Artesunate Switches Monocytes to an Inflammatory Phenotype with the Ability to Kill Leukemic Cells
Abstract
1. Introduction
2. Results
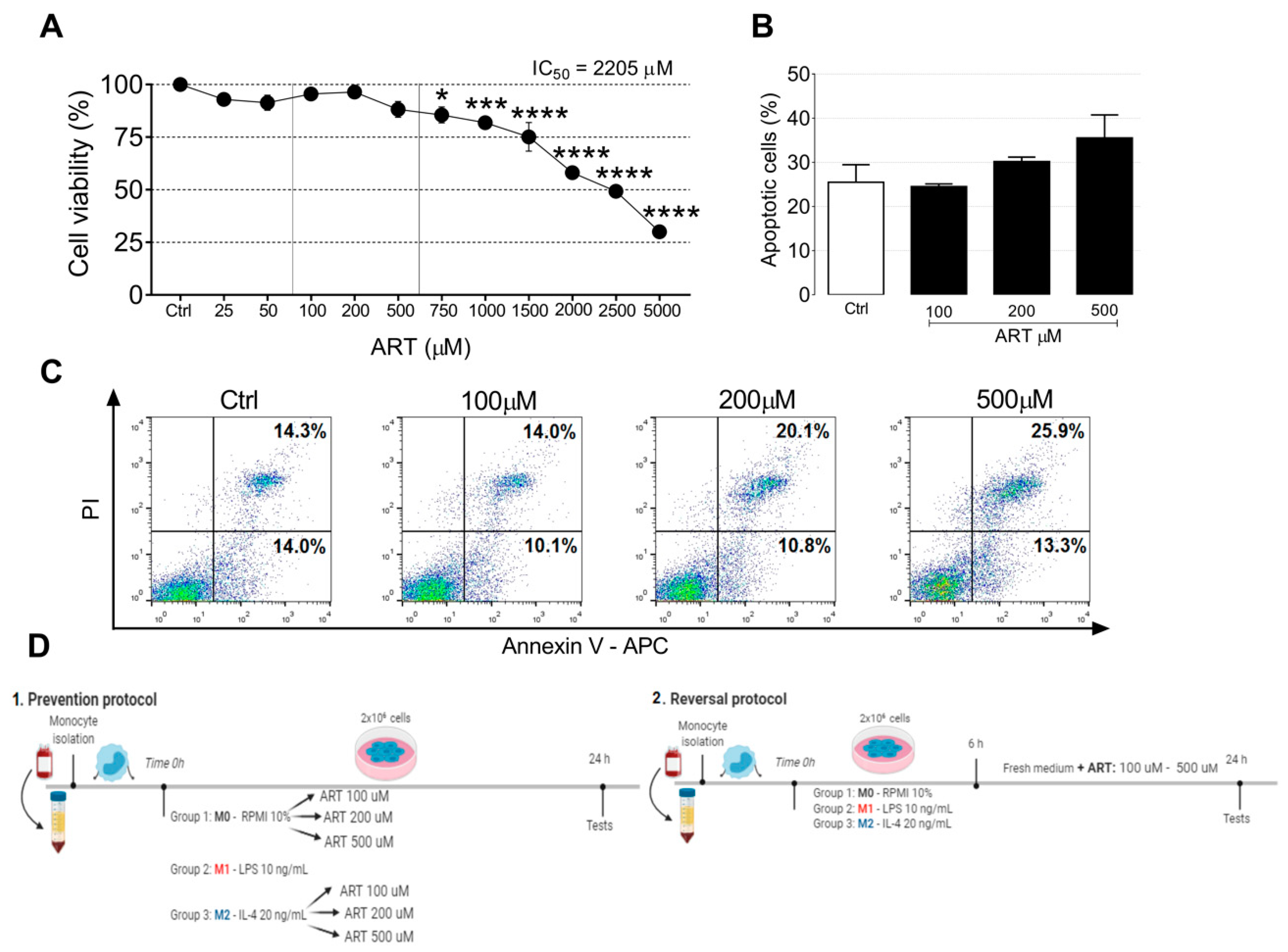
2.1. ART Is Safe for Human Primary Monocytes
2.2. ART Induces Monocytes Phenotypic Changes to a Pro-Inflammatory Phenotype
2.3. ART Induces Pro-Inflammatory Cytokine Release from Monocytes
2.4. ART Downregulates JAK2/STAT3 Pathway
2.5. ART Induces a Tumoricidal Phenotype
3. Discussion
4. Materials and Methods
4.1. Monocytes
4.2. Monocytes Treatment
4.3. Cell Death and Viability Assays
4.3.1. MTT Assay
4.3.2. Apoptosis Assay
4.4. Activation Phenotype Markers
4.4.1. Flow Cytometry
Membrane Markers
Nitric Oxide Production
4.4.2. ELISA
4.5. Western Blot
4.6. Apoptotic Activity of ART
4.6.1. Tumor Cell Lines and Co Culture System
4.6.2. Apoptosis Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Artesunate |
| ATCC | American Type Culture Collection |
| BM | Bone marrow |
| CCL18 | C-C motif chemokine 18 |
| CD80 | Cluster of differentiation 80 (also known as B7) |
| CD86 | Cluster of Differentiation 86 |
| CD206 | Cluster of Differentiation 206 (also known as the mannose receptor) |
| FBS | Fetal bovine serum |
| IL-1β | Interleukin 1 beta |
| IL-4 | Interleukin 4 |
| IL-8 | Interleukin 8 |
| MCP-1 | Monocyte Chemoattractant Protein-1 (also known as CCL2) |
| M1 | Classically activated monocytes |
| M2 | Alternatively activated monocytes |
| NO | Nitric oxide |
| PBMC | Peripheral blood mononuclear cells |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor alpha |
References
- Han, Y.; Liu, Z.; Liu, J.; Yan, W.; Xia, Y.; Yue, S.; Yu, J. Antibody-Based Immunotherapeutic Strategies for the Treatment of Hematological Malignancies. BioMed Res. Int. 2020, 2020, 4956946. [Google Scholar] [CrossRef] [PubMed]
- Lamble, A.J.; Kosaka, Y.; Laderas, T.; Maffit, A.; Kaempf, A.; Brady, L.K.; Wang, W.; Long, N.; Saultz, J.N.; Mori, M.; et al. Reversible suppression of T cell function in the bone marrow microenvironment of acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2020, 117, 14331–14341. [Google Scholar] [CrossRef] [PubMed]
- Forte, D.; Krause, D.S.; Andreeff, M.; Bonnet, D.; Méndez-Ferrer, S. Updates on the hematologic tumor microenvironment and its therapeutic targeting. Haematologica 2019, 104, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Hawkins, E.D.; Lo Celso, C. The interplay of leukemia cells and the bone marrow microenvironment. Blood 2018, 131, 1507–1511. [Google Scholar] [CrossRef]
- Gouveia-Fernandes, S. Monocytes and Macrophages in Cancer: Unsuspected Roles. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1219, pp. 161–185. [Google Scholar]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Yang, X.; Feng, W.; Wang, R.; Yang, F.; Wang, L.; Chen, S.; Ru, Y.; Cheng, T.; Zheng, G. Repolarizing heterogeneous leukemia-associated macrophages with more M1 characteristics eliminates their pro-leukemic effects. Oncoimmunology 2018, 7, e1412910. [Google Scholar] [CrossRef]
- Zyad, A.; Tilaoui, M.; Jaafari, A.; Oukerrou, M.A.; Mouse, H.A. More insights into the pharmacological effects of artemisinin. Phyther. Res. 2018, 32, 216–229. [Google Scholar] [CrossRef]
- Kim, S.K.; Choe, J.Y.; Park, K.Y. Anti-inflammatory effect of artemisinin on uric acid-induced NLRP3 inflammasome activation through blocking interaction between NLRP3 and NEK7. Biochem. Biophys. Res. Commun. 2019, 517, 338–345. [Google Scholar] [CrossRef]
- Wang, K.S.; Li, J.; Wang, Z.; Mi, C.; Ma, J.; Piao, L.X.; Xu, G.H.; Li, X.; Jin, X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol. Immunotoxicol. 2017, 39, 28–36. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Li, Y.; Deng, Z.; Zhou, L.; Long, J.; Tang, Y.; Zuo, Z.; Zhang, Y.; Xie, H. Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. BioMed Pharmacother. 2019, 117, 109181. [Google Scholar] [CrossRef]
- Verma, A.; Ghosh, S.; Salotra, P.; Singh, R. Artemisinin-resistant Leishmania parasite modulates host cell defense mechanism and exhibits altered expression of unfolded protein response genes. Parasitol. Res. 2019, 118, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- De Sarkar, S.; Sarkar, D.; Sarkar, A.; Dighal, A.; Chakrabarti, S.; Staniek, K.; Gille, L.; Chatterjee, M. The leishmanicidal activity of artemisinin is mediated by cleavage of the endoperoxide bridge and mitochondrial dysfunction. Parasitology 2019, 146, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Li, W.; Winters, A.; Liu, R.; Yang, S.H. Artemisinin prevents glutamate-induced neuronal cell death via Akt pathway activation. Front. Cell. Neurosci. 2018, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xu, J.; Zheng, W. Artemisinin protects PC12 cells against β-amyloid-induced apoptosis through activation of the ERK1/2 signaling pathway. Redox Biol. 2017, 12, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Houh, Y.K.; Kim, K.E.; Park, S.; Hur, D.Y.; Kim, S.; Kim, D.; Bang, S.I.; Yang, Y.; Park, H.J.; Cho, D. The effects of artemisinin on the cytolytic activity of natural killer (NK) cells. Int. J. Mol. Sci. 2017, 18, 1600. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.I.; Foglio, M.A.; Olalla Saad, S.T. Artemisinin-type drugs for the treatment of hematological malignancies. Cancer Chemother. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Lam, N.S.; Long, X.; Wong, J.W.; Griffin, R.C.; Doery, J.C.G. Artemisinin and its derivatives: A potential treatment for leukemia. Anticancer Drugs 2019, 30, 1–18. [Google Scholar] [CrossRef]
- Wong, Y.K.; Xu, C.; Kalesh, K.A.; He, Y.; Lin, Q.; Wong, W.S.F.; Shen, H.M.; Wang, J. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med. Res. Rev. 2017, 37, 1492–1517. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Y.J.; Wang, Y.J.; Cai, W.Y.; Weng, X.G.; Li, Q.; Chen, Y.; Yang, Q.; Zhu, X.X. Research progress of effect of artemisinin family drugs on T lymphocytes immunomodulation. Zhongguo Zhongyao Zazhi 2019, 44, 4992–4999. [Google Scholar]
- Cao, Y.; Feng, Y.H.; Gao, L.W.; Li, X.Y.; Jin, Q.X.; Wang, Y.Y.; Xu, Y.Y.; Jin, F.; Lu, S.L.; Wei, M.J. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int. Immunopharmacol. 2019, 70, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Bi, J.; Wan, X. Artemisinin sensitizes tumor cells to NK cell-mediated cytolysis. Biochem. Biophys. Res. Commun. 2020, 524, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadegan, M.; Rajayi, H.; Mofazzal Jahromi, M.A.; Yeganeh, H.; Yousefi, M.; Muhammad Hassan, Z.; Majidi, J. Application of arteether-loaded polyurethane nanomicelles to induce immune response in breast cancer model. Artif. Cells Nanomed. Biotechnol. 2017, 45, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Liu, Z.N.; Ye, J.; Sha, M.; Qian, H.; Bu, X.H.; Luan, Z.Y.; Xu, X.L.; Huang, A.H.; Yuan, D.L.; et al. Artesunate exerts an anti-immunosuppressive effect on cervical cancer by inhibiting PGE2 production and Foxp3 expression. Cell Biol. Int. 2014, 38, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Ożańska, A.; Szymczak, D.; Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol. 2020, 92, e12883. [Google Scholar] [CrossRef]
- Cho, H.; Seo, Y.; Loke, K.M.; Kim, S.W.; Oh, S.M.; Kim, J.H.; Soh, J.; Kim, H.S.; Lee, H.; Kim, J.; et al. Cancer-stimulated CAFs enhance monocyte differentiation and protumoral TAM activation via IL6 and GM-CSF secretion. Clin. Cancer Res. 2018, 24, 5407–5421. [Google Scholar] [CrossRef]
- Mengos, A.E.; Gastineau, D.A.; Gustafson, M.P. The CD14+HLA-DrlO/NEG monocyte: An immunosuppressive phenotype that restrains responses to cancer immunotherapy. Front. Immunol. 2019, 10, 1147. [Google Scholar] [CrossRef]
- Leonard, E.J.; Yoshimura, T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol. Today 1990, 11, 97–101. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 beta—A friend or foe in malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, A.J.; Ye, S.K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019, 52, 415–423. [Google Scholar] [CrossRef]
- Kitamura, H.; Ohno, Y.; Toyoshima, Y.; Ohtake, J.; Homma, S.; Kawamura, H.; Takahashi, N.; Taketomi, A. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017, 108, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Talukder, A.; Savage, N.M.; Singh, N.; Liu, K. JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology 2017, 6, e1291106. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Behme, D.; Pantke, M.; Reiling, N.; Binder, C.; Pukrop, T.; Klemm, F. Isolation of human monocytes by double gradient centrifugation and their differentiation to macrophages in Teflon-coated cell culture bags. J. Vis. Exp. 2014, 91, 51554. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, R.I.; Olalla Saad, S.T.; Azambuja, J.H. Artesunate Switches Monocytes to an Inflammatory Phenotype with the Ability to Kill Leukemic Cells. Int. J. Mol. Sci. 2021, 22, 608. https://doi.org/10.3390/ijms22020608
Mancuso RI, Olalla Saad ST, Azambuja JH. Artesunate Switches Monocytes to an Inflammatory Phenotype with the Ability to Kill Leukemic Cells. International Journal of Molecular Sciences. 2021; 22(2):608. https://doi.org/10.3390/ijms22020608
Chicago/Turabian StyleMancuso, Rubia Isler, Sara Teresinha Olalla Saad, and Juliana Hofstätter Azambuja. 2021. "Artesunate Switches Monocytes to an Inflammatory Phenotype with the Ability to Kill Leukemic Cells" International Journal of Molecular Sciences 22, no. 2: 608. https://doi.org/10.3390/ijms22020608
APA StyleMancuso, R. I., Olalla Saad, S. T., & Azambuja, J. H. (2021). Artesunate Switches Monocytes to an Inflammatory Phenotype with the Ability to Kill Leukemic Cells. International Journal of Molecular Sciences, 22(2), 608. https://doi.org/10.3390/ijms22020608





