TGF-β Activity of a Demineralized Bone Matrix
Abstract
1. Introduction
2. Results
2.1. Cell Viability in Response to Supernatants and Lysates of OraGRAFT®
2.2. TGF-β1 Identified by Immunoassay in Supernatants and Lysates of OraGRAFT®
2.3. TGF-β1 Activity by Bioassay in Supernatants and Lysates of OraGRAFT®
2.4. Blocking the TGF-β Receptor Type I Kinase Activity by SB431542
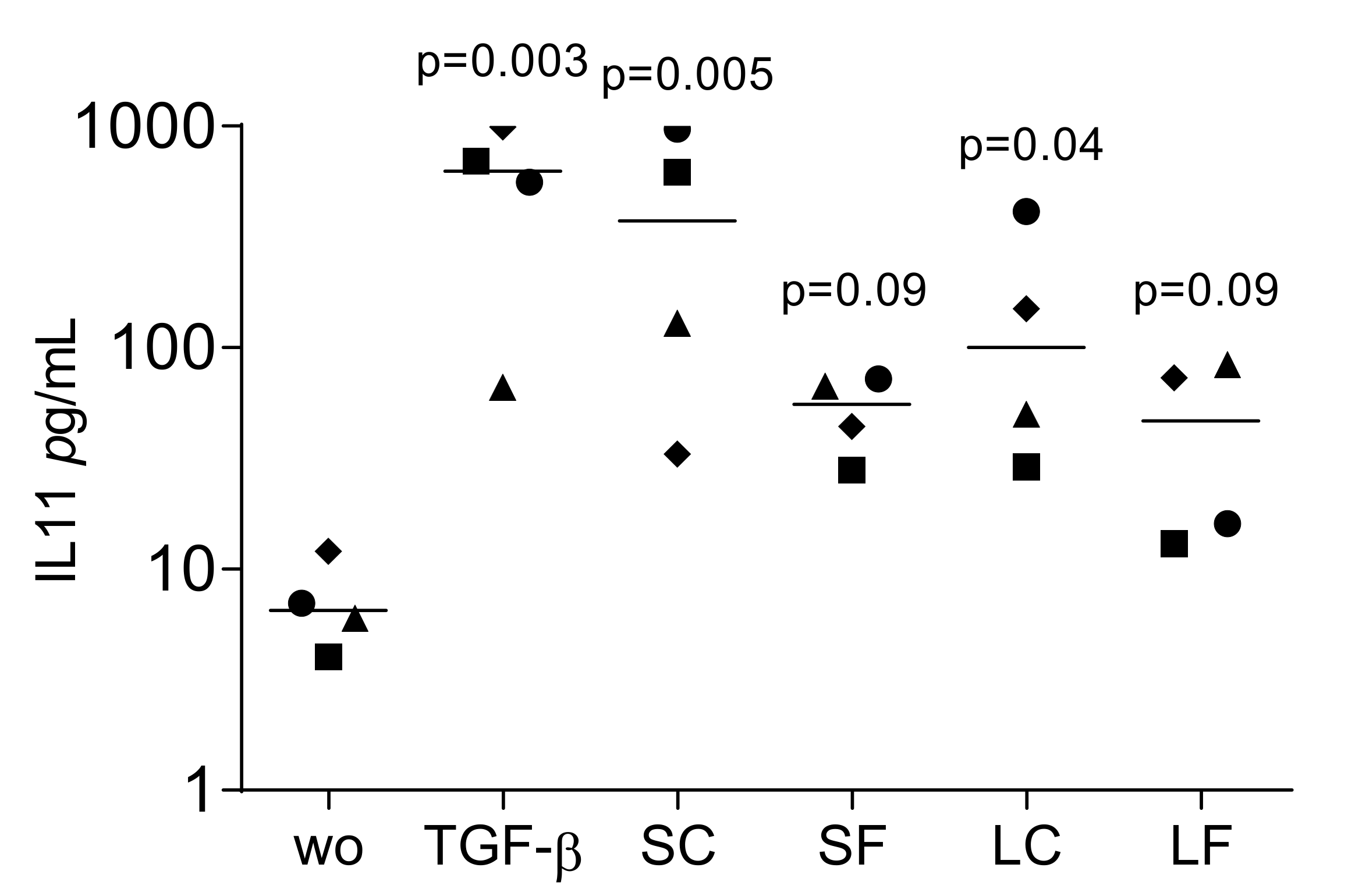
2.5. TGF-β1 Activity by Immunoassay in Supernatants of OraGRAFT®
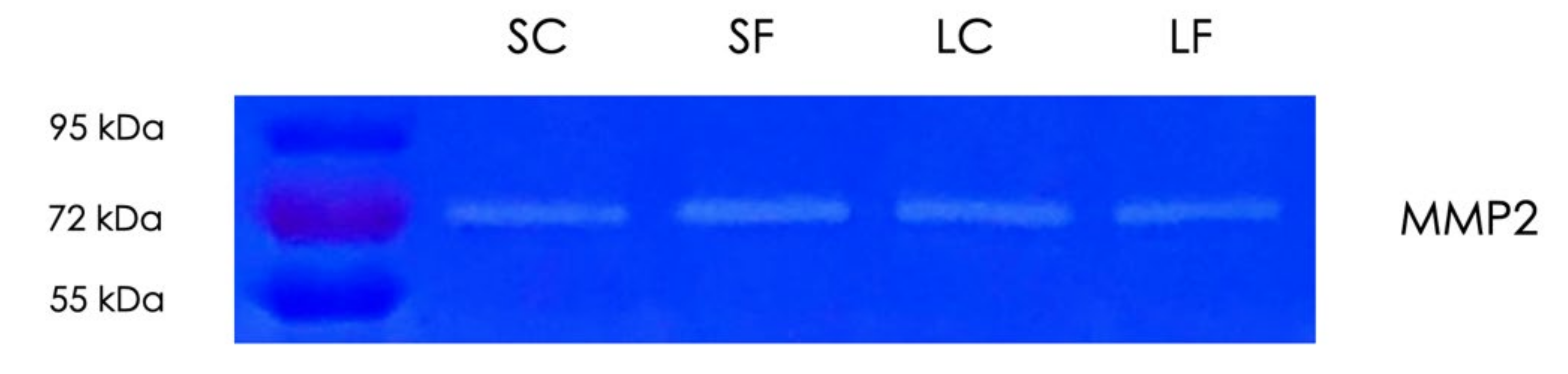
2.6. Gelatin Zymography of Supernatants and Lysates of OraGRAFT®
2.7. Blocking of Protease Activity Reduces TGF-β1 Activity in Supernatants of OraGRAFT®
3. Discussion
4. Materials and Methods
4.1. Preparation of Allograft Supernatants and Lysates
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. RT-PCR and Immunoassay
4.5. Immunofluorescence
4.6. Gelatin Zymography
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferguson, C.; Alpern, E.; Miclau, T.; Helms, J.A. Does adult fracture repair recapitulate embryonic skeletal formation? Mech. Dev. 1999, 87, 57–66. [Google Scholar] [CrossRef]
- Vasak, C.; Busenlechner, D.; Schwarze, U.Y.; Leitner, H.F.; Guzon, F.M.; Hefti, T.; Schlottig, F.; Gruber, R. Early bone apposition to hydrophilic and hydrophobic titanium implant surfaces: A histologic and histomorphometric study in minipigs. Clin. Oral Implant Res. 2014, 25, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 52–69. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implants 2009, 24, 237–259. [Google Scholar]
- Nelson, A.C.; Mealey, B.L. A randomized controlled trial on the impact of healing time on wound healing following ridge preservation using a 70%/30% combination of mineralized and demineralized freeze-dried bone allograft. J. Periodontol. 2020, 91, 1256–1263. [Google Scholar] [CrossRef]
- Guillaume, B. Filling bone defects with beta-TCP in maxillofacial surgery: A review. Morphologie 2017, 101, 113–119. [Google Scholar] [CrossRef]
- Thoma, D.S.; Bienz, S.P.; Figuero, E.; Jung, R.E.; Sanz-Martin, I. Efficacy of lateral bone augmentation performed simultaneously with dental implant placement: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 257–276. [Google Scholar] [CrossRef]
- Cicciu, M.; Cervino, G.; Herford, A.S.; Fama, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Moussa, N.T.; Dym, H. Maxillofacial Bone Grafting Materials. Dent. Clin. N. Am. 2020, 64, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implant. 2014, 7 (Suppl. 2), S203–S217. [Google Scholar]
- Jakoi, A.M.; Iorio, J.A.; Cahill, P.J. Autologous bone graft harvesting: A review of grafts and surgical techniques. Musculoskelet. Surg. 2015, 99, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Wiseman, S.; Reddi, A.H. Transformation of fibroblasts by allogeneic and xenogeneic transplants of demineralized tooth and bone. J. Exp. Med. 1970, 132, 1250–1258. [Google Scholar] [CrossRef]
- Huggins, C.B.; Urist, M.R. Dentin matrix transformation: Rapid induction of alkaline phosphatase and cartilage. Science 1970, 167, 896–898. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef]
- Sampath, T.K.; Coughlin, J.E.; Whetstone, R.M.; Banach, D.; Corbett, C.; Ridge, R.J.; Ozkaynak, E.; Oppermann, H.; Rueger, D.C. Bovine osteogenic protein is composed of dimers of OP-1 and BMP-2A, two members of the transforming growth factor-beta superfamily. J. Biol. Chem. 1990, 265, 13198–13205. [Google Scholar] [CrossRef]
- Sampath, T.K.; Muthukumaran, N.; Reddi, A.H. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc. Natl. Acad. Sci. USA 1987, 84, 7109–7113. [Google Scholar] [CrossRef]
- Seyedin, S.M.; Thomas, T.C.; Thompson, A.Y.; Rosen, D.M.; Piez, K.A. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc. Natl. Acad. Sci. USA 1985, 82, 2267–2271. [Google Scholar] [CrossRef] [PubMed]
- Pfeilschifter, J.; Diel, I.; Scheppach, B.; Bretz, A.; Krempien, R.; Erdmann, J.; Schmid, G.; Reske, N.; Bismar, H.; Seck, T.; et al. Concentration of transforming growth factor beta in human bone tissue: Relationship to age, menopause, bone turnover, and bone volume. J. Bone Miner. Res. 1998, 13, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Strauss, F.J.; Stahli, A.; Beer, L.; Mitulovic, G.; Gilmozzi, V.; Haspel, N.; Schwab, G.; Gruber, R. Acid bone lysate activates TGFbeta signalling in human oral fibroblasts. Sci. Rep. 2018, 8, 16065. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.L.; Cao, X. Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J. Clin. Investig. 2014, 124, 466–472. [Google Scholar] [CrossRef]
- Jann, J.; Gascon, S.; Roux, S.; Faucheux, N. Influence of the TGF-beta Superfamily on Osteoclasts/Osteoblasts Balance in Physiological and Pathological Bone Conditions. Int. J. Mol. Sci. 2020, 21, 7597. [Google Scholar] [CrossRef]
- Wildemann, B.; Kadow-Romacker, A.; Pruss, A.; Haas, N.P.; Schmidmaier, G. Quantification of growth factors in allogenic bone grafts extracted with three different methods. Cell Tissue Bank. 2007, 8, 107–114. [Google Scholar] [CrossRef]
- Wildemann, B.; Kadow-Romacker, A.; Haas, N.P.; Schmidmaier, G. Quantification of various growth factors in different demineralized bone matrix preparations. J. Biomed. Mater. Res. A 2007, 81, 437–442. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.H. The regulation of TGFbeta signal transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef]
- Zimmermann, M.; Caballe-Serrano, J.; Bosshardt, D.D.; Ankersmit, H.J.; Buser, D.; Gruber, R. Bone-Conditioned Medium Changes Gene Expression in Bone-Derived Fibroblasts. Int. J. Oral Maxillofac. Implant. 2015, 30, 953–958. [Google Scholar] [CrossRef]
- Stahli, A.; Bosshardt, D.; Sculean, A.; Gruber, R. Emdogain-regulated gene expression in palatal fibroblasts requires TGF-betaRI kinase signaling. PLoS ONE 2014, 9, e105672. [Google Scholar] [CrossRef]
- Imai, K.; Hiramatsu, A.; Fukushima, D.; Pierschbacher, M.D.; Okada, Y. Degradation of decorin by matrix metalloproteinases: Identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem. J. 1997, 322, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Dangelo, M.; Sarment, D.P.; Billings, P.C.; Pacifici, M. Activation of transforming growth factor beta in chondrocytes undergoing endochondral ossification. J. Bone Miner. Res. 2001, 16, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Triplett, R.G.; Nevins, M.; Marx, R.E.; Spagnoli, D.B.; Oates, T.W.; Moy, P.K.; Boyne, P.J. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. 2009, 67, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, U.; Rudelstorfer, C.M.; Barth, B.; Tepper, G.; Lidinsky, D.; Heimel, P.; Watzek, G.; Gruber, R. Crestal Sinus Augmentation with Recombinant Human Bone Morphogenetic Protein 2: Clinical and Radiographic Outcomes of 2-Year Pilot Trial. Int. J. Oral Maxillofac. Implant. 2017, 32, e213–e220. [Google Scholar] [CrossRef] [PubMed]
- Bodalia, P.N.; Balaji, V.; Kaila, R.; Wilson, L. Effectiveness and safety of recombinant human bone morphogenetic protein-2 for adults with lumbar spine pseudarthrosis following spinal fusion surgery: A systematic review. Bone Joint Res. 2016, 5, 145–152. [Google Scholar] [CrossRef]
- Tejwani, S.G.; Chen, J.; Funahashi, T.T.; Love, R.; Maletis, G.B. Revision Risk After Allograft Anterior Cruciate Ligament Reconstruction: Association With Graft Processing Techniques, Patient Characteristics, and Graft Type. Am. J. Sports Med. 2015, 43, 2696–2705. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, D.; Spinetti, G.; Zhang, J.; Jiang, L.Q.; Pintus, G.; Monticone, R.; Lakatta, E.G. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arter. Thromb Vasc. Biol. 2006, 26, 1503–1509. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Shuang, Y.; Bosshardt, D.D.; Gruber, R.; Buser, D.; Chandad, F.; Zhang, Y. Osteoinductive potential of a novel biphasic calcium phosphate bone graft in comparison with autographs, xenografts, and DFDBA. Clin. Oral Implant. Res. 2016, 27, 668–675. [Google Scholar] [CrossRef]
- Yamaoka, K.; Hirai, R.; Tsugita, A.; Mitsui, H. The purification of an acid- and heat-labile transforming growth factor from an avian sarcoma virus-transformed rat cell line. J. Cell. Physiol. 1984, 119, 307–314. [Google Scholar] [CrossRef]
- Wei, C.; Li, L.; Menon, M.C.; Zhang, W.; Fu, J.; Kidd, B.; Keung, K.L.; Woytovich, C.; Greene, I.; Xiao, W.; et al. Genomic Analysis of Kidney Allograft Injury Identifies Hematopoietic Cell Kinase as a Key Driver of Renal Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 1385–1393. [Google Scholar] [CrossRef]
- Suga, K.; Saitoh, M.; Kokubo, S.; Nozaki, K.; Fukushima, S.; Yasuda, S.; Sasamata, M.; Miyata, K. Synergism between interleukin-11 and bone morphogenetic protein-2 in the healing of segmental bone defects in a rabbit model. J. Interf. Cytokine Res. 2004, 24, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.C.; Ganapathy, S.; Gorin, Y.; Mahadev, K.; Block, K.; Abboud, H.E.; Harris, S.E.; Ghosh-Choudhury, G.; Ghosh-Choudhury, N. Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem. J. 2011, 433, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Novince, C.M.; Michalski, M.N.; Koh, A.J.; Sinder, B.P.; Entezami, P.; Eber, M.R.; Pettway, G.J.; Rosol, T.J.; Wronski, T.J.; Kozloff, K.M.; et al. Proteoglycan 4: A dynamic regulator of skeletogenesis and parathyroid hormone skeletal anabolism. J. Bone Miner. Res. 2012, 27, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Mitulovic, G.; Panahipour, L.; Gruber, R. Proteomic Analysis of Porcine-Derived Collagen Membrane and Matrix. Materials 2020, 13, 5187. [Google Scholar] [CrossRef]





| Concentration | 50% | 25% | 12% | 6% |
|---|---|---|---|---|
| SC | 101.4 ± 0.2 | 97.8 ± 0.1 | 93.7 ± 0.1 | 86.5 ± 0.1 |
| SF | 85.7 ± 0.1 | 100.1 ± 0.3 | 121.7 ± 0.3 | 85.2 ± 0.1 |
| LC | 17.3 ± 0.04 | 13.5 ± 0.04 | 23.3 ± 0.01 | 104.0 ± 0.1 |
| LF | 20.0 ± 0.1 | 37.5 ± 0.2 | 111.5 ± 0.2 | 115.2 ± 0.2 |
| Primers | Sequence_F | Sequence_R |
|---|---|---|
| hNOX4 | TCTTGGCTTACCTCCGAGGA | CTCCTGGTTCTCCTGCTTGG |
| hPRG4 | CAGTTGCAGGTGGCATCTC | TCGTGATTCAGCAAGTTTCATC |
| hGAPDH | AAGCCACATCGCTCAGACAC | GCCCAATACGACCAAATCC |
| h18s | CCGATTGGATGGTTTAGTGAG | AGTTCGACCGTCTTCTCAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panahipour, L.; Omerbasic, A.; Nasirzade, J.; Gruber, R. TGF-β Activity of a Demineralized Bone Matrix. Int. J. Mol. Sci. 2021, 22, 664. https://doi.org/10.3390/ijms22020664
Panahipour L, Omerbasic A, Nasirzade J, Gruber R. TGF-β Activity of a Demineralized Bone Matrix. International Journal of Molecular Sciences. 2021; 22(2):664. https://doi.org/10.3390/ijms22020664
Chicago/Turabian StylePanahipour, Layla, Anes Omerbasic, Jila Nasirzade, and Reinhard Gruber. 2021. "TGF-β Activity of a Demineralized Bone Matrix" International Journal of Molecular Sciences 22, no. 2: 664. https://doi.org/10.3390/ijms22020664
APA StylePanahipour, L., Omerbasic, A., Nasirzade, J., & Gruber, R. (2021). TGF-β Activity of a Demineralized Bone Matrix. International Journal of Molecular Sciences, 22(2), 664. https://doi.org/10.3390/ijms22020664






