS | Innate immune system via lipopolysaccharide |
| FK506 | Tacrolimus |
| PBS | Phosphate-buffered saline |
| IOAG | Artery Graft2 |
| DASH | Disabilities of the Arm, Shoulder and Hand |
References
- Tremp, M.; Zu Schwabedissen, M.M.; Kappos, E.A.; Engels, P.E.; Fischmann, A.; Scherberich, A.; Schaefer, D.J.; Kalbermatten, D.F. The Regeneration Potential after Human and Autologous Stem Cell Transplantation in a Rat Sciatic Nerve Injury Model can be Monitored by MRI. Cell Transplant. 2015, 24, 203–211. [Google Scholar] [CrossRef]
- Matthes, S.M.; Reimers, K.; Janssen, I.; Liebsch, C.; Kocsis, J.D.; Vogt, P.M.; Radtke, C. Intravenous Transplantation of Mesenchymal Stromal Cells to Enhance Peripheral Nerve Regeneration. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Yousefi, F.; Arab, F.L.; Nikkhah, K.; Amiri, H.; Mahmoudi, M. Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life Sci. 2019, 221, 99–108. [Google Scholar] [CrossRef]
- Moattari, M.; Kouchesfehani, H.M.; Kaka, G.; Sadraie, S.H.; Naghdi, M. Evaluation of nerve growth factor (NGF) treated mesenchymal stem cells for recovery in neurotmesis model of peripheral nerve injury. J. Cranio-Maxillofac. Surg. 2018, 46, 898–904. [Google Scholar] [CrossRef] [PubMed]
- De Albornoz, P.M.; Delgado, P.J.; Forriol, F.; Maffulli, N. Non-surgical therapies for peripheral nerve injury. Br. Med. Bull. 2011, 100, 73–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titolo, P.; Lavorato, A.; Isoardo, G.; Vincitorio, F.; Garbossa, D.; Battiston, B. Transfer of the peroneal component of the sciatic nerve in total brachial plexus lesion: An anatomical feasibility study. Injury 2020, 51, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jones, S.; Jia, X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int. J. Mol. Sci. 2017, 18, 94. [Google Scholar] [CrossRef]
- Uz, M.; Das, S.R.; Ding, S.; Sakaguchi, D.S.; Hondred, J.A.; Mallapragada, S.K. Advances in Controlling Differentiation of Adult Stem Cells for Peripheral Nerve Regeneration. Adv. Healthc. Mater. 2018, 7, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C.V. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int. J. Mol. Sci. 2016, 17, 2101. [Google Scholar] [CrossRef]
- Mathot, F.; Shin, A.Y.; Van Wijnen, A.J. Targeted stimulation of MSCs in peripheral nerve repair. Gene 2019, 710, 17–23. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I. Adult Mesenchymal Stem Cells: When, Where, and How. Stem Cells Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I.; Hariri, R.J. Body Management: Mesenchymal Stem Cells Control the Internal Regenerator. Stem Cells Transl. Med. 2015, 4, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Linee guida per il reporting di revisioni sistematiche e meta-analisi: Il PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Vahabzadeh, B.; Amini, K. Sciatic nerve regeneration induced by transplantation of in vitro bone marrow stromal cells into an inside-out artery graft in rat. J. Cranio-Maxillofac. Surg. 2014, 42, 1389–1396. [Google Scholar] [CrossRef]
- Matsuse, D.; Kitada, M.; Kohama, M.; Nishikawa, K.; Makinoshima, H.; Wakao, S.; Fujiyoshi, Y.; Heike, T.; Nakahata, T.; Akutsu, H.; et al. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Differentiate into Functional Schwann Cells That Sustain Peripheral Nerve Regeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 973–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Nguyen, P.; Xu, Q.; Park, W.; Lee, S.; Furuhashi, A.; Le, A.D. Neural Progenitor-Like Cells Induced from Human Gingiva-Derived Mesenchymal Stem Cells Regulate Myelination of Schwann Cells in Rat Sciatic Nerve Regeneration. Stem Cells Transl. Med. 2016, 6, 458–470. [Google Scholar] [CrossRef]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef] [Green Version]
- Resch, A.; Wolf, S.; Mann, A.; Weiss, T.; Stetco, A.-L.; Radtke, C. Co-Culturing Human Adipose Derived Stem Cells and Schwann Cells on Spider Silk—A New Approach as Prerequisite for Enhanced Nerve Regeneration. Int. J. Mol. Sci. 2019, 20, 71. [Google Scholar] [CrossRef] [Green Version]
- Santiago, L.Y.; Clavijo-Alvarez, J.; Brayfield, C.; Rubin, J.P.; Marra, K.G. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009, 18, 145–158. [Google Scholar] [CrossRef]
- Monje, P.V.; Usach, V.; Soto, P.A.; Monje, P.V.; Setton-Avruj, P.C. EGFP transgene: A useful tool to track transplanted bone marrow mononuclear cell contribution to peripheral remyelination. Transgenic Res. 2018, 27, 135–153. [Google Scholar] [CrossRef]
- Pan, H.-C.; Yang, D.-Y.; Chiu, Y.-T.; Lai, S.-Z.; Wang, Y.-C.; Chang, M.-H.; Cheng, F.-C. Enhanced regeneration in injured sciatic nerve by human amniotic mesenchymal stem cell. J. Clin. Neurosci. 2006, 13, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, G.; Pisciotta, A.; Riccio, M.; Bertoni, L.; De Biasi, S.; Gibellini, L.; Zordani, A.; Cavallini, G.M.; La Sala, G.B.; Bruzzesi, G.; et al. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e774–e785. [Google Scholar] [CrossRef] [PubMed]
- di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Eren, F.; Oksuz, S.; Küçükodaci, Z.; Kendırlı, M.T.; Cesur, C.; Alarçın, E.; ırem Bektaş, E.; Karagöz, H.; Kerımoğlu, O.; Köse, G.T.; et al. Targeted mesenchymal stem cell and vascular endothelial growth factor strategies for repair of nerve defects with nerve tissue implanted autogenous vein graft conduits. Microsurgery 2016, 36, 578–585. [Google Scholar] [CrossRef]
- Pan, H.-C.; Cheng, F.-C.; Chen, C.-J.; Lai, S.-Z.; Lee, C.-W.; Yang, D.-Y.; Chang, M.-H.; Ho, S.-P. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J. Clin. Neurosci. 2007, 14, 1089–1098. [Google Scholar] [CrossRef]
- Armaiz Flores, A.; Wang, H. The Use and Delivery of Stem Cells in Nerve Regeneration: Preclinical Evidence and Regulatory Considerations. Ann. Plast. Surg. 2018, 80, 448–456. [Google Scholar] [CrossRef]
- Tong, X.; Liu, G.; Cheng, Y.; Guo, S.; Feng, Y.; Li, Q.; Jia, H.; Wang, Y. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int. J. Mol. Med. 2011, 28, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Kemp, S.W.P.; Walsh, S.K.; Midha, R. Growth factor and stem cell enhanced conduits in peripheral nerve regeneration and repair. Neurol. Res. 2008, 30, 1030–1038. [Google Scholar] [CrossRef]
- Widgerow, A.D.; Salibian, A.A.; Lalezari, S.; Evans, G.R. Neuromodulatory nerve regeneration: Adipose tissue-derived stem cells and neurotrophic mediation in peripheral nerve regeneration. J. Neurosci. Res. 2013, 91, 1517–1524. [Google Scholar] [CrossRef]
- Dezawa, M.; Takahashi, I.; Esaki, M.; Takano, M.; Sawada, H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 2001, 14, 1771–1776. [Google Scholar] [CrossRef]
- Jones, S.; Eisenberg, H.M.; Jia, X. Advances and Future Applications of Augmented Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2016, 17, 1494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Guo, Y.-C.; Wang, D.-R.; Liu, J.-Y.; Pan, J. Adipose Stem Cell-Based Clinical Strategy for Neural Regeneration: A Review of Current Opinion. Stem Cells Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Dadon-Nachum, M.; Sadan, O.; Srugo, I.; Melamed, E.; Offen, D. Differentiated Mesenchymal Stem Cells for Sciatic Nerve Injury. Stem Cell Rev. Rep. 2011, 7, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zheng, C.; Zhang, F.; Lin, B.; Cao, M.; Tian, X.; Zhang, J.; Zhang, X.; Shen, J. Magnetic resonance imaging of enhanced nerve repair with mesenchymal stem cells combined with microenvironment immunomodulation in neurotmesis. Muscle Nerve 2020, 61, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, N.; Zhang, N.; Xiong, Y.; Wang, Z.; Liang, S.; Zhao, D.; Huang, F.; Zhang, C. Repair of Peripheral Nerve Sensory Impairments via the Transplantation of Bone Marrow Neural Tissue-Committed Stem Cell-Derived Sensory Neurons. Cell. Mol. Neurobiol. 2019, 39, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Sebben, A.D.; Lichtenfels, M.; Da Silva, J.L.B. Peripheral nerve regeneration: Cell therapy and neurotrophic factors. Rev. Bras. Ortop. Engl. Ed. 2011, 46, 643–649. [Google Scholar] [CrossRef]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W. Stem-cell–based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020, 61, 449–459. [Google Scholar] [CrossRef]
- Zhu, M.; Heydarkhan-Hagvall, S.; Hedrick, M.; Benhaim, P.; Zuk, P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J. Vis. Exp. 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Drela, K.; Lech, W.; Figiel-Dabrowska, A.; Zychowicz, M.; Mikula, M.; Sarnowska, A.; Domanska-Janik, K. Enhanced neuro-therapeutic potential of Wharton’s Jelly–derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy 2016, 18, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.M.; El-Shal, A.S.; Ahmed, F.E.; Shaban, S.F.; Wahdan, R.A.; Kandel, W.A.; Senger, M.S. Combined Wharton’s jelly derived mesenchymal stem cells and nerve guidance conduit: A potential promising therapy for peripheral nerve injuries. Int. J. Biochem. Cell Biol. 2017, 86, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Tsutsui, T. Human dental mesenchymal stem cells and neural regeneration. Hum. Cell 2013, 26, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; He, Y.; Wang, X.; Key, B.; Lee, B.H.; Li, H.; Ye, Q. Potential Roles of Dental Pulp Stem Cells in Neural Regeneration and Repair. Stem Cells Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Editor, D. A new, Custom-Made Device for Flap Protection in. Microsurgery 2009, 504–506. [Google Scholar] [CrossRef]
- Grimoldi, N.; Colleoni, F.; Tiberio, F.; Vetrano, I.G.; Cappellari, A.; Costa, A.; Belicchi, M.; Razini, P.; Giordano, R.; Spagnoli, D.; et al. Stem Cell Salvage of Injured Peripheral Nerve. Cell Transplant. 2015, 24, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.S.; Ahn, S.Y.; Sung, S.I.; Ahn, J.-Y.; Chang, Y.S. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr. Res. 2017, 83, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, G.; Pisciotta, A.; Bertoni, L.; Vallarola, A.; Bertani, G.; Mecugni, D. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen. Res. 2020, 15, 373–381. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Huang, H.; Zhu, Y.; Qin, T. Design of a Smart Nerve Conduit Based on a Shape-Memory Polymer. Adv. Mater. Technol. 2016, 1. [Google Scholar] [CrossRef]
- Siemionow, M.; Bozkurt, M.; Zor, F. Regeneration and repair of peripheral nerves with different biomaterials: Review. Microsurgery 2010, 30, 574–588. [Google Scholar] [CrossRef]
- Duan, X.; Cheng, L.-N.; Zhang, F.; Liu, J.; Guo, R.-M.; Zhong, X.; Wen, X.-H.; Shen, J. In vivo MRI monitoring nerve regeneration of acute peripheral nerve traction injury following mesenchymal stem cell transplantation. Eur. J. Radiol. 2012, 81, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Brohlin, M.; Mahay, D.; Novikov, L.N.; Terenghi, G.; Wiberg, M.; Shawcross, S.G.; Novikova, L.N. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci. Res. 2009, 64, 41–49. [Google Scholar] [CrossRef]
- Boido, M.; Garbossa, D.; Fontanella, M.; Ducati, A.; Vercelli, A. Mesenchymal Stem Cell Transplantation Reduces Glial Cyst and Improves Functional Outcome After Spinal Cord Compression. World Neurosurg. 2014, 81, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, B.M.; Lavorato, A.; Titolo, P.; Colonna, M.R.; Vincitorio, F.; Colzani, G.; Garbossa, D.; Battiston, B. Principles of Cortical Plasticity in Peripheral Nerve Surgery. Surg. Technol. Int. 2020, 36, 444–452. [Google Scholar] [PubMed]
- Kehoe, S.; Zhang, X.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Kanaya, F.; Firrell, J.C.; Breidenbach, W.C. Sciatic Function Index, Nerve Conduction Tests, Muscle Contraction, and Axon Morphometry as Indicators of Regeneration. Plast. Reconstr. Surg. 1996, 98, 1264–1271. [Google Scholar] [CrossRef]
- Dellon, A.L.; MacKinnon, S.E. Sciatic nerve regeneration in the rat. Validity of walking track assessment in the presence of chronic contractures. Microsurgery 1989, 10, 220–225. [Google Scholar] [CrossRef]
- Shen, N.; Zhu, J. Application of sciatic functional index in nerve functional assessment. Microsurgery 1995, 16, 552–555. [Google Scholar] [CrossRef]
- Hobson, M.I. Increased vascularisation enhances axonal regeneration within an acellular nerve conduit. Ann. R. Coll. Surg. Engl. 2002, 84, 47–53. [Google Scholar]
- Li, Y.; Guo, L.; Ahn, H.S.; Kim, M.H.; Kim, S. Amniotic mesenchymal stem cells display neurovascular tropism and aid in the recovery of injured peripheral nerves. J. Cell. Mol. Med. 2014, 18, 1028–1034. [Google Scholar] [CrossRef]
| ADSC | BMSC | FetalSC | DPSC | MSC (More Than One Source) |
|---|---|---|---|---|
| Tremp et al., 2015 [1] | Mohammadi et al., 2014 [15] | Matsuse et al., 2010 [16] | Zhang et al., 2016 [17] | Cofano et al., 2019 [18] |
| Resch et al., 2019 [19] Santiago et al., 2009 [20] | Chen, et al., 2016 [21] | Pan et al., 2006 [22] | Carnevale et al., 2016 [23] | Yousefi et al., 2019 [3] |
| Di Summa et al., 2010 [24] | Eren et al., 2015 [25] | Pan et al., 2007 [26] | Flores et al., 2017 [27] | |
| Liu et al., 2011 [28] | Sullivan et al., 2016 [9] | Moattari et al., 2018 [4] | Kemp et al., 2008 [29] | |
| Widgerow et al., 2013 [30] | Dezawa et al., 2001 [31] | De Albornoz et al., 2011 [5] | Jones et al., 2016 [32] | |
| Wang et al., 2019 [33] | Dadon-Nachum et al., 2011 [34] | Yang et al., 2020 [35] | ||
| Yu et al., 2019 [36] | Jiang et al., 2017 [7] | |||
| Matthes et al., 2013 [2] | Mathot et al., 2019 [10] | |||
| Sebben et al., 201 [37] | ||||
| Uz et al., 2018 [8] | ||||
| Kubiak et al., 2019 [38] |
| Availability [24,28,30,32,37,38,39,40] | Invasive Procedure of Collection [24,28,30,32,37,38,39,40] | Paracrine Growth Factors [7,30,32,33,38,40,41,42] | Immunogenicity [16,28,33,38,41,43] | Use in Pre-Clinical Studies [1,2,4,15,17,19,20,22,23,30,31,35,44] | Axonal Growth [1,15,20,22,31] | Survival [7,20,27,28,32,38] | |
|---|---|---|---|---|---|---|---|
| BMSC | ++ | +++ | +++ | / | ++ | +++ | / |
| ADSC | +++ | + (minimally invasive) | +++ | --- | +++ | +++ | ++ |
| UMDSC | ++ | Not invasive | + | Immunologically inert | + | / | / |
| AMSC | ++ | Not invasive | + | -- | + | ++ | + |
| DPSC | + | + | ++ | -- | + | / | + |
| SKSC | + | + | / | / | / | / | / |
| Cell Type | Differentiated Cell Type/Differentiation Factor | Animal Nerve Model | Type of Procedure | Nerve Gap | Postoperative Time | Analysis | Results | Reference |
|---|---|---|---|---|---|---|---|---|
| BMSC | Astrocyte-like | Rat sciatic nerve | Intramuscular injection | 3 weeks | Motor function rotarod test, lateral reflex measurements, electrophysiological study, immunohistochemistry | Increased motor performance, full reflex response, CMAP, and conduction latency were restored in the treated group. | Dadon-Nachum, 2011 [34] | |
| BMSC | Schwann cell-like | Rat sciatic nerve | Group 1: artificial grafts (Matrigel) + cells Group 2: artificial graft (Matrigel) | 3 weeks | Immunohistochemistry | In the differentiated MSC graft, the distance of regrowth was 2.2 mm at 1 week and reached up to 8 ± 10 mm at 3 weeks, whereas in the undifferentiated MSC graft, only a growth of 2.5 mm at 3 weeks was achieved. | Dezawa, 2001 [31] | |
| BMSCs | Rats Rightperoneal nerve | Group 1: nerve excised—saline filled vein graft Group 2: nerve excised, reversed and used as an autogenous nerve graft. Groups 3–6: nerve discarded | 15/16 mm | 8 weeks | Gait analysis, PFI, axon counts, EMG | For PFI and EMG, no statistical differences between group 2 and 5 were found. For axon counts, no statistical differences between 2 and 5 and between 5 and 6 were found. | Eren et al., 2015 [25] | |
| BMSC | Rat | Sham-operated group (SHAM), sciatic nerve transection group (SNTG), Artery graft (IOAG) | 10 mm | 4–8; 8–12 weeks | Sciatic functional index (SFI), Static sciatic index (SSI), Electrophysiological measurement | Nerve conduction velocity in BMSC-treated animals was significantly higher than that in the IOAG group. | Mohammadi, 2014 [15] | |
| BMSC | Rat | injected femoral vein | 3 weeks | Determination of the walking track with analysis of the sciatic functional index | The locomotor improvement was observed in 14 days. The functional improvement in the MSC group was significant in 7 days, but the rate of change in improvement from 14 to 21 days. | Matthes, 2013 [2] | ||
| ADSC | Rat Sciatic nerve | Group 1: ANA injected with ADSC Group 2: ANA injected with DMEM medium | 10 mm | 12 weeks | SFI, electrophysiological study, muscle weight measurement (anterior tibial muscle), histological examination, tissue preparation, immunofluorescence staining | The SFI of the ADSC group was significantly improved compared to the DMEM group, but there was no obvious difference in comparison with the autograft group Histological examination showed regeneration of the nerve tissue in the ADSC group. | Liu, 2011[28] | |
| ADSC | Schwann cells | Rat sciatic nerve | Group 1: fibrin conduit + ADSC Group 2: fibrin conduit + MSC Group 3: fibrin conduit + Schwann cells | 10 mm | 2 week | Quantification of regeneration length | In the short term, the fibrin conduit can optimize peripheral nerve regeneration. ADSCs promote regeneration in the same manner as MSCs. | Di Summa, 2010 [24] |
| ADSC | Schwann cells | Rat | Group 1: nerve conduit + APCs Group2: conduit Group3: autograft Group4: empty | 6 mm | 3 weeks | SFI, immunohistochemistry, gastrocnemius muscle weight ratio, histological analysis | The best SFI improvements were observed 3 weeks after surgery in group 1. No difference was observed among groups after 12 weeks. | Santiago, 2009 [20] |
| ADSC, Schwann cells | - | Co-culture of human Schwann cells and ADSCs on spider silk scaffold | - | 3 weeks | Microscope analysis, immunochemistry | Early cell was attached to the spider silk fibers (within 24 h). ADSCs and Schwann cells migrated and proliferated equally along the silk fibers. Spider silk fibers in a long-distance peripheral nerve gap enhance Schwann cell migration. | Resch, 2019 [19] | |
| human-ADSC, rat-ADSC, Schwann cells | FSK, FGF, GGF, PDGF | Rat sciatic nerve | fibrin conduit filled with cells Group 1: control Group 2: r-ADSCs Group 3: h-ADSCs (deep layer) Group 4: h-ADSCs (superficial layer) Group 5: r-Schwann cells-like cells Group 6: h-Stromal Vascular Fraction (SVF) Group 7: r-Schwann cells | 10 mm | 2–4 weeks | MRI, immunocytochemistry | A longer regeneration distance in G7 and inferior results were seen in G4 and G6. A strong correlation between the length of the regenerating axon front measured by MRI and the one measured by immunocytochemistry was observed. | Tremp, 2015 [1] |
| Wharton jelly stem cells | Rat sciatic nerve | Group 1: no intervention Group 2: membrane + cells Group 3: NGF Group 4: NGF + cells Group 5: NGF + membrane Group 6: NGF + membrane + cells | 0 mm transected | 8 weeks | SFI, hot water paw immersion test, electrophysiological evaluation, histological analysis | The reaction time in the hot-water paw immersion test significantly decreased in the therapeutic groups. Most increased the amplitude in electrophysiological studies in group 6. The mean number of nerve fibers increased significantly in group 2 and group 6. | Moattari, 2018 [4] | |
| amniotic MSC | FBS, FGF | Rat sciatic nerve | Group 1: 4-0 silk filled with fibrin glue and Surgicel Group 2: 4-0 silk filled with fibrin glue, Surgicel and MSCs | 5 mm | 8 weeks | Max diameter axons, nerve continuity, disorientation of fibres, fibrotic tissue invasion, ankle kinematics, SFI | Better results were observed in the MSC group. | Pan, 2006 [22] |
| SDSC | - | human (1 case report) radial and median nerve | Right (gap 5 cm): sural nerve graft Left (gap 8–10 cm): neuragen filled with SDSCs + interposed sural nerve graft | 50/100 mm | 3 years | MRI, EMG, clinical | SDSCs were able to differentiate into the GFAP astroglial cell type, glia cells, and Schwann cells. Left biceps and triceps: M2. Better sensor and motor conduction in the right side was observed. | Grimoldi, 2015 [45] |
| Human gingival MSC (GMSC) | EGF, bFGF, BNF | Rat sciatic nerve | Group 1: GMSC seeded on GelFoam. Group 2: GMSC—derived neural progenitor cells (iNPCs) seeded on GelFoam Group 3: GelFoam alone as the control group | 0 mm (crushed) | 4 weeks | Histological, immunohistochemical, gastrocnemius muscle weight | GMSCs can be directly induced to multipotent and expandable NPC-like cells. GMSCs and iNPCs could differentiate into both neuronal and Schwann cells. iNPCs possess enhanced therapeutic potential to facilitate regeneration of injured peripheral nerves. GMSCs and iNPCs might delay the demyelination process after injury and might promote remyelination. | Zhang, 2016 [17] |
| MSC | Rat sciatic nerve | Group A-D: MSC + LPS + FK506 Group B-E: MSC Group C-F: PBS control group | 0 mm transected | 2–4–8 weeks | Groups A–C: MRI, SFI Groups D–F: histological analysis | Group A: more rapid recovery of fibers at MRI and higher SFIs than other groups were observed. Group D: the best axonal regeneration and faster continuity of nerve fibers in 8 weeks were observed. | Yang, 2020 [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavorato, A.; Raimondo, S.; Boido, M.; Muratori, L.; Durante, G.; Cofano, F.; Vincitorio, F.; Petrone, S.; Titolo, P.; Tartara, F.; et al. Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. Int. J. Mol. Sci. 2021, 22, 572. https://doi.org/10.3390/ijms22020572
Lavorato A, Raimondo S, Boido M, Muratori L, Durante G, Cofano F, Vincitorio F, Petrone S, Titolo P, Tartara F, et al. Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. International Journal of Molecular Sciences. 2021; 22(2):572. https://doi.org/10.3390/ijms22020572
Chicago/Turabian StyleLavorato, Andrea, Stefania Raimondo, Marina Boido, Luisa Muratori, Giorgia Durante, Fabio Cofano, Francesca Vincitorio, Salvatore Petrone, Paolo Titolo, Fulvio Tartara, and et al. 2021. "Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review" International Journal of Molecular Sciences 22, no. 2: 572. https://doi.org/10.3390/ijms22020572
APA StyleLavorato, A., Raimondo, S., Boido, M., Muratori, L., Durante, G., Cofano, F., Vincitorio, F., Petrone, S., Titolo, P., Tartara, F., Vercelli, A., & Garbossa, D. (2021). Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. International Journal of Molecular Sciences, 22(2), 572. https://doi.org/10.3390/ijms22020572









