Effects of Survival Motor Neuron Protein on Germ Cell Development in Mouse and Human
Abstract
1. Introduction
2. Results
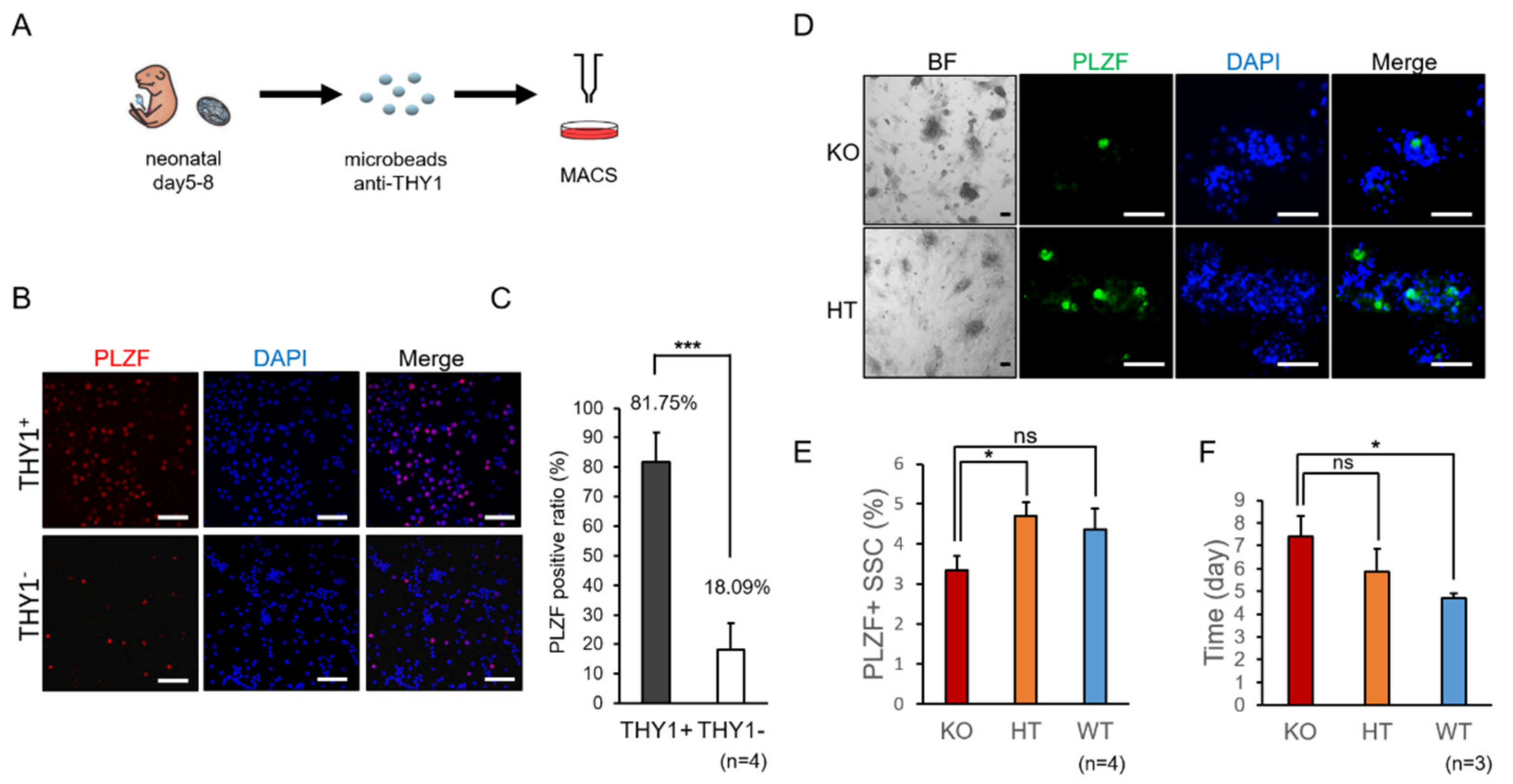
2.1. Decreased Propagation of Spermatogonia in SMA-Like Mice during In Vitro Culture
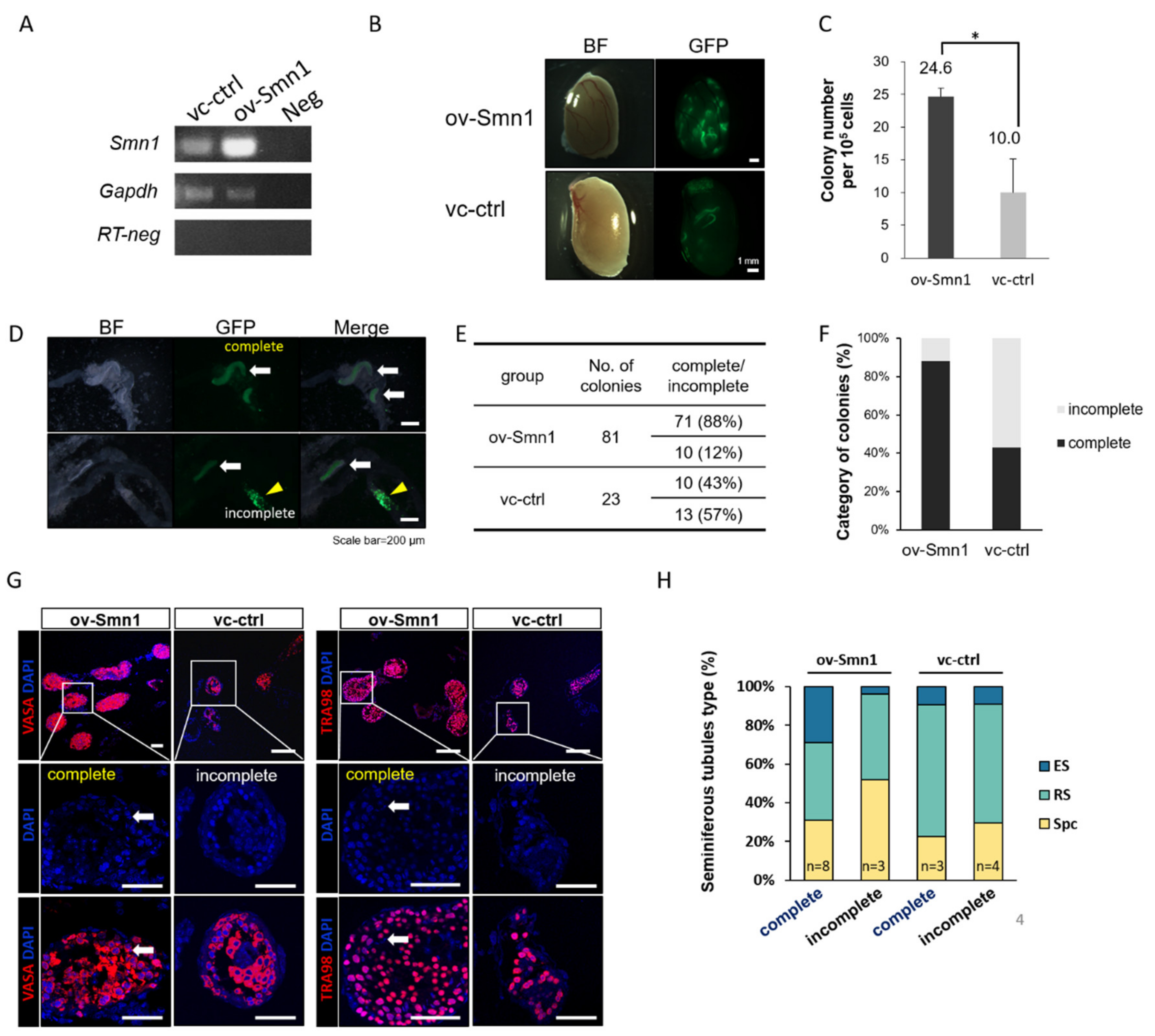
2.2. Overexpression of SMN1 Promotes Self-Renewal and Homing Ability of Mouse SSCs
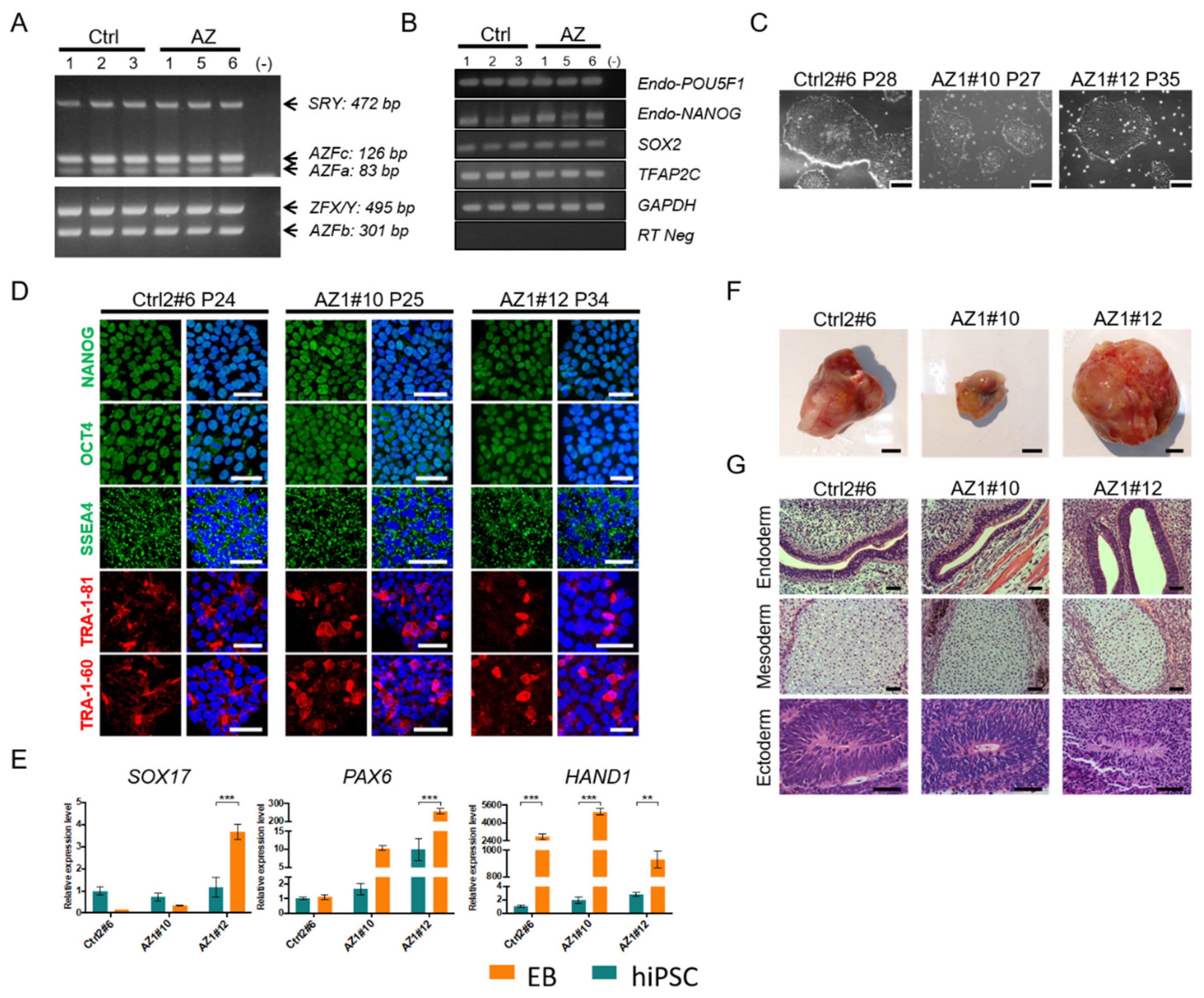
2.3. Generation and In Vitro Characterization of Human Induced Pluripotent Stem Cells (hiPSCs) from Non-Obstructive Azoospermic Patients
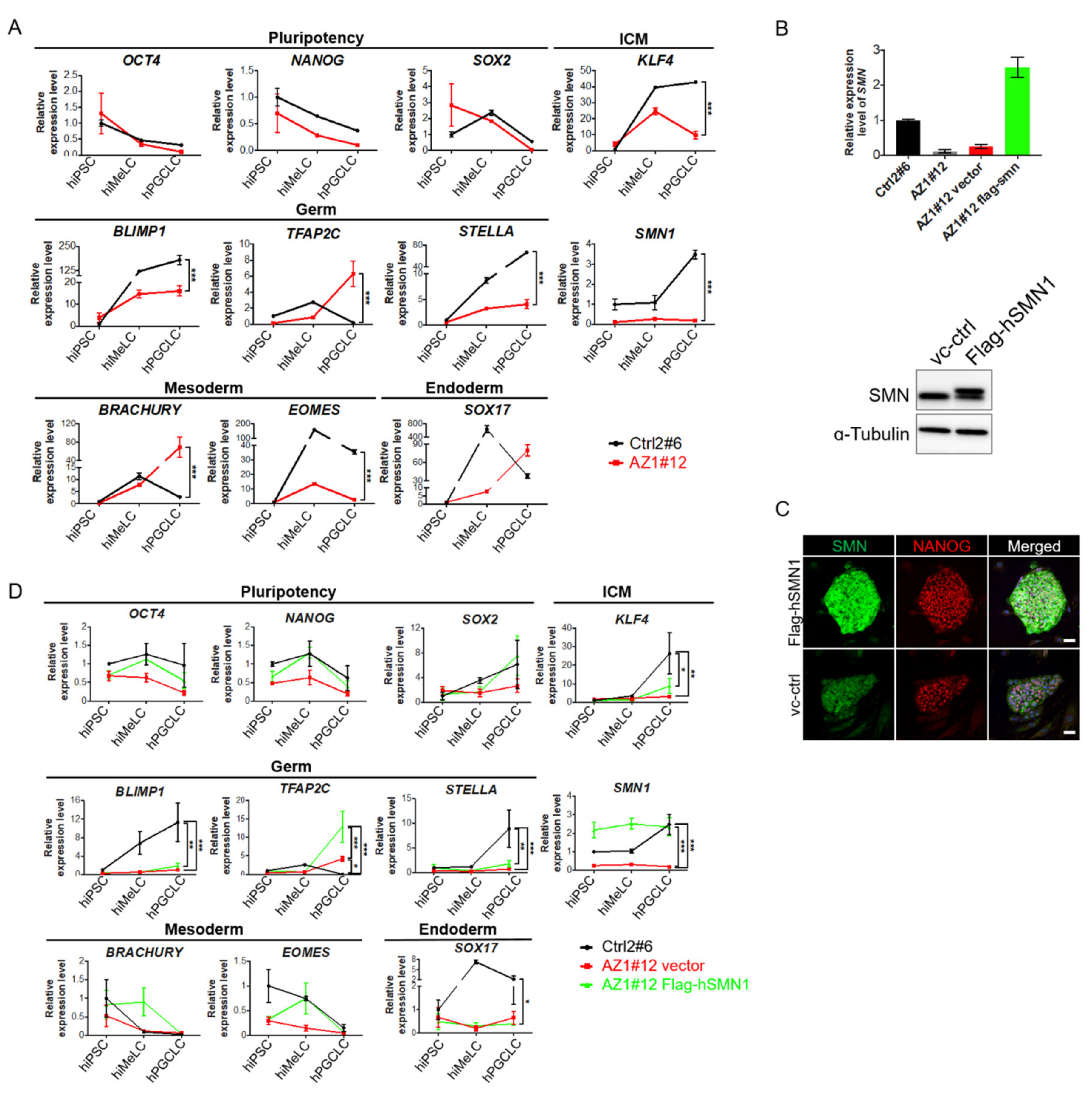
2.4. Induction of Human Primordial Germ Cell-Like Cells (hPGCLCs) in SMN Overexpressed Azoospermia hiPSCs
3. Discussion
4. Materials and Methods
4.1. Availability of Data and Materials
4.2. Generation and Culture of hiPSCs from Azoospermic Patients
4.3. Transduction of SMN into hiPSCs or Mouse SSCs with Lentivirus
4.4. Induction of hiMeLCs, hPGCLC
4.5. In Vitro Proliferation of Mouse SSCs
4.6. Immunofluorescent Staining and Confocal Microscopy
4.7. Immunohistochemistry (IHC)
4.8. Western Blotting
4.9. Gene Expression Analysis
4.10. Teratoma Assay
4.11. Germ cell Transplantation
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, J.F.; Thoma, M.E.; Sørensen, D.N.; McLain, A.C.; King, R.B.; Sundaram, R.; Keiding, N.; Louis, G.M.B. The prevalence of couple infertility in the United States from a male perspective: Evidence from a nationally representative sample. Androl. US 2013, 1, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Oakley, L.L.; Doyle, P.; Maconochie, N. Lifetime prevalence of infertility and infertility treatment in the UK: Results from a population-based survey of reproduction. Hum. Reprod. 2007, 23, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Dabaja, A.A.; Schlegel, P.N. Medical treatment of male infertility. Transl. Androl. Urol. 2014, 3, 9–16. [Google Scholar] [PubMed]
- Saylam, B.; Efesoy, O.; Çayan, S.; Saylam, B. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertil. Steril. 2011, 95, 809–811. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.C.; Li, X.; A Anguelov, R.; Toth, G.; Cristescu, A.; Acsadi, G. Survival motor neuron protein regulates apoptosis in an in vitro model of spinal muscular atrophy. Neurotox. Res. 2008, 13, 39–48. [Google Scholar] [CrossRef]
- Hayashi, K.; Surani, M.A. Resetting the Epigenome beyond Pluripotency in the Germline. Cell Stem Cell 2009, 4, 493–498. [Google Scholar] [CrossRef][Green Version]
- McLaren, A.; Lawson, K.A. How is the mouse germ-cell lineage established? Differentiation 2005, 73, 435–437. [Google Scholar] [CrossRef]
- Irie, N.; Weinberger, L.; Tang, W.W.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 Is a Critical Specifier of Human Primordial Germ Cell Fate. Cell 2015, 160, 253–268. [Google Scholar] [CrossRef]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H.; et al. Robust In Vitro Induction of Human Germ Cell Fate from Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Song, H.W.; Wilkinson, M.F. In vitro spermatogenesis: A long journey to get tails. Spermatogenesis 2012, 2, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Easley, C.A.; Phillips, B.T.; McGuire, M.M.; Barringer, J.M.; Valli, H.; Hermann, B.P.; Simerly, C.R.; Rajkovic, A.; Miki, T.; Orwig, K.E.; et al. Direct Differentiation of Human Pluripotent Stem Cells into Haploid Spermatogenic Cells. Cell Rep. 2012, 2, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, S.; Liang, D.; Wang, P.; Fu, J.; Ma, Q.; Kong, R.; Shi, L.; Gong, X.; Chen, W.; et al. In Vitro Modeling of Human Germ Cell Development Using Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-F.; Xu, J.; Chang, C.-C.; Yang, S.-H.; Li, H.-Y.; Hsieh-Li, H.M.; Tsai, M.-H.; Wu, S.-C.; Cheng, W.T.K.; Liu, J.-L.; et al. SMN is required for the maintenance of embryonic stem cells and neuronal differentiation in mice. Brain Struct. Funct. 2014, 220, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Grice, S.J.; Liu, J.-L. Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in Drosophila. PLoS Genet. 2011, 7, e1002030. [Google Scholar] [CrossRef]
- Adami, R.; Bottai, D. Spinal Muscular Atrophy Modeling and Treatment Advances by Induced Pluripotent Stem Cells Studies. Stem Cell Rev. Rep. 2019, 15, 795–813. [Google Scholar] [CrossRef]
- Schorling, D.C.; Pechmann, A.; Kirschner, J. Advances in Treatment of Spinal Muscular Atrophy–New Phenotypes, New Challenges, New Implications for Care. J. Neuromuscul. Dis. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Chang, W.-F.; Xu, J.; Lin, T.-Y.; Hsu, J.; Hsieh-Li, H.M.; Hwu, Y.-M.; Liu, J.-L.; Lu, C.-H.; Sung, L.-Y. Survival Motor Neuron Protein Participates in Mouse Germ Cell Development and Spermatogonium Maintenance. Int. J. Mol. Sci. 2020, 21, 794. [Google Scholar] [CrossRef]
- Ehrmann, I.; Crichton, J.H.; Gazzara, M.R.; James, K.; Liu, Y.; Grellscheid, S.N.; Curk, T.; De Rooij, D.G.; Steyn, J.S.; Cockell, S.; et al. An ancient germ cell-specific RNA-binding protein protects the germline from cryptic splice site poisoning. eLife 2019, 8, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, T.; Li, Q.; Liu, C.; Han, F.; Chen, M.; Zhang, L.; Cui, X.; Qin, Y.; Bao, S.; et al. Prmt5 is required for germ cell survival during spermatogenesis in mice. Sci. Rep. 2015, 5, 11031. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Kubota, Y.; Ogawa, T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat. Protoc. 2013, 8, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.S.; Toyooka, Y.; Akasu, R.; Katoh-Fukui, Y.; Nakahara, Y.; Suzuki, R.; Yokoyama, M.; Noce, T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genome Res. 2000, 14, 841–853. [Google Scholar]
- Toyooka, Y.; Tsunekawa, N.; Takahashi, Y.; Matsui, Y.; Satoh, M.; Noce, T. Expression and intracellular localization of mouse Vasa -homologue protein during germ cell development. Mech. Dev. 2000, 93, 139–149. [Google Scholar] [CrossRef]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the Mouse Germ Cell Specification Pathway in Culture by Pluripotent Stem Cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef]
- Nakaki, F.; Hayashi, K.; Ohta, H.; Kurimoto, K.; Yabuta, Y.; Saitou, M. Induction of mouse germ-cell fate by transcription factors in vitro. Nat. Cell Biol. 2013, 501, 222–226. [Google Scholar] [CrossRef]
- Becherel, O.J.; Yeo, A.J.; Stellati, A.; Heng, E.Y.H.; Luff, J.; Suraweera, A.; Woods, R.; Fleming, J.; Carrie, D.; McKinney, K.; et al. Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing. PLoS Genet. 2013, 9, e1003435. [Google Scholar] [CrossRef]
- Maxwell, G.K.; Szunyogova, E.; Shorrock, H.K.; Gillingwater, T.H.; Parson, S.H. Developmental and degenerative cardiac defects in the Taiwanese mouse model of severe spinal muscular atrophy. J. Anat. 2018, 232, 965–978. [Google Scholar] [CrossRef]
- Ogawa, T.; Aréchaga, J.M.; Avarbock, M.R.; Brinster, R.L. Transplantation of testis germinal cells into mouse seminiferous tubules. Int. J. Dev. Biol. 1997, 41, 111–122. [Google Scholar]
- Wu, H.; Sun, L.; Wen, Y.; Liu, Y.; Yu, J.; Mao, F.; Wang, Y.; Tong, C.; Guo, X.; Hu, Z.; et al. Major spliceosome defects cause male infertility and are associated with nonobstructive azoospermia in humans. Proc. Natl. Acad. Sci. USA 2016, 113, 4134–4139. [Google Scholar] [CrossRef]
- Verma, B.; Akinyi, M.V.; Norppa, A.J.; Frilander, M.J. Minor spliceosome and disease. Semin. Cell Dev. Biol. 2018, 79, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.N.; Ottesen, E.W.; Singh, N.N. The First Orally Deliverable Small Molecule for the Treatment of Spinal Muscular Atrophy. Neurosci. Insights 2020, 15, 2633105520973985. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, F.S.-H.; Cheng, C.-C.; Peng, S.-Y.; Huang, H.-Y.; Lian, W.S.; Jan, M.-L.; Fang, Y.-T.; Cheng, E.C.-H.; Lee, K.-H.; Cheng, W.T.-K.; et al. Isolation of therapeutically functional mouse bone marrow mesenchymal stem cells within 3 h by an effective single-step plastic-adherent method. Cell Prolif. 2010, 43, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Li, H.M.; Chang, J.-G.; Jong, Y.-J.; Wu, M.-H.; Wang, N.M.; Tsai, C.H.; Li, H. A mouse model for spinal muscular atrophy. Nat. Genet. 2000, 24, 66–70. [Google Scholar] [CrossRef]
- Liao, H.-F.; Chen, W.S.C.; Chen, Y.-H.; Kao, T.-H.; Tseng, Y.-T.; Lee, C.-Y.; Chiu, Y.-C.; Lee, P.-L.; Lin, Q.-J.; Ching, Y.-H.; et al. DNMT3L promotes quiescence in postnatal spermatogonial progenitor cells. Development 2014, 141, 2402–2413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-F.; Peng, M.; Hsu, J.; Xu, J.; Cho, H.-C.; Hsieh-Li, H.-M.; Liu, J.-L.; Lu, C.-H.; Sung, L.-Y. Effects of Survival Motor Neuron Protein on Germ Cell Development in Mouse and Human. Int. J. Mol. Sci. 2021, 22, 661. https://doi.org/10.3390/ijms22020661
Chang W-F, Peng M, Hsu J, Xu J, Cho H-C, Hsieh-Li H-M, Liu J-L, Lu C-H, Sung L-Y. Effects of Survival Motor Neuron Protein on Germ Cell Development in Mouse and Human. International Journal of Molecular Sciences. 2021; 22(2):661. https://doi.org/10.3390/ijms22020661
Chicago/Turabian StyleChang, Wei-Fang, Min Peng, Jing Hsu, Jie Xu, Huan-Chieh Cho, Hsiu-Mei Hsieh-Li, Ji-Long Liu, Chung-Hao Lu, and Li-Ying Sung. 2021. "Effects of Survival Motor Neuron Protein on Germ Cell Development in Mouse and Human" International Journal of Molecular Sciences 22, no. 2: 661. https://doi.org/10.3390/ijms22020661
APA StyleChang, W.-F., Peng, M., Hsu, J., Xu, J., Cho, H.-C., Hsieh-Li, H.-M., Liu, J.-L., Lu, C.-H., & Sung, L.-Y. (2021). Effects of Survival Motor Neuron Protein on Germ Cell Development in Mouse and Human. International Journal of Molecular Sciences, 22(2), 661. https://doi.org/10.3390/ijms22020661





