Human Embryo Models and Drug Discovery
Abstract
1. Animal Models and Immortalized 2D Human Cell Culture Models
2. Human Pluripotent Stem Cells
3. Human Organoids
4. Human Embryoids
4.1. Gastrulation Micropatterned Colonies
4.2. Asymmetric Early Post-Implantation Epiblasts
4.3. Post-Implantation Amniotic Sac Embryoids
4.4. Gastruloids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donowitz, M.; Turner, J.R.; Verkam, A.S.; Zachos, N.C. Current and potential future applications of human stem cell models in drug development. J. Clin. Investig. 2020, 130, 3342–3344. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Frum, T.; Spence, J.R. hPSC-derived organoids: Models of human development and disease. J. Mol. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sances, S.; Workman, M.J.; Svendsen, C.N. Multi-lineage human iPSC-derived platforms for disease modeling and drug discovery. Cell Stem Cell 2020, 26, 309–329. [Google Scholar] [CrossRef]
- Lui, J.H.; Hansen, D.V.; Kriegstein, A.R. Development and evolution of the human neocortex. Cell 2011, 146, 18–36. [Google Scholar] [CrossRef]
- Kuzawa, C.W.; Chugani, H.T.; Grossman, L.I.; Lipovich, L.; Muzik, O.; Hof, P.R.; Wildman, D.E.; Sherwood, C.C.; Leonard, W.R.; Lange, N. Metabolic costs and evolutionary implications of human brain development. Proc. Natl. Acad. Sci. USA 2014, 111, 13010–13015. [Google Scholar] [CrossRef]
- Salick, M.R.; Lubeck, E.; Riesselman, A.; Kaykas, A. The future of cerebral organoids in drug discovery. Sem. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.I.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef]
- Shahbazi, M.N.; Zernicka-Goetz, M. Deconstructing the mouse and human early embryo. Nat. Cell Biol. 2018, 20, 878–887. [Google Scholar] [CrossRef]
- Baillie-Benson, P.; Moris, N.; Arias, A.M. Pluripotent stem cell models of early mammalian development. Curr. Opin. Cell Biol. 2020, 66, 89–96. [Google Scholar] [CrossRef]
- Fu, J.; Warmflash, A.; Lutolf, M.P. Stem-cell-based embryo models for fundamental research and translation. Nat. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Seruga, B.; Ocana, A.; Amir, E.; Tannock, I.F. Failures in phase III: Causes and consequences. Clin. Cancer Res. 2015, 21, 4552–4560. [Google Scholar] [CrossRef] [PubMed]
- Fabre, K.; Berridge, B.; Proctor, W.R.; Ralston, S.; Will, Y.; Baran, S.W.; Yoder, G.; Van Vleet, T.R. Introduction to a manuscript series on the characterization and use of microphysiological systems (MPS) in pharmaceutical safety and ADME applications. Lab Chip 2020, 20, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Kasendra, M.; Luc, R.; Yin, J.; Manatakis, D.V.; Kulkarni, G.; Lucchesi, C.; Sliz, J.; Apostolou, A.; Sunuwar, L.; Obrigewitch, J.; et al. Duodenum intestine-chip for preclinical drug assessment in a human relevant model. eLife 2020, 9, e50135. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Fentem, J.; Chamberlain, M.; Sangster, B. The feasibility of replacing animal testing for assessing consumer safety: A suggested future direction. Altern. Lab. Anim. 2004, 32, 617–623. [Google Scholar] [CrossRef]
- Heuberger, R.; Petty, M.; Huntingford, J. Companion animal owner perception, knowledge, and beliefs regarding pain management in end-of-life care. Top. Companion Anim. Med. 2016, 31, 152–159. [Google Scholar] [CrossRef]
- Bailey, J.; Balls, M. Recent efforts to elucidate the scientific validity of animal-based drug tests by the pharmaceutical industry, pro-testing lobby groups, and animal welfare organisations. BMC Med. Ethics 2019, 20, 16. [Google Scholar] [CrossRef]
- Weatherbee, B.A.T.; Cui, T.; Zernicka-Goetz, M. Modeling human embryo development with embryonic and extra-embryonic stem cells. Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Gillet, J.P.; Varma, S.; Gottesman, M.M. The clinical relevance of cancer cell lines. J. Natl. Cancer Inst. 2012, 105, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lee, H.-A. Trends in the development of human stem cell-based non-animal drug testing models. Korean J. Physiol. Pharmacol. 2020, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, P.; Cai, S.; Peng, J.; Hua, G. Organoid based personalized medicine: From bench to bedside. Cell Regen. 2020, 9, 21. [Google Scholar] [PubMed]
- Morton, J.J.; Alzofon, N.; Jimeno, A. The humanized mouse: Emerging translational potential. Mol. Carcinog. 2020, 59, 830–838. [Google Scholar] [CrossRef]
- Toolan, H.W. Growth of human tumors in cortisone-treated laboratory animals: The possibility of obtaining permanently transplantable human tumors. Cancer Res. 1953, 13, 389–394. [Google Scholar]
- Brendel, C.; Rio, P.; Verhoeyen, E. Humanized mice are precious tools for evaluation of hematopoietic gene therapies and preclinical modeling to move towards a clinical trial. Biochem. Pharmacol. 2020, 174, 113711. [Google Scholar] [CrossRef]
- Byrne, A.T.; Alferez, D.G.; Amant, F.; Annibali, D.; Arribas, J.; Biankin, A.V.; Bruna, A.; Budinska, E.; Caldas, C.; Chang, D.K.; et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 2017, 17, 254–268. [Google Scholar] [CrossRef]
- Fujii, E.; Kato, A.; Suzuki, M. Patient-derived xenograft (PDX) models: Characteristics and points to consider for the process of establishment. J. Toxicol. Pathol. 2020, 33, 153–160. [Google Scholar] [CrossRef]
- Thompson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocyst. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Takahasi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Chen, K.G.; Mallon, B.S.; Park, K.; Robey, P.G.; McKay, R.D.G.; Gottesman, M.M.; Zheng, W. Pluripotent stem cell platforms for drug discovery. Trends Mol. Med. 2018, 24, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.; Schipany, K.; Hengstschläger, M. The decision on the “optimal” human pluripotent stem cell. Stem Cell Transl. Med. 2014, 3, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Pereira Daoud, A.M.; Popovic, M.; Dondorp, W.J.; Bustos, M.T.; Bredenoord, A.L.; Chuva de Sousa Lopes, S.M.; van den Brink, S.C.; Roelen, B.A.J.; de Wert, G.M.W.R.; Heindryckx, B. Modelling human embryogenesis: Embryo-like structure spark ethical and policy debate. Hum. Reprod. Update 2020, 26, 779–798. [Google Scholar] [CrossRef] [PubMed]
- De Masi, C.; Spitaleri, P.; Murdocca, M.; Novelli, G.; Sangiuolo, F. Application of CRISPR/Cas9 to human-induced pluripotent stem cells: From gene editing to drug discovery. Hum. Genom. 2020, 14, 25. [Google Scholar] [CrossRef]
- Rosner, M.; Siegel, N.; Fuchs, C.; Slabina, N.; Dolznig, H.; Hengstschläger, M. Efficient siRNA-mediated prolonged gene silencing in human amniotic fluid stem cells. Nat. Protoc. 2010, 5, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Rosner, M.; Kovacic, B.; Hengstschläger, M. Full biological characterization of human pluripotent stem cells will open the door to translational research. Arch. Toxicol. 2016, 90, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.G.; Daley, G.O. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef]
- Zahumenska, R.; Nosal, V.; Smolar, M.; Okajcekova, T.; Skovierova, H.; Strnadel, J.; Halasova, E. Induced pluripotency: A powerful tool for in vitro modeling. Int. J. Mol. Sci. 2020, 21, 8910. [Google Scholar] [CrossRef]
- Ruillier, V.; Tournois, J.; Boissart, C.; Lasbareilles, M.; Mahe, G.; Chatrousse, L.; Cailleret, M.; Peschanski, M.; Benchoua, A. Rescuing compounds for Lesch-Nyhan disease identified using stem cell-based phenotypic screening. JCI Insight 2020, 5, e132094. [Google Scholar] [CrossRef]
- Vijayaraj, P.; Minasyan, A.; Durra, A.; Karumbayaram, S.; Mehrabi, M.; Aros, C.J.; Ahadome, S.D.; Shia, D.W.; Chung, K.; Sandlin, J.M.; et al. Modeling progressive fibrosis with pluripotent stem cells identifies an anti-fibrotic small molecule. Cell Rep. 2019, 29, 3488–3505. [Google Scholar] [CrossRef]
- Young, J.E.; Fong, L.K.; Frankowski, H.; Petsko, G.A.; Small, S.A.; Goldstein, L.S.B. Stabilizing the retromer complex in a human stem cell model of alzheimer’s disease reduces TAU phosphorylation independently of amyloid precursor protein. Stem Cell Rep. 2018, 10, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Readhead, B.; Hartley, B.J.; Eastwood, B.J.; Collier, D.A.; Evans, D.; Farias, R.; He, C.; Hoffman, G.; Sklar, P.; Dudley, J.T.; et al. Expression-based drug screening of neural progenitor cells from individuals with schizophrenia. Nat. Commun. 2018, 9, 4412. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Izumi, Y.; Watanabe, A.; Tsukita, K.; Woltjen, K.; Yamamoto, T.; Hotta, A.; Kondo, T.; Kitaoka, S.; Ohta, A.; et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci. Transl. Med. 2017, 9, eaaf3962. [Google Scholar] [CrossRef] [PubMed]
- Cayo, M.A.; Mallanna, S.K.; Di Furio, F.; Jing, R.; Tolliver, L.B.; Bures, M.; Urick, A.; Noto, F.K.; Pashos, E.E.; Greseth, M.D.; et al. A drug screen using human iPSC-derived hepatocyte-like cells reveals cardiac glycosides as a potential treatment for hypercholesterolemia. Cell Stem Cell 2017, 20, 478–489. [Google Scholar] [CrossRef]
- Sala, L.; Yu, Z.; Ward-van Oosterwaard, D.; van Veldhoven, J.P.; Moretti, A.; Laugwitz, K.L.; Mummery, C.L.; Ijzerman, A.P.; Bellin, M. A new hERG allosteric modulator rescues genetic and drug-induced long-QT syndrome phenotypes in cardiomyocytes from isogenic pairs of patient induced pluripotent stem cells. EMBO Mol. Med. 2016, 8, 1065–1081. [Google Scholar] [CrossRef]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef]
- Kovacic, B.; Rosner, M.; Schlangen, K.; Kramer, N.; Hengstschläger, M. DRUGPATH—A novel bioinformatic approach identifies DNA-damage pathway as a regulator of size maintenance in human ESCs and iPSCs. Sci. Rep. 2019, 9, 1897. [Google Scholar] [CrossRef]
- Rosner, M.; Hengstschläger, M. Human pluripotent stem cells release oncogenic soluble E-cadherin. Stem Cells 2016, 34, 2443–2446. [Google Scholar] [CrossRef]
- Rosner, M.; Pham, T.T.; Moriggl, R.; Hengstschläger, M. Human stem cells alter the invasive properties of somatic cells via paracrine activation of mTORC1. Nat. Commun. 2017, 8, 595. [Google Scholar] [CrossRef]
- Unger, C.; Kramer, N.; Walzl, A.; Scherzer, M.; Hengstschläger, M.; Dolznig, H. Modelling human carcinomas: Physiologically relevant 3D models to improve anti-cancer drug development. Adv. Drug Deliv. Rev. 2014, 79, 50–67. [Google Scholar] [CrossRef]
- Unterleuthner, D.; Kramer, N.; Pudelko, K.; Burian, A.; Hengstschläger, M.; Dolznig, H. An optimized 3D coculture assay for preclinical testing of pro-and antiangiogenic drugs. SLAS Discov. 2017, 22, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000, 6, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zeevart, K.; Mabrouk, M.H.E.; Wagner, W.; Goetzke, R. Cell mechanics in embryoid bodies. Cells 2020, 9, 2270. [Google Scholar] [CrossRef]
- Valli, A.; Rosner, M.; Fuchs, C.; Siegel, N.; Bishop, C.E.; Dolznig, H.; Mädel, U.; Feichtinger, W.; Atala, A.; Hengstschläger, M. Embryoid body formation of human amniotic fluid stem cells depends on mTOR. Oncogene 2010, 29, 966–977. [Google Scholar] [CrossRef]
- Khoo, M.L.M.; McQuade, L.R.; Smith, M.S.R.; Lees, J.G.; Sidhu, K.S.; Tuch, B.E. Growth and differentiation of embryoid bodies derived from human embryonic stem cells: Effect of glucose and basic fibroblast growth factor. Biol. Reprod. 2005, 73, 1147–1156. [Google Scholar] [CrossRef]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Xia, Y.; Belmonte, J.C.I. Design approaches for generating organ constructs. Cell Stem Cell 2019, 24, 877–894. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2008, 3, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Artegiani, B.; Clevers, H. Use of application of 3D-organoid technology. Hum. Mol. Genet. 2018, 27, R99–R107. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Manfrin, A.; Lutolf, M. Progress and potential in organoid research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Baretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef]
- Doudna, J. The promise and challenge of therapeutic genome editing. Nature 2020, 578, 229–236. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Wiegerinck, C.L.; de Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; de Winter-de Groot, K.M.; Brandsma, A.M.; de Jong, N.W.; Bijvelds, M.J.; Scholte, B.J.; et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 2013, 19, 939–945. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Berkers, G.; Kruisselbrink, E.; Vonk, A.; de Jonge, H.R.; Janssens, H.M.; Bronsveld, I.; van de Graaf, E.A.; Nieuwenhuis, E.E.; Houwen, R.H.; et al. Characterization responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 2016, 8, 344ra84. [Google Scholar] [CrossRef] [PubMed]
- Mellios, N.; Feldman, D.A.; Sheridian, S.D.; Ip, J.P.K.; Kwok, S.; Amoah, S.K.; Rosen, B.; Rodriguez, B.A.; Crawford, B.; Swaminathan, R.; et al. MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol. Psychiatry 2018, 23, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.A.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.S.; Brooks, C.R.; Lam, A.Q.; Fu, H.; Morizane, R.; Agrawal, V.; Saad, A.F.; Li, M.K.; Hughes, M.R.; Werff, R.V.; et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human epiblast spheroids. Nat. Commun. 2015, 6, 8715. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.M.; Song, X.; Czerniecki, S.M.; Gulieva, R.E.; Churchill, A.J.; Kim, Y.K.; Winston, R.E.; Churchill, A.J.; Kim, Y.K.; Winston, K.; et al. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat. Mater. 2017, 16, 1112–1119. [Google Scholar] [CrossRef]
- Korogi, Y.; Gotoh, S.; Ikeo, S.; Yamamoto, Y.; Sone, N.; Tamai, K.; Konishi, S.; Nagasaki, T.; Matsumoto, H.; Ito, I.; et al. In vitro disease modeling of Hermansky-Pudlak syndrome type 2 using human induced pluripotent stem cell-derived alveolar organoids. Stem Cell Rep. 2019, 12, 431–440. [Google Scholar] [CrossRef]
- Lane, A.; Jovanovic, K.; Shortall, C.; Ottaviani, D.; Panes, A.B.; Schwarz, N.; Guarascio, R.; Hayes, M.J.; Palfi, A.; Chadderton, N.; et al. Modeling and rescue of RP2 retinitis pigmentosa using iPSC-derived retinal organoids. Stem Cell Rep. 2020, 15, 67–79. [Google Scholar] [CrossRef]
- Crespo, M.; Vilar, E.; Tsai, S.Y.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.; Witherspoon, M.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef]
- Fleischer, A.; Vallejo-Diez, S.; Martin-Fernandez, J.M.; Sanchez-Gilabert, A.; Castresana, M.; Del Pozo, A.; Esquisabel, A.; Avila, S.; Castrillo, J.L.; Gainza, E.; et al. iPSC-derived intestinal organoids from cystic fibrosis patients acquire CFTR activity upon TALEN-mediated repair of the p-F508del mutation. Mol. Ther. Methods Clin. Dev. 2020, 17, 858–870. [Google Scholar] [CrossRef]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Bois, A.; Kriegstein, A.R. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 2017, 20, 435–449. [Google Scholar] [CrossRef]
- Xu, R.; Brawner, A.T.; Li, S.; Liu, J.J.; Kim, H.; Xue, H.; Pang, Z.P.; Kim, W.Y.; Hart, R.P.; Liu, Y.; et al. OLIG2 drives abnormal neurodevelopmental phenotypes in human iPSC-based organoid and chimeric mouse models of down syndrome. Cell Stem Cell 2019, 24, 908–926.e8. [Google Scholar] [CrossRef] [PubMed]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.-L.; Qu, L.; et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Garcez, P.P.; Loiola, E.C.; da Costa, R.M.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef]
- Xu, M.; Lee, E.M.; Wen, Z.; Cheng, Y.; Huang, W.K.; Qian, X.; Tcw, J.; Kouznetsova, J.; Ogden, S.C.; Hammack, C.; et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016, 22, 1101–1107. [Google Scholar] [CrossRef]
- Finkbeiner, S.R.; Zeng, X.-L.; Utama, B.; Atmar, R.L.; Shroyer, N.F.; Estes, M.K. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio 2012, 3, e00159-12. [Google Scholar] [CrossRef]
- Leslie, J.L.; Huang, S.; Opp, J.S.; Nagy, M.S.; Kobayashi, M.; Young, V.B.; Spence, J.R. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 2015, 83, 138–145. [Google Scholar] [CrossRef]
- Forbester, J.L.; Goulding, D.; Vallier, L.; Hannan, N.; Hale, C.; Pickard, D.; Mukhopadhyay, S.; Dougan, G. Interaction of Salmonella enterica serovar typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect. Immun. 2015, 83, 2926–2934. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Sachs, N.; Chiu, M.C.; Wong, B.H.-Y.; Chu, H.; Poon, V.K.-M.; Wang, D.; Zhao, X.; Wen, L.; et al. Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc. Natl. Acad. Sci. USA 2018, 115, 6822–6827. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Ching, R.H.H.; Chan, S.K.H.; Nicholls, J.M.; Sachs, N.; Clevers, H.; Peiris, J.S.M.; Chan, M.C.W. Tropism, replication competence, and innate immune responses of influenza virus: An analysis of human airway organoids and ex-vivo bronchus cultures. Lancet Respir. Med. 2018, 6, 846–854. [Google Scholar] [CrossRef]
- Berkers, G.; van Mourik, P.; Vonk, A.M.; Kruisselbrink, E.; Dekkers, J.F.; de Winter-de Groot, K.M.; Arets, H.G.M.; Marck-van der Wilt, R.E.P.; Dijkema, J.S.; Vanderschuren, M.M.; et al. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep. 2019, 26, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Heemskerk, I.; Gumucio, D.L. Opening the black box: Stem cell-based modelling of human post-implantation development. J. Cell. Biol. 2018, 218, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.N.; Siggia, E.D.; Zernicka-Goetz, M. Self-organization of stem cells into embryos: A window on early mammalian development. Science 2019, 364, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Vianello, S.; Lutolf, M.P. Understanding the mechanobiology of early mammalian development through bioengineered models. Dev. Cell 2019, 48, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Fu, J. Synthetic human embryology: Towards a quantitative future. Curr. Opin. Genet. Dev. 2020, 63, 30–35. [Google Scholar] [CrossRef]
- Mole, M.A.; Weberling, A.; Zernicka-Goetz, M. Comparative analysis of human and mouse development. From zygote to pre-gastrulation. Curr. Top. Dev. Biol. 2020, 136, 113–138. [Google Scholar]
- Rivron, N.; Pera, M.; Rossant, J.; Arias, A.M.; Zernicka-Goetz, M.; Fu, J.; van den Brink, S.; Bredenoord, A.; Dondorp, W.; de Wert, G.; et al. Debate ethics of embryo models from stem cells. Nature 2018, 564, 183–185. [Google Scholar] [CrossRef]
- O’Leary, T.; Heindryckx, B.; Lierman, S.; van Bruggen, D.; Goeman, J.J.; Vandewoestyne, M.; Deforce, D.; de Sousa Lopes, S.M.; De Sutter, P. Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat. Biotechnol. 2012, 30, 278–282. [Google Scholar] [CrossRef]
- Deglincerti, A.; Croft, G.F.; Pietila, L.N.; Zernicka-Goetz, M.; Siggia, E.D.; Brivanlou, A.H. Self-organization of the in vitro attached human embryo. Nature 2016, 533, 251–254. [Google Scholar] [CrossRef]
- Shahbazi, M.N.; Jedrusik, A.; Vuoristo, S.; Recher, G.; Hupalowska, A.; Bolton, V.; Fogarty, N.M.E.; Campbell, A.; Devito, L.G.; Ilic, D.; et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016, 18, 700–708. [Google Scholar] [CrossRef]
- Hyun, I.; Wilkerson, A.; Johnston, J. Embryology policy: Revisit the 14-day rule. Nature 2016, 533, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Hurlblut, J.B.; Hyun, I.; Levine, A.D.; Lovell-Badge, R.; Lunshof, J.E.; Matthews, K.R.W.; Mills, P.; Murdoch, A.; Pera, M.F.; Scott, C.T.; et al. Revisiting the Warnock rule. Nat. Biotechnol. 2017, 35, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Weatherbee, B.A.T.; Glover, D.M.; Zernicka-Goetz, M. Expression of SARS-CoV-2 receptor ACE2 and the protease TMPRSS2 suggests susceptibility of the human embryo in the first trimester. Open Biol. 2020, 10, 200162. [Google Scholar] [CrossRef] [PubMed]
- Warmflash, A.; Sorre, B.; Etoc, F.; Siggia, E.D.; Brivanlou, A.H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 2014, 11, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Deglincerti, A.; Etoc, F.; Guerra, M.C.; Martyn, I.; Metzger, J.; Ruzo, A.; Simunovic, M.; Yoney, A.; Brivanlou, A.H.; Siggia, E.; et al. Self-organization of human embryonic stem cells on micropatterns. Nat. Prot. 2016, 11, 2223–2232. [Google Scholar] [CrossRef]
- Simunovic, M.; Metzger, J.J.; Etoc, F.; Yoney, A.; Ruzo, A.; Martyn, I.; Croft, G.; You, D.S.; Brivanlou, A.H.; Siggia, E.D. A 3D model of a human epiblast reveals MBP4-driven symmetry breaking. Nat. Cell Biol. 2019, 21, 900–910. [Google Scholar] [CrossRef]
- Shao, Y.; Taniguchi, K.; Gurdziel, K.; Townshend, R.F.; Xue, X.; Yong, K.M.A.; Sang, J.; Spence, J.R.; Gumucio, D.L.; Fu, J. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater. 2017, 16, 419–425. [Google Scholar] [CrossRef]
- Shao, Y.; Taniguchi, K.; Townshend, R.F.; Miki, T.; Gumucio, D.L.; Fu, J. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat. Commun. 2017, 8, 208. [Google Scholar] [CrossRef]
- Zheng, Y.; Xue, X.; Shao, Y.; Wang, S.; Esfahani, S.N.; Li, Z.; Muncie, J.M.; Lakins, J.N.; Weaver, V.M.; Gumucio, D.L.; et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature 2019, 573, 421–425. [Google Scholar] [CrossRef]
- Esfahani, S.N.; Shao, Y.; Irizarry, A.M.R.; Li, Z.; Xue, X.; Gumucio, D.L.; Fu, J. Microengineered human amniotic ectoderm tissue array for high-content development phenotyping. Biomaterials 2019, 216, 119244. [Google Scholar] [CrossRef]
- Moris, N.; Anlas, K.; van den Brink, S.C.; Alemany, A.; Schröder, J.; Ghimire, S.; Balayo, T.; van Oudenaarden, A.; Arias, A.M. An in vitro model of early anteroposterior organization during human development. Nature 2020, 582, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Tewary, M.; Ostblom, J.; Prochazka, L.; Zulueta-Coarasa, T.; Shakiba, N.; Fernandez-Gonzalez, R.; Zandstra, P.W. A stepwise model of reaction-diffusion and positional information governs self-organized human peri-gastrulation-like patterning. Development 2017, 144, 4298–4312. [Google Scholar] [CrossRef] [PubMed]
- Etoc, F.; Metzger, J.; Ruzo, A.; Kirst, C.; Yoney, A.; Ozair, M.Z.; Brivanlou, A.H.; Siggia, E.D. A balance between secreted inhibitors and edge sensing controls gastruloid self-organization. Dev. Cell 2016, 39, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, I.; Burt, K.; Miller, M.; Chhabra, S.; Guerra, M.C.; Liu, L.; Warmflash, A. Rapid changes in morphogen concentration control self-organized patterning in human embryonic stem cells. eLife 2019, 8, e40526. [Google Scholar] [CrossRef]
- Nemashkalo, A.; Ruzo, A.; Heemskerk, I.; Warmflash, A. Morphogen and community effects determine cell fates in response to BMP4 signaling in human embryonic stem cells. Development 2017, 144, 3042–3053. [Google Scholar] [CrossRef]
- Chhabra, S.; Liu, L.; Goh, R.; Kong, X.; Warmflash, A. Dissecting the dynamics of signaling events in the BMP, WNT, and NODAL cascade during self-organized fate patterning in human gastruloids. PLoS Biol. 2019, 17, e3000498. [Google Scholar] [CrossRef]
- Martyn, I.; Brivanlou, A.H.; Siggia, E.D. A wave of WNT signaling balanced by secreted inhibitors controls primitive streak formation in micropattern colonies of human embryonic stem cells. Development 2019, 146, dev172791. [Google Scholar] [CrossRef]
- Minn, K.T.; Fu, Y.C.; He, S.; Dietmann, S.; George, S.C.; Anastasio, M.A.; Morris, S.A.; Solnica-Krezel, L. High-resolution transcriptional and morphogenetic profiling of cells from micropatterned human ESC gastruloid cultures. eLife 2020, 9, e59445. [Google Scholar] [CrossRef]
- Pera, M.F.; de Wert, G.; Dondorp, W.; Lovell-Badge, R.; Mummery, C.L.; Munsie, M.; Tan, P.P. What if stem cells turn into embryos in a dish? Nat. Methods 2015, 12, 917–919. [Google Scholar] [CrossRef]
- Hyun, I.; Munsie, M.; Pera, M.F.; Rivron, N.C.; Rossant, J. Toward guidelines for research on human embryo models formed from stem cells. Stem Cell Rep. 2020, 14, 169–174. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Schust, D.J.; Fisher, S.J. Implantation and the survival of early pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Macklon, N.S.; Geraedts, J.; Fauser, B.C.J.M. Conception to ongoing pregnancy: The black box of early pregnancy loss. Hum. Reprod. Update 2002, 8, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Burton, G.J. Pathophysiology of histological changes in early pregnancy loss. Placenta 2005, 26, 114–123. [Google Scholar] [CrossRef] [PubMed]
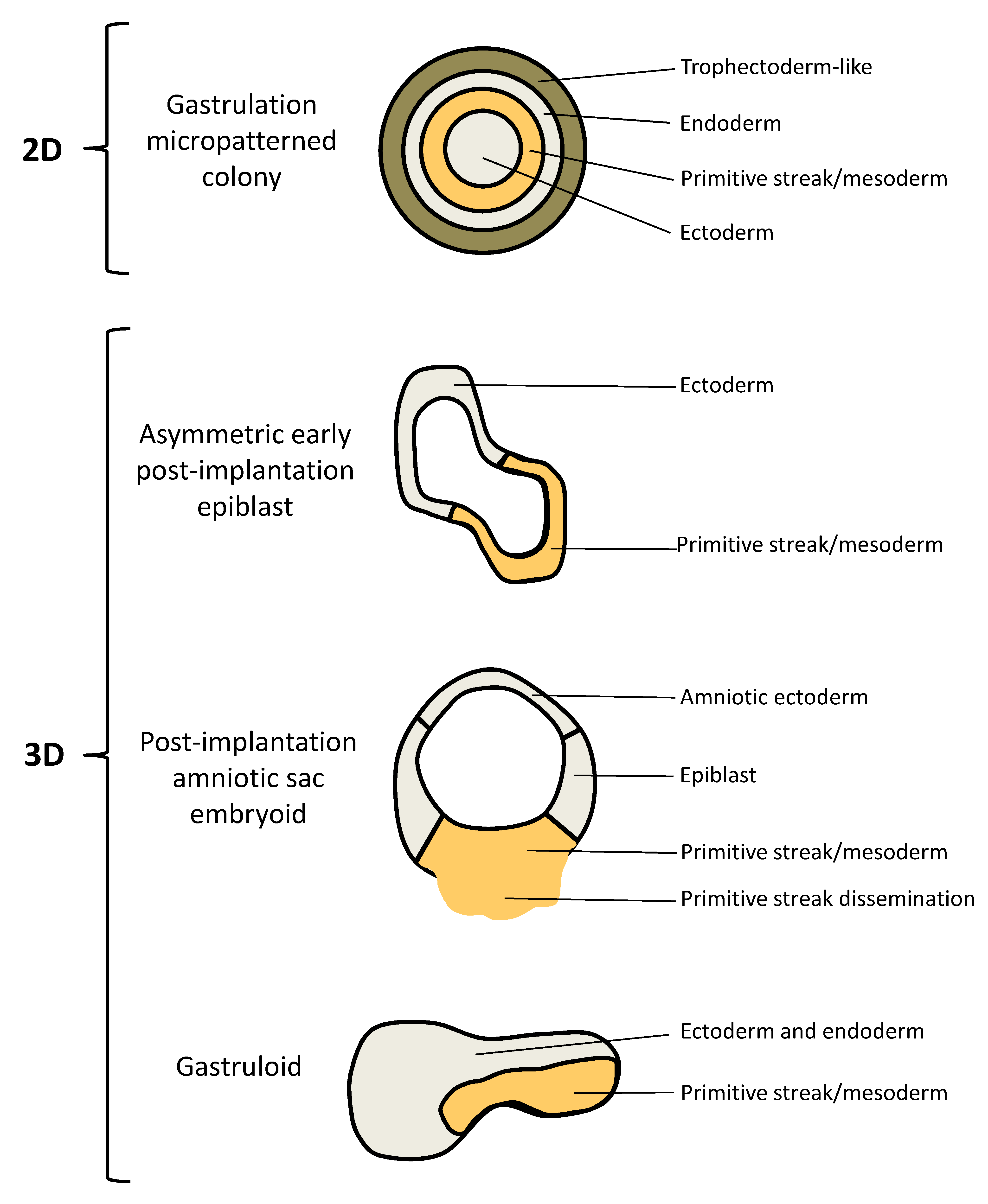
| Embryo Model | Reference | Structure | In Vivo Equivalent | In Vitro Development |
|---|---|---|---|---|
| Gastrulation micropatterned colony | [104,105] | 2D | Gastrulation: germ layer patterning and gastrulation “movements” | hESCs are forced to grow in a confined geometry on micropatterned coverslips; treatment of hESCs with BMP4 triggers self-organized spatial patterns of an outer trophectoderm-like ring, an inner ectodermal circle and a ring of mesendoderm in between, where cells undergo EMT and migrate inwards mimicking gastrulation |
| Asymmetric early post-implantation epiblast | [106] | 3D | Early post-implantation, pre-gastrulation: day 10 human epiblast | hESCs dispersed in hydrogel supplemented with Matrigel form lumenal sacs, which break the anterior–posterior symmetry upon BMP4 treatment and polarize into ectoderm and mesoderm |
| Post-implantation amniotic sac embryoid (PASE) | [107,108,109,110] | 3D | Post-implantation, gastrulation: post-implantation development until days 13–19 (including epiblast cyst formation at days 6–7, asymmetric sac development at days 7–13 and the onset of gastrulation at days 13-19) | hESCs form an amniotic sac, with amniotic ectoderm, an amniotic cavity and an embryonic disc; the embryonic disc develops into a primitive streak-like region with cells undergoing EMT and disseminating into the microenvironment |
| Gastruloid | [111] | 3D | Post-gastrulation: 72 h gastruloids show features of late Carnegie-stage 8 embryos (days 17–19) to early Carnegie stage 9 embryos (days 19–21) | hESCs are treated with Chiron, a WNT agonist, before seeding in low-adherence plates; gene expression studies of the so formed gastruloids reveal evidence for derivatives of the three germ layers organized in a spatiotemporal manner |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosner, M.; Reithofer, M.; Fink, D.; Hengstschläger, M. Human Embryo Models and Drug Discovery. Int. J. Mol. Sci. 2021, 22, 637. https://doi.org/10.3390/ijms22020637
Rosner M, Reithofer M, Fink D, Hengstschläger M. Human Embryo Models and Drug Discovery. International Journal of Molecular Sciences. 2021; 22(2):637. https://doi.org/10.3390/ijms22020637
Chicago/Turabian StyleRosner, Margit, Manuel Reithofer, Dieter Fink, and Markus Hengstschläger. 2021. "Human Embryo Models and Drug Discovery" International Journal of Molecular Sciences 22, no. 2: 637. https://doi.org/10.3390/ijms22020637
APA StyleRosner, M., Reithofer, M., Fink, D., & Hengstschläger, M. (2021). Human Embryo Models and Drug Discovery. International Journal of Molecular Sciences, 22(2), 637. https://doi.org/10.3390/ijms22020637





