Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis
Abstract
1. Introduction
2. Circadian Rhythm and Arteriosclerosis
3. Circadian Disruption and Vascular Complications
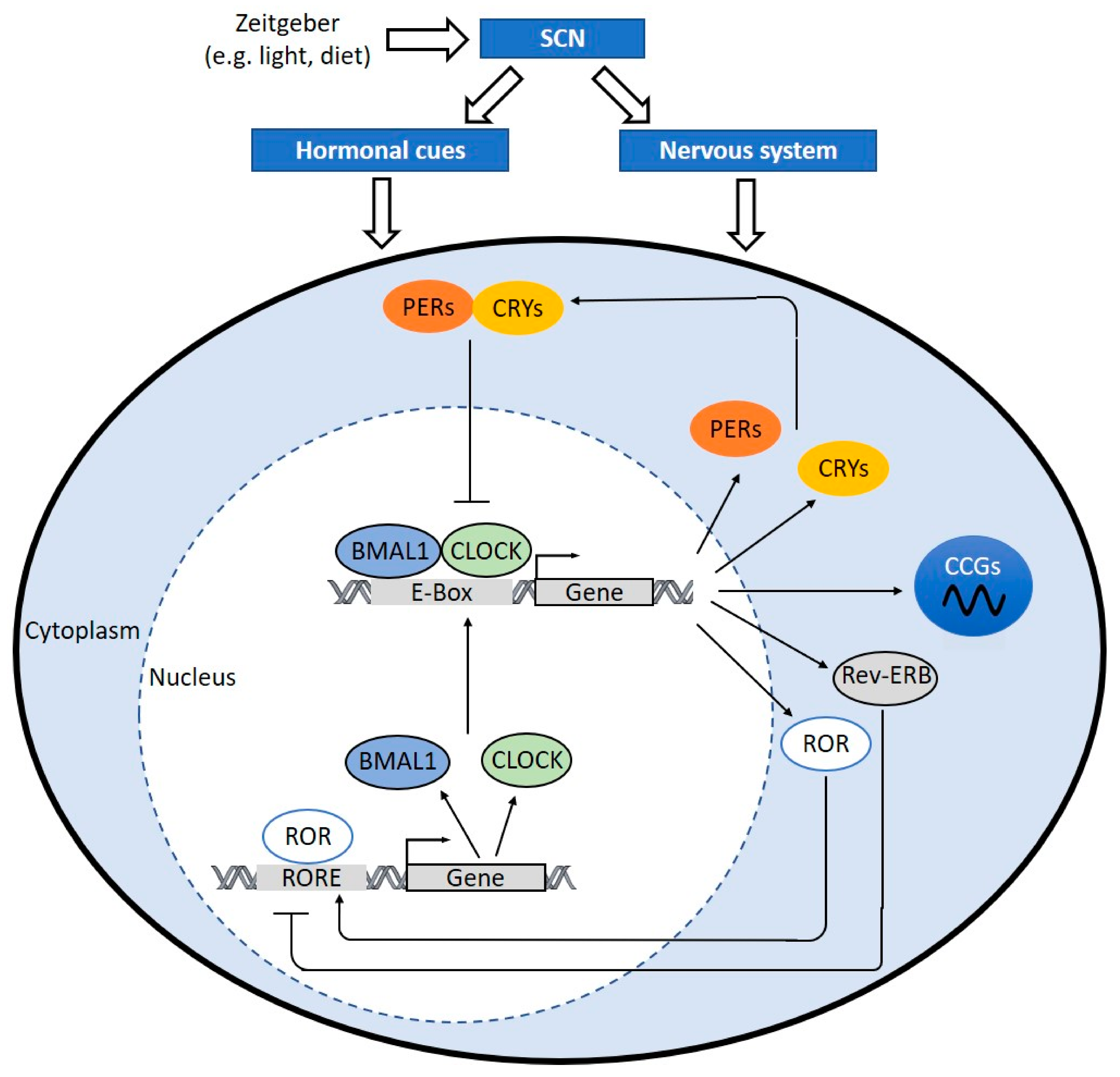
4. Clock Components
5. Clock Components and Vascular Complications
5.1. BMAL1 and CLOCK
5.2. CRY1/2
5.3. PER1/2
5.4. REV-ERB
6. Targeting the Circadian Clock for the Treatment of Atherosclerosis
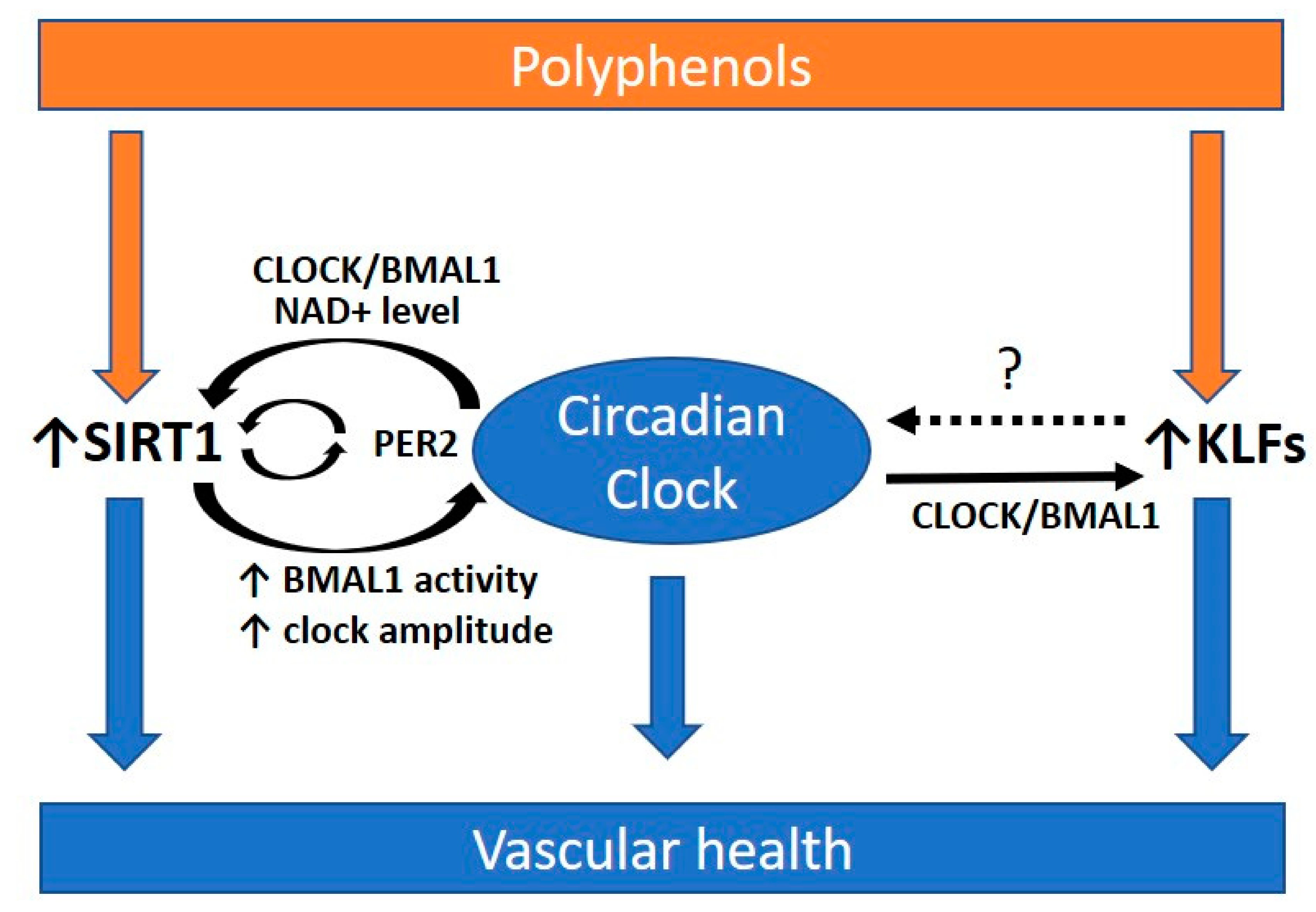
6.1. The Role of SIRT1 in Regulating the Circadian Rhythm
6.2. Krüppel-Like Factors (KLFs), Circadian Rhythms and Atherosclerosis
6.3. Polyphenols and the Circadian Clock
7. Summary and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 2016, 315, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.L.; Feskanich, D.; Sanchez, B.N.; Rexrode, K.M.; Schernhammer, E.S.; Lisabeth, L.D. Rotating night shift work and the risk of ischemic stroke. Am. J. Epidemiol. 2009, 169, 1370–1377. [Google Scholar] [CrossRef]
- Deng, N.; Kohn, T.P.; Lipshultz, L.I.; Pastuszak, A.W. The Relationship Between Shift Work and Men’s Health. Sex. Med. Rev. 2018, 6, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Suwazono, Y.; Sakata, K.; Okubo, Y.; Harada, H.; Kobayashi, E.; Uetani, M.; Nogawa, K. A longitudinal study on the relationship between shift work and the progression of hypertension in male Japanese workers. J. Hypertens. 2005, 23, 2173–2178. [Google Scholar] [CrossRef]
- Nazri, S.M.; Tengku, M.A.; Winn, T. The association of shift work and hypertension among male factory workers in Kota Bharu, Kelantan, Malaysia. Southeast Asian J. Trop. Med. Public Health 2008, 39, 176–183. [Google Scholar]
- Esquirol, Y.; Perret, B.; Ruidavets, J.B.; Marquie, J.C.; Dienne, E.; Niezborala, M.; Ferrieres, J. Shift work and cardiovascular risk factors: New knowledge from the past decade. Arch. Cardiovasc. Dis. 2011, 104, 636–668. [Google Scholar] [CrossRef]
- Haus, E.; Smolensky, M. Biological clocks and shift work: Circadian dysregulation and potential long-term effects. Cancer Causes Control 2006, 17, 489–500. [Google Scholar] [CrossRef]
- Brum, M.C.; Filho, F.F.; Schnorr, C.C.; Bottega, G.B.; Rodrigues, T.C. Shift work and its association with metabolic disorders. Diabetol. Metab. Syndr. 2015, 7, 45. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; Cardoso, C.R.; Salles, G.F. Prognostic value of nocturnal blood pressure reduction in resistant hypertension. Arch. Intern. Med. 2009, 169, 874–880. [Google Scholar] [CrossRef]
- Ayala, D.E.; Moya, A.; Crespo, J.J.; Castineira, C.; Dominguez-Sardina, M.; Gomara, S.; Sineiro, E.; Mojon, A.; Fontao, M.J.; Hermida, R.C.; et al. Circadian pattern of ambulatory blood pressure in hypertensive patients with and without type 2 diabetes. Chronobiol. Int. 2013, 30, 99–115. [Google Scholar] [CrossRef]
- Ohlander, J.; Keskin, M.C.; Stork, J.; Radon, K. Shift work and hypertension: Prevalence and analysis of disease pathways in a German car manufacturing company. Am. J. Ind. Med. 2015, 58, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Kobiita, A.; Damara, M.; Misra, N.; Meziane, H.; Champy, M.F.; Chambon, P. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, E6691–E6698. [Google Scholar] [CrossRef]
- Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.D.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Nutrition and the circadian system. Br. J. Nutr. 2016, 116, 434–442. [Google Scholar] [CrossRef]
- Monk, T.H. Enhancing circadian zeitgebers. Sleep 2010, 33, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 2001, 63, 647–676. [Google Scholar] [CrossRef]
- Coomans, C.P.; van den Berg, S.A.; Houben, T.; van Klinken, J.B.; van den Berg, R.; Pronk, A.C.; Havekes, L.M.; Romijn, J.A.; van Dijk, K.W.; Biermasz, N.R.; et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013, 27, 1721–1732. [Google Scholar] [CrossRef]
- Kraves, S.; Weitz, C.J. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat. Neurosci. 2006, 9, 212–219. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Bullock, C.M.; Li, C.; Lee, A.G.; Bermak, J.C.; Belluzzi, J.; Weaver, D.R.; Leslie, F.M.; Zhou, Q.Y. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 2002, 417, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Schibler, U.; Ripperger, J.; Brown, S.A. Peripheral circadian oscillators in mammals: Time and food. J. Biol. Rhythm. 2003, 18, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Azzi, A. Peripheral circadian oscillators in mammals. In Circadian Clocks; Springer: Berlin/Heidelberg, Germany, 2013; pp. 45–66. [Google Scholar]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. Plos Med. 2011, 8, e1001141. [Google Scholar] [CrossRef]
- Parkes, K.R. Shift work and age as interactive predictors of body mass index among offshore workers. Scand. J. Work Environ. Health 2002, 28, 64–71. [Google Scholar] [CrossRef]
- Reutrakul, S.; Van Cauter, E. Interactions between sleep, circadian function, and glucose metabolism: Implications for risk and severity of diabetes. Ann. N. Y. Acad Sci. 2014, 1311, 151–173. [Google Scholar] [CrossRef]
- Kawano, H.; Motoyama, T.; Yasue, H.; Hirai, N.; Waly, H.M.; Kugiyama, K.; Ogawa, H. Endothelial function fluctuates with diurnal variation in the frequency of ischemic episodes in patients with variant angina. J. Am. Coll. Cardiol. 2002, 40, 266–270. [Google Scholar] [CrossRef]
- Otto, M.E.; Svatikova, A.; Barretto, R.B.; Santos, S.; Hoffmann, M.; Khandheria, B.; Somers, V. Early morning attenuation of endothelial function in healthy humans. Circulation 2004, 109, 2507–2510. [Google Scholar] [CrossRef]
- Walters, J.; Skene, D.; Hampton, S.M.; Ferns, G.A. Biological rhythms, endothelial health and cardiovascular disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2003, 9, RA1-8. [Google Scholar]
- Singh, R.B.; Cornelissen, G.; Weydahl, A.; Schwartzkopff, O.; Katinas, G.; Otsuka, K.; Watanabe, Y.; Yano, S.; Mori, H.; Ichimaru, Y.; et al. Circadian heart rate and blood pressure variability considered for research and patient care. Int. J. Cardiol. 2003, 87, 9–28. [Google Scholar] [CrossRef]
- Panza, J.A.; Epstein, S.E.; Quyyumi, A.A. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N. Engl. J. Med. 1991, 325, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Marler, J.R.; Price, T.R.; Clark, G.L.; Muller, J.E.; Robertson, T.; Mohr, J.P.; Hier, D.B.; Wolf, P.A.; Caplan, L.R.; Foulkes, M.A. Morning increase in onset of ischemic stroke. Stroke 1989, 20, 473–476. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, N.; Kumar, H.; Niazi, R.A.; Rashid, M.F. Circadian Variation In The Onset Of Acute Myocardial Infarction In Diabetics. J. Ayub Med. Coll. Abbottabad Jamc 2018, 30, 71–73. [Google Scholar]
- Chua, E.C.; Shui, G.; Lee, I.T.; Lau, P.; Tan, L.C.; Yeo, S.C.; Lam, B.D.; Bulchand, S.; Summers, S.A.; Puvanendran, K.; et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 14468–14473. [Google Scholar] [CrossRef]
- Lange, T.; Dimitrov, S.; Born, J. Effects of sleep and circadian rhythm on the human immune system. Ann. N. Y. Acad Sci. 2010, 1193, 48–59. [Google Scholar] [CrossRef]
- Lucassen, E.A.; Coomans, C.P.; van Putten, M.; de Kreij, S.R.; van Genugten, J.H.; Sutorius, R.P.; de Rooij, K.E.; van der Velde, M.; Verhoeve, S.L.; Smit, J.W.; et al. Environmental 24-h Cycles Are Essential for Health. Curr. Biol. 2016, 26, 1843–1853. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Murray, V.; Berge, E.; del Zoppo, G.J. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst. Rev. 2014, 7, CD000213. [Google Scholar] [CrossRef] [PubMed]
- Carmona, P.; Mendez, N.; Ili, C.G.; Brebi, P. The Role of Clock Genes in Fibrinolysis Regulation: Circadian Disturbance and Its Effect on Fibrinolytic Activity. Front. Physiol. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Butler, M.P.; Shea, S.A. Role of the circadian system in cardiovascular disease. J. Clin. Invest. 2018, 128, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Anderson, T.J. Fundamentals of endothelial function for the clinical cardiologist. Circulation 2002, 105, 546–549. [Google Scholar] [CrossRef]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Paschos, G.K.; FitzGerald, G.A. Circadian clocks and vascular function. Circ. Res. 2010, 106, 833–841. [Google Scholar] [CrossRef]
- Nernpermpisooth, N.; Qiu, S.; Mintz, J.D.; Suvitayavat, W.; Thirawarapan, S.; Rudic, D.R.; Fulton, D.J.; Stepp, D.W. Obesity alters the peripheral circadian clock in the aorta and microcirculation. Microcirculation 2015, 22, 257–266. [Google Scholar] [CrossRef]
- Kanabrocki, E.L.; George, M.; Hermida, R.C.; Messmore, H.L.; Ryan, M.D.; Ayala, D.E.; Hoppensteadt, D.A.; Fareed, J.; Bremner, F.W.; Third, J.L.; et al. Day-night variations in blood levels of nitric oxide, T-TFPI, and E-selectin. Clin. Appl. Thromb. Hemost. Off. J. Int. Acad. Clin. Appl. Thromb. Hemost. 2001, 7, 339–345. [Google Scholar] [CrossRef]
- Tabas, I.; Bornfeldt, K.E. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.J.; Durrington, H.J.; Gibbs, J.E.; Blaikley, J.; Loudon, A.S.; Ray, D.W.; Sabroe, I. A matter of time: Study of circadian clocks and their role in inflammation. J. Leukoc. Biol. 2016, 99, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Schloss, M.J.; Horckmans, M.; Nitz, K.; Duchene, J.; Drechsler, M.; Bidzhekov, K.; Scheiermann, C.; Weber, C.; Soehnlein, O.; Steffens, S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol. Med. 2016, 8, 937–948. [Google Scholar] [CrossRef]
- Fearnley, G.R.; Balmforth, G.; Fearnley, E. Evidence of a diurnal fibrinolytic rhythm; with a simple method of measuring natural fibrinolysis. Clin. Sci. 1957, 16, 645–650. [Google Scholar]
- Haus, E.; Cusulos, M.; Sackett-Lundeen, L.; Swoyer, J. Circadian variations in blood coagulation parameters, alpha-antitrypsin antigen and platelet aggregation and retention in clinically healthy subjects. Chronobiol. Int. 1990, 7, 203–216. [Google Scholar] [CrossRef]
- Undar, L.; Turkay, C.; Korkmaz, L. Circadian variation in circulating platelet aggregates. Ann. Med. 1989, 21, 429–433. [Google Scholar] [CrossRef]
- Jafri, S.M.; VanRollins, M.; Ozawa, T.; Mammen, E.F.; Goldberg, A.D.; Goldstein, S. Circadian variation in platelet function in healthy volunteers. Am. J. Cardiol. 1992, 69, 951–954. [Google Scholar] [CrossRef]
- Kapiotis, S.; Jilma, B.; Quehenberger, P.; Ruzicka, K.; Handler, S.; Speiser, W. Morning hypercoagulability and hypofibrinolysis. Diurnal variations in circulating activated factor VII, prothrombin fragment F1+2, and plasmin-plasmin inhibitor complex. Circulation 1997, 96, 19–21. [Google Scholar] [CrossRef]
- Rudnicka, A.R.; Rumley, A.; Lowe, G.D.; Strachan, D.P. Diurnal, seasonal, and blood-processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation 2007, 115, 996–1003. [Google Scholar] [CrossRef]
- Kanabrocki, E.L.; Sothern, R.B.; Messmore, H.L.; Roitman-Johnson, B.; McCormick, J.B.; Dawson, S.; Bremner, F.W.; Third, J.L.; Nemchausky, B.A.; Shirazi, P.; et al. Circadian interrelationships among levels of plasma fibrinogen, blood platelets, and serum interleukin-6. Clin. Appl. Thromb. Hemost. Off. J. Int. Acad. Clin. Appl. Thromb. Hemost. 1999, 5, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, F.; Kluft, C. Circadian variation of fibrinolytic activity in blood. Chronobiol. Int. 1991, 8, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Shea, S.A. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 2014, 123, 590–593. [Google Scholar] [CrossRef]
- Undar, L.; Ertugrul, C.; Altunbas, H.; Akca, S. Circadian variations in natural coagulation inhibitors protein C, protein S and antithrombin in healthy men: A possible association with interleukin-6. Thromb. Haemost. 1999, 81, 571–575. [Google Scholar] [PubMed]
- Takeda, N.; Maemura, K.; Horie, S.; Oishi, K.; Imai, Y.; Harada, T.; Saito, T.; Shiga, T.; Amiya, E.; Manabe, I.; et al. Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J. Biol. Chem. 2007, 282, 32561–32567. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.A.; Martino, T.A.; Tata, N.; Ralph, M.R.; Sole, M.J.; Belsham, D.D. Vascular circadian rhythms in a mouse vascular smooth muscle cell line (Movas-1). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1529–R1538. [Google Scholar] [CrossRef] [PubMed]
- Haus, E. Chronobiology of hemostasis and inferences for the chronotherapy of coagulation disorders and thrombosis prevention. Adv. Drug Deliv Rev. 2007, 59, 966–984. [Google Scholar] [CrossRef]
- Farajnia, S.; Deboer, T.; Rohling, J.H.; Meijer, J.H.; Michel, S. Aging of the suprachiasmatic clock. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2014, 20, 44–55. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Hood, S.; Amir, S. The aging clock: Circadian rhythms and later life. J. Clin. Invest. 2017, 127, 437–446. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, B.; Wang, X.; Luo, C.; Zhou, T.; Zheng, X.; Ding, J. Circadian rhythm and atherosclerosis (Review). Exp. Ther. Med. 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, C.S.; Swirski, F.K. Circadian Influence on Metabolism and Inflammation in Atherosclerosis. Circ. Res. 2016, 119, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Crnko, S.; Du Pre, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Bunger, M.K.; Walisser, J.A.; Sullivan, R.; Manley, P.A.; Moran, S.M.; Kalscheur, V.L.; Colman, R.J.; Bradfield, C.A. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis 2005, 41, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Buurma, M.; van Diemen, J.J.K.; Thijs, A.; Numans, M.E.; Bonten, T.N. Circadian Rhythm of Cardiovascular Disease: The Potential of Chronotherapy With Aspirin. Front. Cardiovasc. Med. 2019, 6, 84. [Google Scholar] [CrossRef]
- Anea, C.B.; Zhang, M.; Stepp, D.W.; Simkins, G.B.; Reed, G.; Fulton, D.J.; Rudic, R.D. Vascular disease in mice with a dysfunctional circadian clock. Circulation 2009, 119, 1510–1517. [Google Scholar] [CrossRef]
- Kunieda, T.; Minamino, T.; Katsuno, T.; Tateno, K.; Nishi, J.; Miyauchi, H.; Orimo, M.; Okada, S.; Komuro, I. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ. Res. 2006, 98, 532–539. [Google Scholar] [CrossRef]
- Wang, Y.; Pati, P.; Xu, Y.; Chen, F.; Stepp, D.W.; Huo, Y.; Rudic, R.D.; Fulton, D.J. Endotoxin Disrupts Circadian Rhythms in Macrophages via Reactive Oxygen Species. PLoS ONE 2016, 11, e0155075. [Google Scholar] [CrossRef]
- Chen, S.; Fuller, K.K.; Dunlap, J.C.; Loros, J.J. A Pro- and Anti-inflammatory Axis Modulates the Macrophage Circadian Clock. Front. Immunol. 2020, 11, 867. [Google Scholar] [CrossRef]
- Ramesh, V.; Nair, D.; Zhang, S.X.; Hakim, F.; Kaushal, N.; Kayali, F.; Wang, Y.; Li, R.C.; Carreras, A.; Gozal, D. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-alpha pathway. J. Neuroinflamm. 2012, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Schauer, I.E.; Knaub, L.A.; Lloyd, M.; Watson, P.A.; Gliwa, C.; Lewis, K.E.; Chait, A.; Klemm, D.J.; Gunter, J.M.; Bouchard, R.; et al. CREB downregulation in vascular disease: A common response to cardiovascular risk. Arter. Thromb Vasc. Biol. 2010, 30, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, G.; Schlage, W.K.; Boue, S.; Veljkovic, E.; Peitsch, M.C.; Hoeng, J. The Apoe(-/-) mouse model: A suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction. J. Transl. Med. 2016, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lu, C.; Hua, L.; Jin, H.; Yin, L.; Chen, S.; Qian, R. Rhythm changes of clock genes, apoptosis-related genes and atherosclerosis-related genes in apolipoprotein E knockout mice. Can. J. Cardiol. 2009, 25, 473–479. [Google Scholar] [CrossRef][Green Version]
- Chalfant, J.M.; Howatt, D.A.; Tannock, L.R.; Daugherty, A.; Pendergast, J.S. Circadian disruption with constant light exposure exacerbates atherosclerosis in male ApolipoproteinE-deficient mice. Sci. Rep. 2020, 10, 9920. [Google Scholar] [CrossRef] [PubMed]
- Schilperoort, M.; van den Berg, R.; Bosmans, L.A.; van Os, B.W.; Dolle, M.E.T.; Smits, N.A.M.; Guichelaar, T.; van Baarle, D.; Koemans, L.; Berbee, J.F.P.; et al. Disruption of circadian rhythm by alternating light-dark cycles aggravates atherosclerosis development in APOE*3-Leiden.CETP mice. J. Pineal Res. 2020, 68, e12614. [Google Scholar] [CrossRef]
- Nosaka, M.; Ishida, Y.; Kimura, A. Influence of Circadian Rhythm on Thrombus Formation of Murine Deep Vein Thrombosis Model. Ann. Hematol. Oncol. 2017, 4, 1171. [Google Scholar] [CrossRef][Green Version]
- Buhr, E.D.; Takahashi, J.S. Molecular components of the Mammalian circadian clock. Handb. Exp. Pharmacol. 2013, 217, 3–27. [Google Scholar] [CrossRef]
- Reilly, D.F.; Westgate, E.J.; FitzGerald, G.A. Peripheral circadian clocks in the vasculature. Arter. Thromb. Vasc. Biol. 2007, 27, 1694–1705. [Google Scholar] [CrossRef]
- Froy, O.; Chang, D.C.; Reppert, S.M. Redox potential: Differential roles in dCRY and mCRY1 functions. Curr. Biol. 2002, 12, 147–152. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Ueda, H.R.; Hayashi, S.; Chen, W.; Sano, M.; Machida, M.; Shigeyoshi, Y.; Iino, M.; Hashimoto, S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 2005, 37, 187–192. [Google Scholar] [CrossRef]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; FitzGerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Fentress, S.J.; Qiu, Y.; Yun, K.; Cox, J.S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 2013, 341, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Silver, A.C.; Arjona, A.; Walker, W.E.; Fikrig, E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 2012, 36, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Onder, Y.; Green, C.B. Rhythms of metabolism in adipose tissue and mitochondria. Neurobiol. Sleep Circadian Rhythm. 2018, 4, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Cheng, Y.; Kapoor, S.; Reilly, D.; Price, T.S.; FitzGerald, G.A. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc. Natl. Acad. Sci. USA 2007, 104, 3450–3455. [Google Scholar] [CrossRef]
- Cheng, B.; Anea, C.B.; Yao, L.; Chen, F.; Patel, V.; Merloiu, A.; Pati, P.; Caldwell, R.W.; Fulton, D.J.; Rudic, R.D. Tissue-intrinsic dysfunction of circadian clock confers transplant arteriosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108, 17147–17152. [Google Scholar] [CrossRef]
- Yang, G.; Chen, L.; Grant, G.R.; Paschos, G.; Song, W.L.; Musiek, E.S.; Lee, V.; McLoughlin, S.C.; Grosser, T.; Cotsarelis, G.; et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci. Transl. Med. 2016, 8, 324ra316. [Google Scholar] [CrossRef]
- Huo, M.; Huang, Y.; Qu, D.; Zhang, H.; Wong, W.T.; Chawla, A.; Huang, Y.; Tian, X.Y. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2017, 31, 1097–1106. [Google Scholar] [CrossRef]
- Somanath, P.R.; Podrez, E.A.; Chen, J.; Ma, Y.; Marchant, K.; Antoch, M.; Byzova, T.V. Deficiency in core circadian protein Bmal1 is associated with a prothrombotic and vascular phenotype. J. Cell Physiol. 2011, 226, 132–140. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimba, S.; Tezuka, M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol. Pharm Bull. 2007, 30, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Fagundes, C.T.; Yang, G.; Palsson-McDermott, E.M.; Wochal, P.; McGettrick, A.F.; Foley, N.H.; Early, J.O.; Chen, L.; Zhang, H.; et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA 2015, 112, 7231–7236. [Google Scholar] [CrossRef] [PubMed]
- Spengler, M.L.; Kuropatwinski, K.K.; Comas, M.; Gasparian, A.V.; Fedtsova, N.; Gleiberman, A.S.; Gitlin, I.I.; Artemicheva, N.M.; Deluca, K.A.; Gudkov, A.V.; et al. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc. Natl. Acad. Sci. USA 2012, 109, E2457–E2465. [Google Scholar] [CrossRef] [PubMed]
- Westgate, E.J.; Cheng, Y.; Reilly, D.F.; Price, T.S.; Walisser, J.A.; Bradfield, C.A.; FitzGerald, G.A. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation 2008, 117, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Bhatwadekar, A.D.; Beli, E.; Diao, Y.; Chen, J.; Luo, Q.; Alex, A.; Caballero, S.; Dominguez, J.M., II; Salazar, T.E.; Busik, J.V.; et al. Conditional Deletion of Bmal1 Accentuates Microvascular and Macrovascular Injury. Am. J. Pathol. 2017, 187, 1426–1435. [Google Scholar] [CrossRef]
- Xie, Z.; Su, W.; Liu, S.; Zhao, G.; Esser, K.; Schroder, E.A.; Lefta, M.; Stauss, H.M.; Guo, Z.; Gong, M.C. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Invest. 2015, 125, 324–336. [Google Scholar] [CrossRef]
- Dubrovsky, Y.V.; Samsa, W.E.; Kondratov, R.V. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging Us 2010, 2, 936–944. [Google Scholar] [CrossRef]
- Antoch, M.P.; Gorbacheva, V.Y.; Vykhovanets, O.; Toshkov, I.A.; Kondratov, R.V.; Kondratova, A.A.; Lee, C.; Nikitin, A.Y. Disruption of the circadian clock due to the Clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle 2008, 7, 1197–1204. [Google Scholar] [CrossRef]
- Rudic, R.D.; McNamara, P.; Curtis, A.M.; Boston, R.C.; Panda, S.; Hogenesch, J.B.; FitzGerald, G.A. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. Plos Biol. 2004, 2, 1893–1899. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Jiang, X.C.; Hussain, M.M. Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. Circulation 2013, 128, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tong, X.; Arthurs, B.; Guha, A.; Rui, L.; Kamath, A.; Inoki, K.; Yin, L. Liver clock protein BMAL1 promotes de novo lipogenesis through insulin-mTORC2-AKT signaling. J. Biol. Chem. 2014, 289, 25925–25935. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Ishii, N.; Ohta, Y.; Ohno, T.; Watabe, Y.; Hayashi, M.; Wada, T.; Aoyagi, T.; Tezuka, M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 12071–12076. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chu, Y.; Wang, L.; Wang, Y.; Zhao, X.; He, W.; Zhang, P.; Yang, X.; Liu, X.; Tian, L.; et al. Overexpression of CRY1 protects against the development of atherosclerosis via the TLR/NF-kappaB pathway. Int. Immunopharmacol. 2015, 28, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Narasimamurthy, R.; Hatori, M.; Nayak, S.K.; Liu, F.; Panda, S.; Verma, I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 12662–12667. [Google Scholar] [CrossRef]
- Lamia, K.A.; Papp, S.J.; Yu, R.T.; Barish, G.D.; Uhlenhaut, N.H.; Jonker, J.W.; Downes, M.; Evans, R.M. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 2011, 480, 552–556. [Google Scholar] [CrossRef]
- Miki, T.; Chen-Goodspeed, M.; Zhao, Z.; Lee, C.C. Circadian behavior of mice deficient in PER1/PML or PER2/PML. J. Circadian Rhythm. 2013, 11, 9. [Google Scholar] [CrossRef]
- Lowrey, P.L.; Takahashi, J.S. Genetics of Circadian Rhythms in Mammalian Model Organisms. Adv. Genet. 2011, 74, 175–230. [Google Scholar] [CrossRef]
- Lee, C.C. The circadian clock and tumor suppression by mammalian Period genes. Method Enzym. 2005, 393, 852–861. [Google Scholar] [CrossRef]
- Albrecht, U.; Zheng, B.H.; Larkin, D.; Sun, Z.S.; Lee, C.C. mPer1 and mPer2 are essential for normal resetting of the circadian clock. J. Biol. Rhythm. 2001, 16, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, B.; Bellet, M.M.; Katada, S.; Astarita, G.; Hirayama, J.; Amin, R.H.; Granneman, J.G.; Piomelli, D.; Leff, T.; Sassone-Corsi, P. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010, 12, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Rousso-Noori, L.; Zwighaft, Z.; Neufeld-Cohen, A.; Golik, M.; Kraut-Cohen, J.; Wang, M.; Han, X.; Asher, G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014, 19, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wen, M.S.; Wang, H.W.; Hsieh, I.C.; Li, Y.X.; Liu, P.Y.; Lin, F.C.; Liao, J.K. Increased Vascular Senescence and Impaired Endothelial Progenitor Cell Function Mediated by Mutation of Circadian Gene Per2. Circulation 2008, 118, 2166–2173. [Google Scholar] [CrossRef]
- Viswambharan, H.; Carvas, J.M.; Antic, V.; Marecic, A.; Jud, C.; Zaugg, C.E.; Ming, X.F.; Montani, J.P.; Albrecht, U.; Yang, Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 2007, 115, 2188–2195. [Google Scholar] [CrossRef]
- Bhatwadekar, A.D.; Yan, Y.; Qi, X.; Thinschmidt, J.S.; Neu, M.B.; Li Calzi, S.; Shaw, L.C.; Dominiguez, J.M.; Busik, J.V.; Lee, C.; et al. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes 2013, 62, 273–282. [Google Scholar] [CrossRef]
- Delezie, J.; Dumont, S.; Dardente, H.; Oudart, H.; Grechez-Cassiau, A.; Klosen, P.; Teboul, M.; Delaunay, F.; Pevet, P.; Challet, E. The nuclear receptor REV-ERBalpha is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012, 26, 3321–3335. [Google Scholar] [CrossRef]
- Jacobi, D.; Liu, S.; Burkewitz, K.; Kory, N.; Knudsen, N.H.; Alexander, R.K.; Unluturk, U.; Li, X.; Kong, X.; Hyde, A.L.; et al. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab. 2015, 22, 709–720. [Google Scholar] [CrossRef]
- Sato, S.; Sakurai, T.; Ogasawara, J.; Takahashi, M.; Izawa, T.; Imaizumi, K.; Taniguchi, N.; Ohno, H.; Kizaki, T. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J. Immunol. 2014, 192, 407–417. [Google Scholar] [CrossRef]
- Ma, H.; Zhong, W.; Jiang, Y.; Fontaine, C.; Li, S.; Fu, J.; Olkkonen, V.M.; Staels, B.; Yan, D. Increased atherosclerotic lesions in LDL receptor deficient mice with hematopoietic nuclear receptor Rev-erbalpha knock- down. J. Am. Heart Assoc. 2013, 2, e000235. [Google Scholar] [CrossRef]
- Sitaula, S.; Billon, C.; Kamenecka, T.M.; Solt, L.A.; Burris, T.P. Suppression of atherosclerosis by synthetic REV-ERB agonist. Biochem. Biophys. Res. Commun. 2015, 460, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Mia, S.; Kane, M.S.; Latimer, M.N.; Reitz, C.J.; Sonkar, R.; Benavides, G.A.; Smith, S.R.; Frank, S.J.; Martino, T.A.; Zhang, J.; et al. Differential effects of REV-ERBalpha/beta agonism on cardiac gene expression, metabolism, and contractile function in a mouse model of circadian disruption. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1487–H1508. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, P.; Emmett, M.J.; Jiang, C.; Uehara, K.; Liu, M.; Adlanmerini, M.; Lazar, M.A. SR9009 has REV-ERB-independent effects on cell proliferation and metabolism. Proc. Natl. Acad. Sci. USA 2019, 116, 12147–12152. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging (Albany N. Y.) 2016, 8, 2290–2307. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Schenk, S.; Imamura, T.; Babendure, J.L.; Sonoda, N.; Bae, E.J.; Oh, D.Y.; Lu, M.; Milne, J.C.; Westphal, C.; et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E419–E428. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Yang, S.R.; Kinnula, V.L.; Rahman, I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir Crit Care Med. 2008, 177, 861–870. [Google Scholar] [CrossRef]
- Schug, T.T.; Xu, Q.; Gao, H.; Peres-da-Silva, A.; Draper, D.W.; Fessler, M.B.; Purushotham, A.; Li, X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell. Biol. 2010, 30, 4712–4721. [Google Scholar] [CrossRef]
- Stein, S.; Schafer, N.; Breitenstein, A.; Besler, C.; Winnik, S.; Lohmann, C.; Heinrich, K.; Brokopp, C.E.; Handschin, C.; Landmesser, U.; et al. SIRT1 reduces endothelial activation without affecting vascular function in ApoE-/- mice. Aging (Albany N. Y.) 2010, 2, 353–360. [Google Scholar] [CrossRef]
- Zeng, H.T.; Fu, Y.C.; Yu, W.; Lin, J.M.; Zhou, L.; Liu, L.; Wang, W. SIRT1 prevents atherosclerosis via liverXreceptor and NFkappaB signaling in a U937 cell model. Mol. Med. Rep. 2013, 8, 23–28. [Google Scholar] [CrossRef]
- Masri, S.; Orozco-Solis, R.; Aguilar-Arnal, L.; Cervantes, M.; Sassone-Corsi, P. Coupling circadian rhythms of metabolism and chromatin remodelling. DiabetesObes. Metab. 2015, 17, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Zhao, T.; Cui, K.; Hu, G.; Chen, Q.; Chen, W.; Wang, X.W.; Soto-Gutierrez, A.; Zhao, K.; Deng, C.X. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci. Rep. 2016, 6, 28633. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Guarente, L. SIRT1 Mediates Central Circadian Control in the SCN by a Mechanism that Decays with Aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Belden, W.J.; Dunlap, J.C. SIRT1 is a circadian deacetylase for core clock components. Cell 2008, 134, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD(+) Biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef]
- Sahar, S.; Masubuchi, S.; Eckel-Mahan, K.; Vollmer, S.; Galla, L.; Ceglia, N.; Masri, S.; Barth, T.K.; Grimaldi, B.; Oluyemi, O.; et al. Circadian control of fatty acid elongation by SIRT1 protein-mediated deacetylation of acetyl-coenzyme A synthetase 1. J. Biol. Chem. 2014, 289, 6091–6097. [Google Scholar] [CrossRef]
- Zhu, Z.; Hua, B.; Shang, Z.; Yuan, G.; Xu, L.; Li, E.; Li, X.; Sun, N.; Yan, Z.; Qian, R.; et al. Altered Clock and Lipid Metabolism-Related Genes in Atherosclerotic Mice Kept with Abnormal Lighting Condition. Biomed. Res. Int. 2016, 2016, 5438589. [Google Scholar] [CrossRef]
- Pearson, R.; Fleetwood, J.; Eaton, S.; Crossley, M.; Bao, S. Kruppel-like transcription factors: A functional family. Int. J. Biochem. Cell Biol. 2008, 40, 1996–2001. [Google Scholar] [CrossRef]
- Mahabeleshwar, G.H.; Kawanami, D.; Sharma, N.; Takami, Y.; Zhou, G.; Shi, H.; Nayak, L.; Jeyaraj, D.; Grealy, R.; White, M.; et al. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity 2011, 34, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Yoshitane, H.; Ozaki, H.; Terajima, H.; Du, N.H.; Suzuki, Y.; Fujimori, T.; Kosaka, N.; Shimba, S.; Sugano, S.; Takagi, T.; et al. CLOCK-controlled polyphonic regulation of circadian rhythms through canonical and noncanonical E-boxes. Mol. Cell. Biol. 2014, 34, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Mattick, J.S.; Yang, Q.; Orman, M.A.; Ierapetritou, M.G.; Berthiaume, F.; Androulakis, I.P. Bioinformatics analysis of transcriptional regulation of circadian genes in rat liver. BMC Bioinform. 2014, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Hergenreider, E.; Heydt, S.; Treguer, K.; Boettger, T.; Horrevoets, A.J.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef]
- Pathak, R.; Shao, L.; Chafekar, S.M.; Feng, W.; Ponnappan, U.; Fink, L.M.; Zhou, D.; Hauer-Jensen, M. IKKbeta regulates endothelial thrombomodulin in a Klf2-dependent manner. J. Thromb. Haemost. 2014, 12, 1533–1544. [Google Scholar] [CrossRef]
- Lin, Z.; Hamik, A.; Jain, R.; Kumar, A.; Jain, M.K. Kruppel-like factor 2 inhibits protease activated receptor-1 expression and thrombin-mediated endothelial activation. Arter. Thromb. Vasc. Biol. 2006, 26, 1185–1189. [Google Scholar] [CrossRef]
- Atkins, G.B.; Wang, Y.; Mahabeleshwar, G.H.; Shi, H.; Gao, H.; Kawanami, D.; Natesan, V.; Lin, Z.; Simon, D.I.; Jain, M.K. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ. Res. 2008, 103, 690–693. [Google Scholar] [CrossRef]
- Nayak, L.; Shi, H.; Atkins, G.B.; Lin, Z.; Schmaier, A.H.; Jain, M.K. The thromboprotective effect of bortezomib is dependent on the transcription factor Kruppel-like factor 2 (KLF2). Blood 2014, 123, 3828–3831. [Google Scholar] [CrossRef]
- Lingrel, J.B.; Pilcher-Roberts, R.; Basford, J.E.; Manoharan, P.; Neumann, J.; Konaniah, E.S.; Srinivasan, R.; Bogdanov, V.Y.; Hui, D.Y. Myeloid-specific Kruppel-like factor 2 inactivation increases macrophage and neutrophil adhesion and promotes atherosclerosis. Circ. Res. 2012, 110, 1294–1302. [Google Scholar] [CrossRef]
- Das, H.; Kumar, A.; Lin, Z.; Patino, W.D.; Hwang, P.M.; Feinberg, M.W.; Majumder, P.K.; Jain, M.K. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 6653–6658. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Martin, M.; Zhang, J.; Huang, H.Y.; Bai, L.; Zhang, J.; Kang, J.; He, M.; Li, J.; Maurya, M.R.; et al. Kruppel-Like Factor 4 Regulation of Cholesterol-25-Hydroxylase and Liver X Receptor Mitigates Atherosclerosis Susceptibility. Circulation 2017, 136, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Hamik, A.; Nayak, L.; Tian, H.; Shi, H.; Lu, Y.; Sharma, N.; Liao, X.; Hale, A.; Boerboom, L.; et al. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J. Clin. Invest. 2012, 122, 4727–4731. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Lu, Y.; Zhou, G.; Liao, X.; Kapil, P.; Anand, P.; Mahabeleshwar, G.H.; Stamler, J.S.; Jain, M.K. Myeloid Kruppel-like factor 4 deficiency augments atherogenesis in ApoE-/- mice--brief report. Arter. Thromb. Vasc. Biol. 2012, 32, 2836–2838. [Google Scholar] [CrossRef] [PubMed]
- Guillaumond, F.; Grechez-Cassiau, A.; Subramaniam, M.; Brangolo, S.; Peteri-Brunback, B.; Staels, B.; Fievet, C.; Spelsberg, T.C.; Delaunay, F.; Teboul, M. Kruppel-like factor KLF10 is a link between the circadian clock and metabolism in liver. Mol. Cell. Biol. 2010, 30, 3059–3070. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Sun, L.; Zhang, H.; Yao, L.; Cui, X.; Gao, Y.; Fang, F.; Chang, Y. KLF10 transcription factor regulates hepatic glucose metabolism in mice. Diabetologia 2017, 60, 2443–2452. [Google Scholar] [CrossRef]
- Wei, X.; Yang, R.; Wang, C.; Jian, X.; Li, L.; Liu, H.; Yang, G.; Li, Z. A novel role for the Kruppel-like factor 14 on macrophage inflammatory response and atherosclerosis development. Cardiovasc. Pathol. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Tabas, I. Consequences of cellular cholesterol accumulation: Basic concepts and physiological implications. J. Clin. Investig. 2002, 110, 905–911. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Liao, X.; Sangwung, P.; Prosdocimo, D.A.; Zhou, G.; Votruba, A.R.; Brian, L.; Han, Y.J.; Gao, H.; et al. Kruppel-like factor 15 is critical for vascular inflammation. J. Clin. Investig. 2013, 123, 4232–4241. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Tien, C.L.; Jain, M.K.; Zhang, L. Kruppel-Like Factor 15 Regulates the Circadian Susceptibility to Ischemia Reperfusion Injury in the Heart. Circulation 2020, 141, 1427–1429. [Google Scholar] [CrossRef]
- Man, A.W.C.; Xia, N.; Daiber, A.; Li, H. The roles of gut microbiota and circadian rhythm in the cardiovascular protective effects of polyphenols. Br. J. Pharm. 2020, 177, 1278–1293. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Sheen, J.M.; Hu, W.L.; Hung, Y.C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Portillo, M.P.; Madrid, J.A.; Arias, N.; Macarulla, M.T.; Garaulet, M. Effects of resveratrol on changes induced by high-fat feeding on clock genes in rats. Br. J. Nutr. 2013, 110, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.Y.; Mi, Y.S.; Liu, Z.G.; Fan, R.; Qiao, Q.L.; Sun, Y.L.; Ren, B.; Liu, X.B. Dietary tea polyphenols ameliorate metabolic syndrome and memory impairment via circadian clock related mechanisms. J. Funct. Foods 2017, 34, 168–180. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvado, M.J.; Arola, L.; Blade, C. Chronic consumption of dietary proanthocyanidins modulates peripheral clocks in healthy and obese rats. J. Nutr. Biochem. 2015, 26, 112–119. [Google Scholar] [CrossRef]
- Guo, T.; Ho, C.T.; Zhang, X.; Cao, J.; Wang, H.; Shao, X.; Pan, D.; Wu, Z. Oolong Tea Polyphenols Ameliorate Circadian Rhythm of Intestinal Microbiome and Liver Clock Genes in Mouse Model. J. Agric. Food Chem. 2019, 67, 11969–11976. [Google Scholar] [CrossRef]
- Song, D.; Yang, C.S.; Zhang, X.; Wang, Y. The relationship between host circadian rhythms and intestinal microbiota: A new cue to improve health by tea polyphenols. Crit. Rev. Food Sci. Nutr. 2021, 61, 139–148. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Zhao, B.; Tan, X.; Wang, L.; Liu, X. Role of Food Phytochemicals in the Modulation of Circadian Clocks. J. Agric. Food Chem. 2019, 67, 8735–8739. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Song, Y.; Cheng, X.R.; Xia, S.; Rahman, M.R.; Shi, Y.; Le, G. Resveratrol restores the circadian rhythmic disorder of lipid metabolism induced by high-fat diet in mice. Biochem. Biophys. Res. Commun. 2015, 458, 86–91. [Google Scholar] [CrossRef]
- Zhou, L.; Long, J.; Sun, Y.; Chen, W.; Qiu, R.; Yuan, D. Resveratrol ameliorates atherosclerosis induced by high-fat diet and LPS in ApoE(-/-) mice and inhibits the activation of CD4(+) T cells. Nutr. Metab. 2020, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, Q.; Shi, L.; Qin, L.; Zhang, Q.; Mi, M. Equol Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice by Inhibiting Endoplasmic Reticulum Stress via Activation of Nrf2 in Endothelial Cells. PLoS ONE 2016, 11, e0167020. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, P.; Xu, S.; Koroleva, M.; Zhang, S.; Si, S.; Jin, Z.G. Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci. Rep. 2017, 7, 6686. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Shen, Y.A.; Hung, P.H.; Yu, Y.B.; Chen, Y.J. Epigallocathechin gallate, polyphenol present in green tea, inhibits stem-like characteristics and epithelial-mesenchymal transition in nasopharyngeal cancer cell lines. BMC Complementary Altern. Med. 2012, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Suarez, M.; Bravo, F.I.; Muguerza, B. Changes in arterial blood pressure caused by long-term administration of grape seed proanthocyanidins in rats with established hypertension. Food Funct. 2020, 11, 8735–8742. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Moore, M.W.; Ha, N.P.; Egbejimi, O.; Fields, A.; Mbawuike, U.; Egbejimi, A.; Shaw, C.A.; Bray, M.S.; Nannegari, V.; et al. Circadian rhythms in myocardial metabolism and contractile function: Influence of workload and oleate. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2385–H2393. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, A.W.C.; Li, H.; Xia, N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. Int. J. Mol. Sci. 2021, 22, 676. https://doi.org/10.3390/ijms22020676
Man AWC, Li H, Xia N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. International Journal of Molecular Sciences. 2021; 22(2):676. https://doi.org/10.3390/ijms22020676
Chicago/Turabian StyleMan, Andy W. C., Huige Li, and Ning Xia. 2021. "Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis" International Journal of Molecular Sciences 22, no. 2: 676. https://doi.org/10.3390/ijms22020676
APA StyleMan, A. W. C., Li, H., & Xia, N. (2021). Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. International Journal of Molecular Sciences, 22(2), 676. https://doi.org/10.3390/ijms22020676




