Inhibitory Neural Network’s Impairments at Hippocampal CA1 LTP in an Aged Transgenic Mouse Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
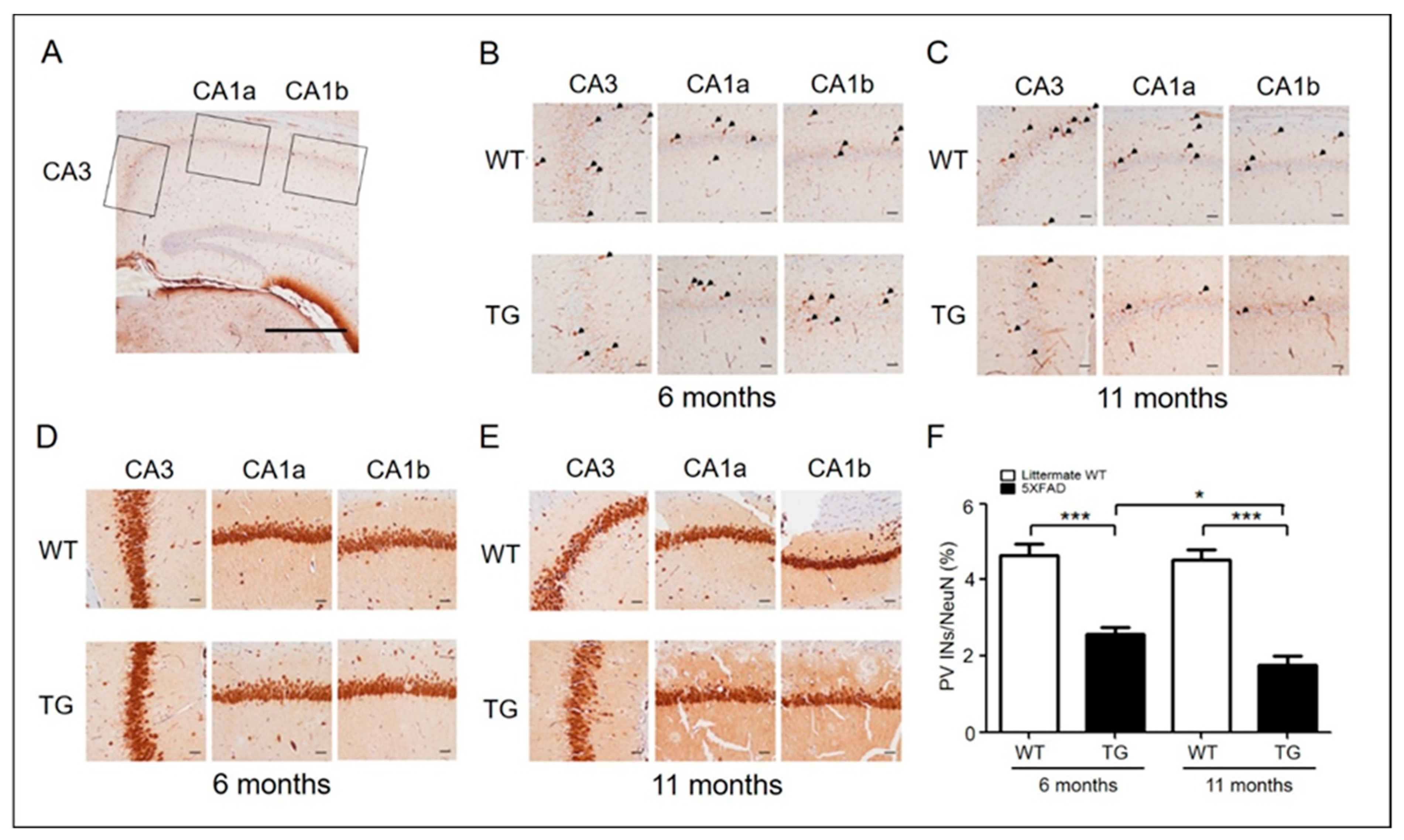
2.1. PV Interneurons Decreased in Hippocampus CA1-3 Region of Aged 5xFAD Mice
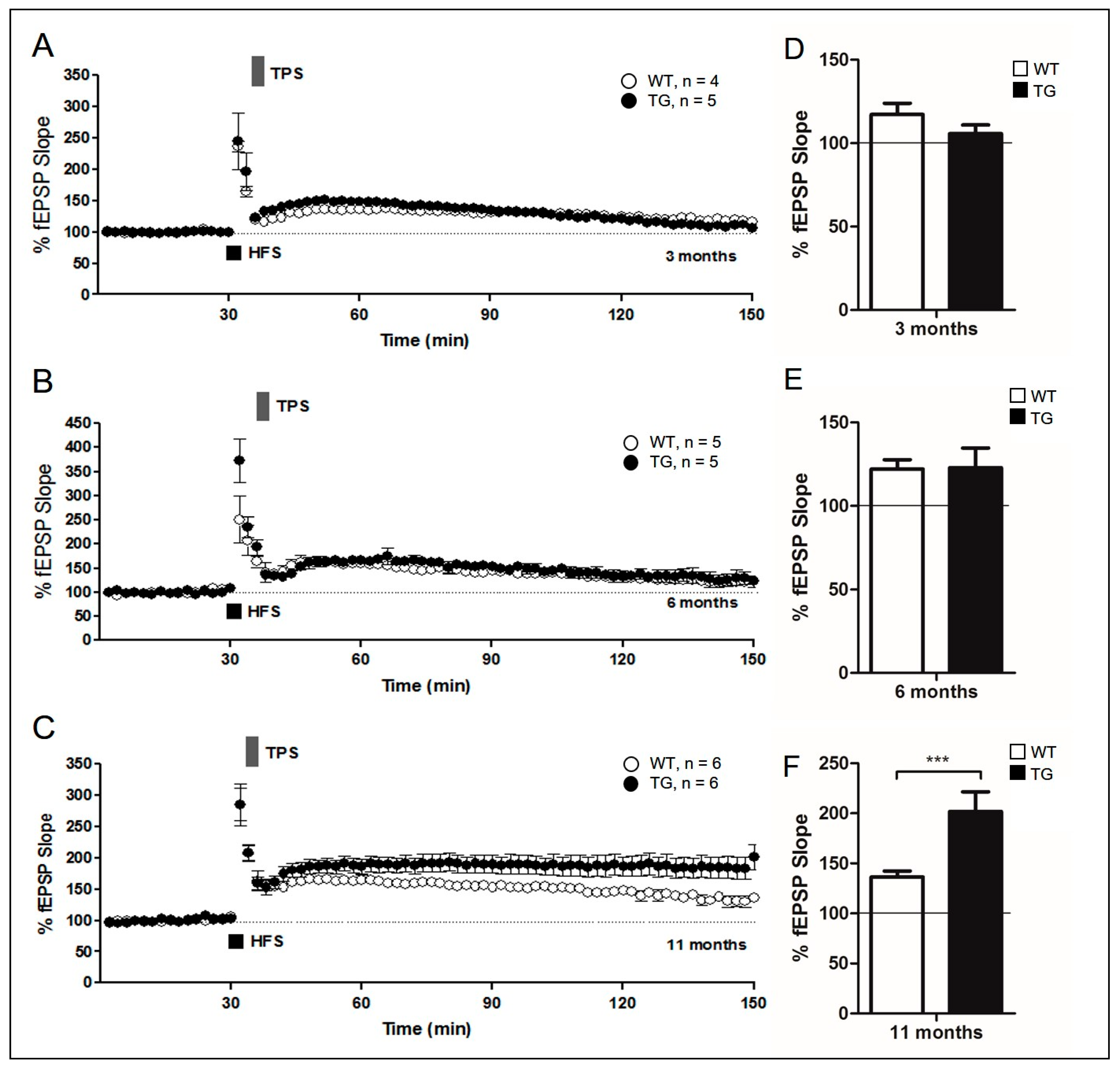
2.2. Evaluation of Synaptic Plasticity in Aged AD Mice
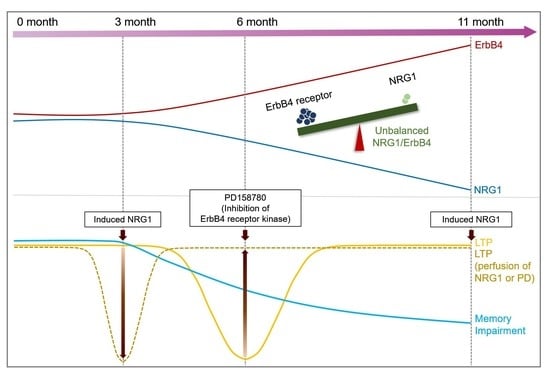
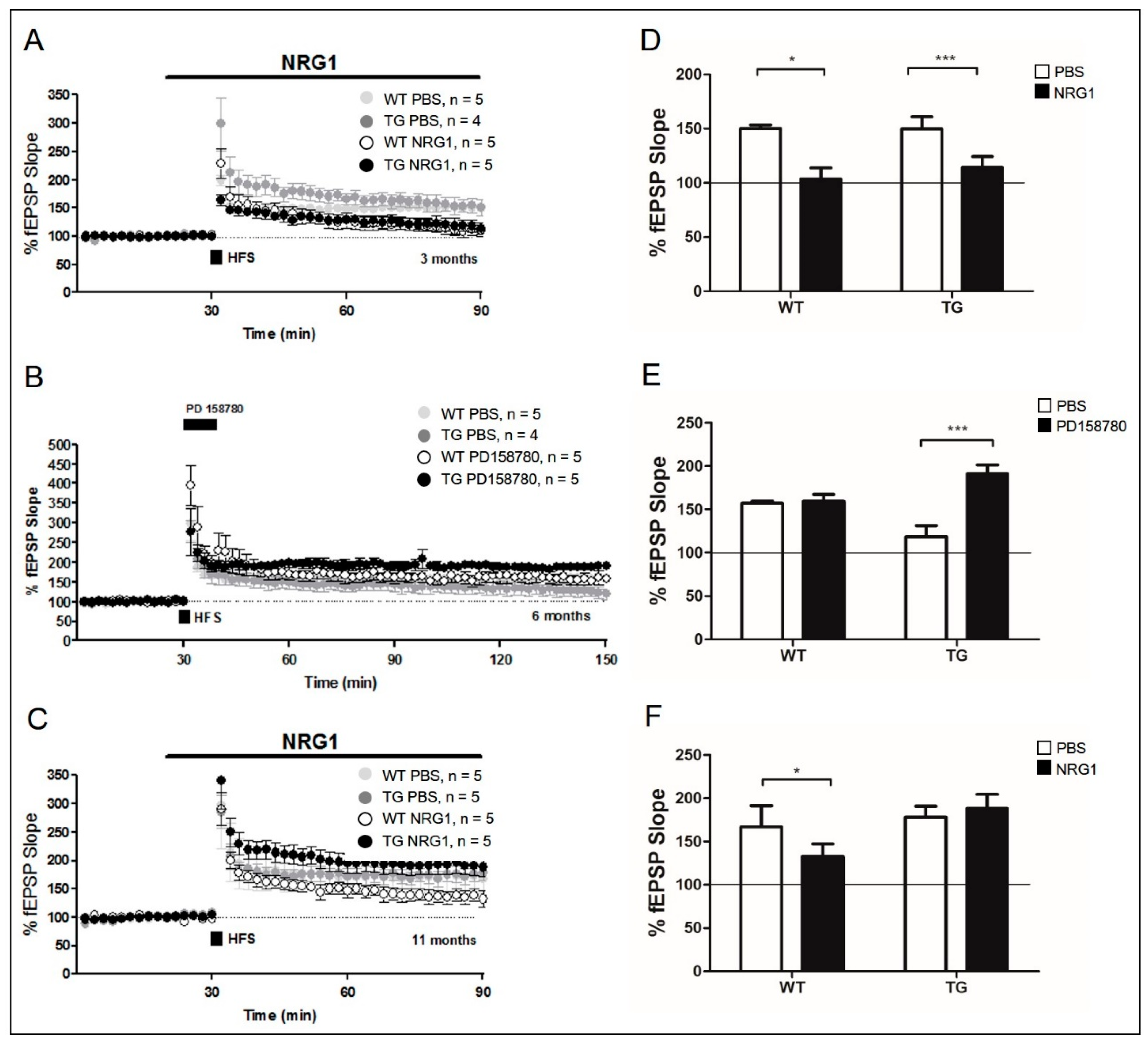
2.3. Effect of NRG1-ErbB Signaling in Aged AD Mice
2.4. Evaluation of Depontentiation-Induced NRG1-ErbB Signaling in Aged 5xFAD Mice
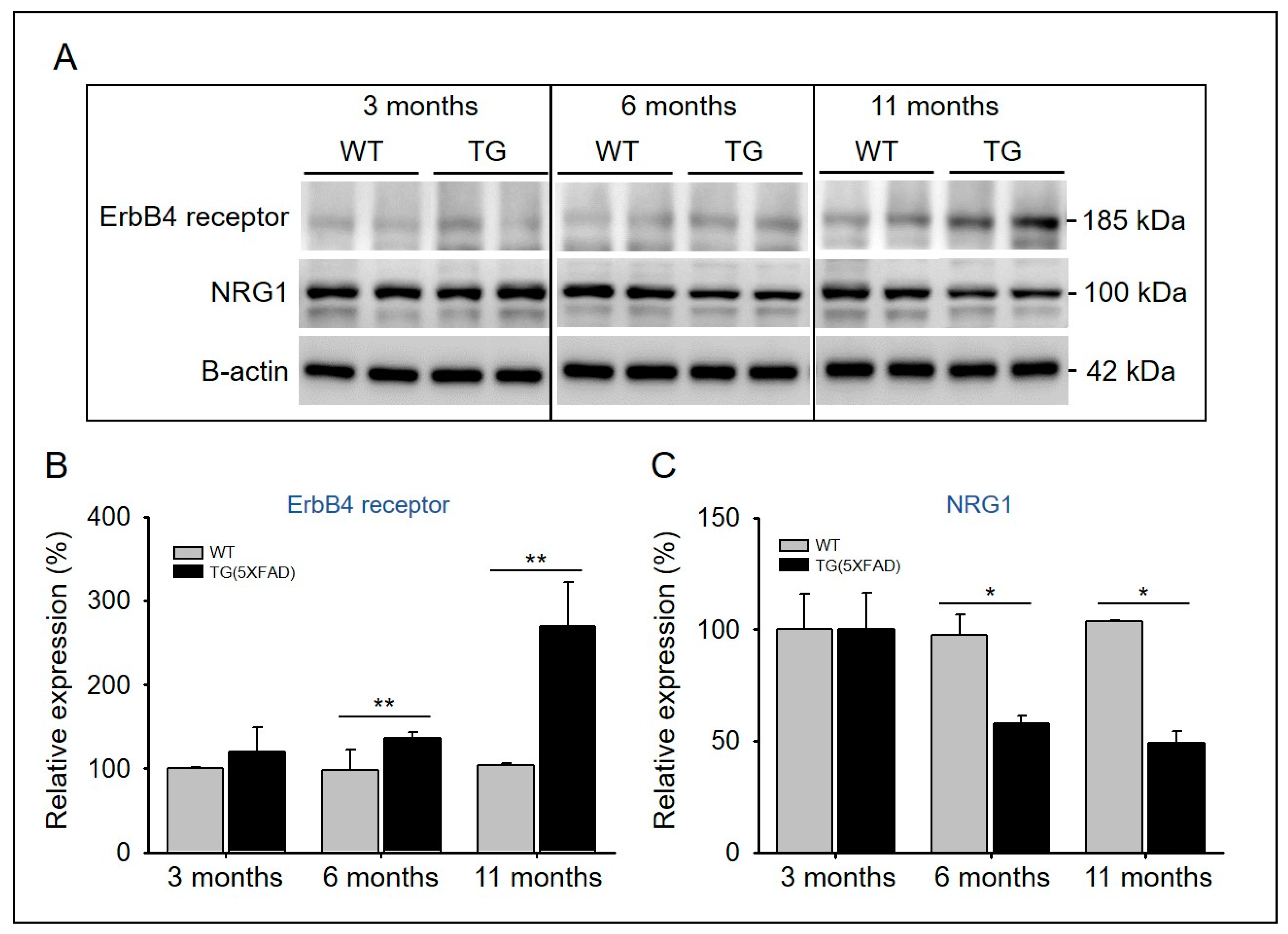
2.5. Imbalance between NRG1 and ErbB4 Receptor Proteins in Aged 5xFAD Mice
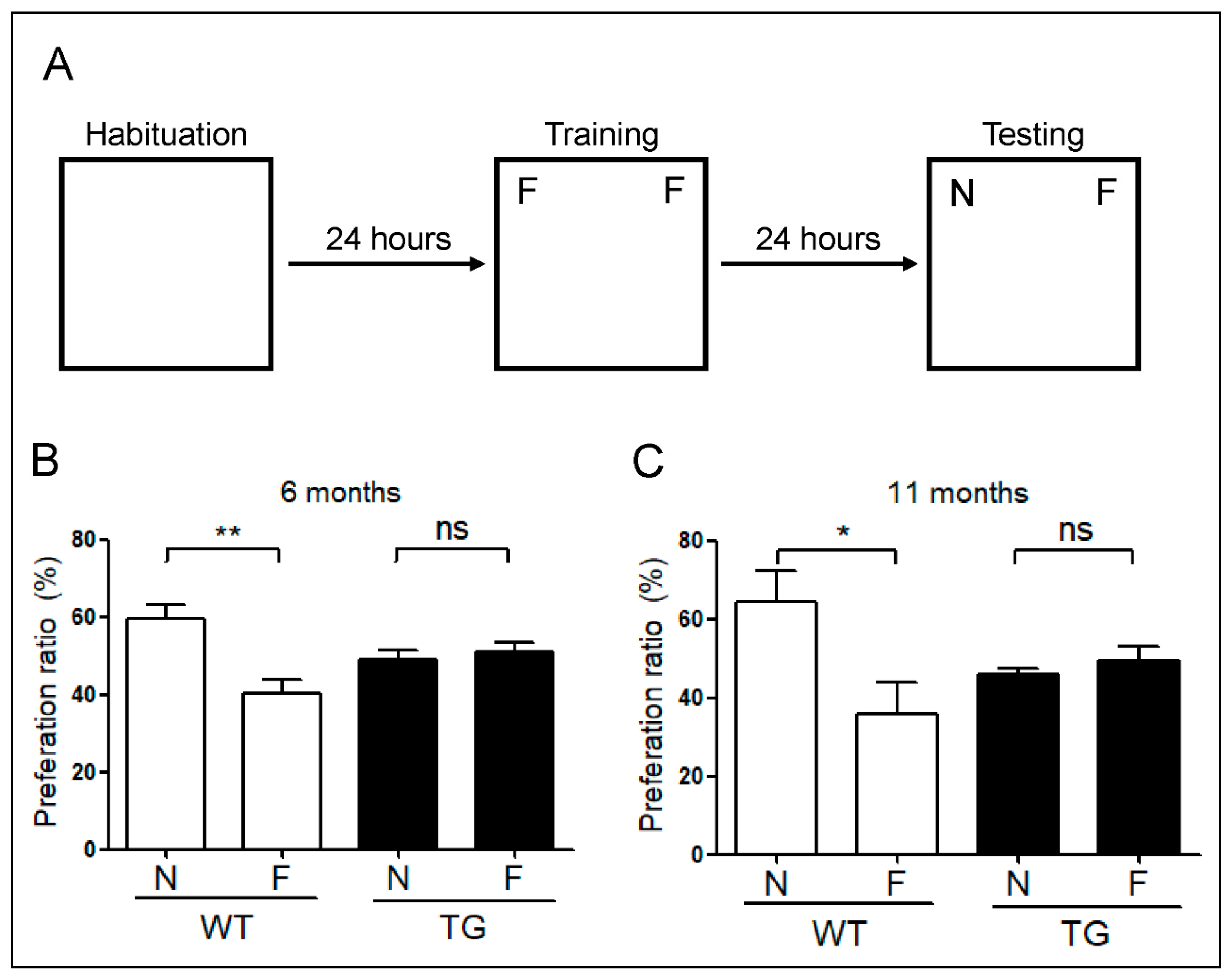
2.6. Evaluation of Memory Impairment in Aged 5xFAD Mice
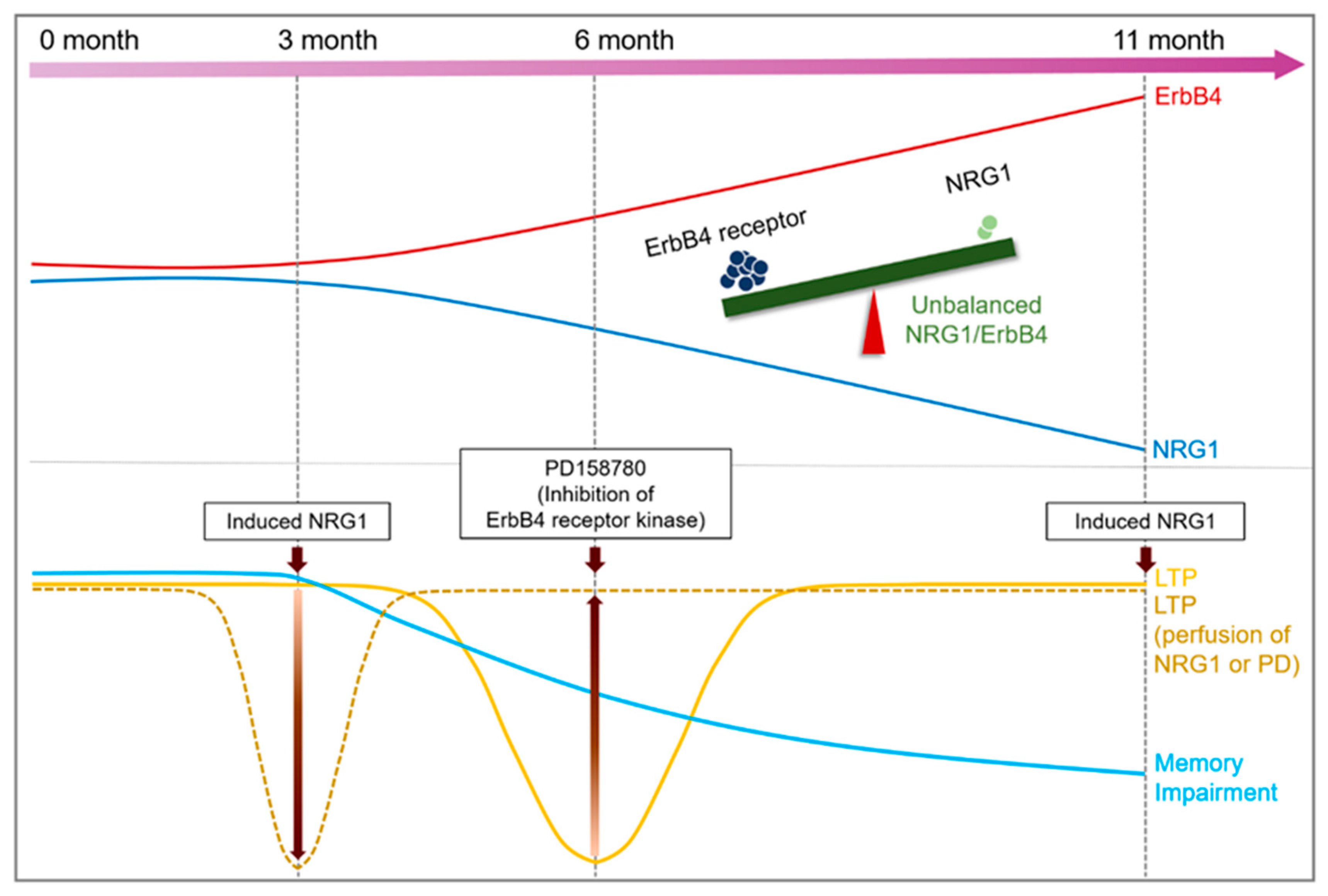
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Hippocampal Slice Preparation
4.4. Electrophysiology
4.5. Western Blot Analysis
4.6. Immunohistochemistry
4.7. Object Recognition Test
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid β |
| aCSF | Artificial cerebrospinal fluid |
| APP | Amyloid precursor protein |
| CA | Cornu ammonis |
| EDTA | Ethylene diamine tetraacetic acid |
| FAD | Familial Alzheimer’s disease |
| fEPSP | Field excitatory postsynaptic potential |
| GABA | Gamma amino butyric acid |
| HFS | High-frequency stimulation |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| NRG1 | Neuregulin 1 |
| NeuN | Neuronal nuclei |
| PV | Parvalbumin |
| PSEN1 | Presenilin-1 |
| PSEN2 | Presenilin-2 |
| SEM | Standard error of the mean |
| TPS | Theta pulse stimulation |
| TG | Transgenic |
| WT | Wild type |
References
- Van Hoesen, G.W.; Hyman, B.T.; Damasio, A.R. Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus 1991, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol. Scand. 1996, 94, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Isla, T.; Price, J.L.; McKeel, D.W., Jr.; Morris, J.C.; Growdon, J.H.; Hyman, B.T. Profound Loss of Layer II Entorhinal Cortex Neurons Occurs in Very Mild Alzheimer’s Disease. J. Neurosci. 1996, 16, 4491–4500. [Google Scholar] [CrossRef] [PubMed]
- Perl, D.P. Neuropathology of Alzheimer’s Disease. Mt. Sinai J. Med. J. Transl. Pers. Med. 2010, 77, 32–42. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef]
- DeTure, M.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Maarouf, C.L.; Kokjohn, T.A.; Whiteside, C.M.; Macias, M.P.; Kalback, W.M.; Sabbagh, M.N.; Beach, T.G.; Vassar, R.; E Roher, A. Molecular Differences and Similarities between Alzheimer’s Disease and the 5XFAD Transgenic Mouse Model of Amyloidosis. Biochem. Insights 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Satoh, J.; Tabira, T.; Sano, M.; Nakayama, H.; Tateishi, J. Parvalbumin-immunoreactive neurons in the human central nervous system are decreased in Alzheimer’s disease. Acta Neuropathol. 1991, 81, 388–395. [Google Scholar] [CrossRef]
- Zallo, F.; Gardenal, E.; Verkhratsky, A.; Rodríguez, J.J. Loss of calretinin and parvalbumin positive interneurones in the hippocampal CA1 of aged Alzheimer’s disease mice. Neurosci. Lett. 2018, 681, 19–25. [Google Scholar] [CrossRef]
- Flanigan, T.J.; Xue, Y.; Rao, S.K.; Dhanushkodi, A.; McDonald, M.P. Abnormal vibrissa-related behavior and loss of barrel field inhibitory neurons in 5xFAD transgenics. Genes Brain Behav. 2014, 13, 488–500. [Google Scholar] [CrossRef]
- Takahashi, H.; Brasnjevic, I.; Rutten, B.P.F.; Van Der Kolk, N.; Perl, D.P.; Bouras, C.; Steinbusch, H.W.M.; Schmitz, C.; Hof, P.R.; Dickstein, D.L. Hippocampal interneuron loss in an APP/PS1 double mutant mouse and in Alzheimer’s disease. Brain Struct. Funct. 2010, 214, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mejias, E.; Nuñez-Diaz, C.; Sanchez-Varo, R.; Gomez-Arboledas, A.; Garcia-Leon, J.A.; Fernandez-Valenzuela, J.J.; Mejias-Ortega, M.; Trujillo-Estrada, L.; Baglietto-Vargas, D.; Moreno-Gonzalez, I.; et al. Distinct disease-sensitive GABAergic neurons in the perirhinal cortex of Alzheimer’s mice and patients. Brain Pathol. 2019, 30, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Ribak, C.E.; Nitsch, R.; Seress, L. Proportion of parvalbumin-positive basket cells in the GABAergic innervation of pyramidal and granule cells of the rat hippocampal formation. J. Comp. Neurol. 1990, 300, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Klausberger, T.; Somogyi, P. Neuronal Diversity and Temporal Dynamics: The Unity of Hippocampal Circuit Operations. Science 2008, 321, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Klausberger, T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur. J. Neurosci. 2009, 30, 947–957. [Google Scholar] [CrossRef]
- Lodge, D.J.; Behrens, M.M.; Grace, A.A. A Loss of Parvalbumin-Containing Interneurons Is Associated with Diminished Oscillatory Activity in an Animal Model of Schizophrenia. J. Neurosci. 2009, 29, 2344–2354. [Google Scholar] [CrossRef]
- Stelzer, A.; Slater, N.T.; Bruggencate, G.T. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature 1987, 326, 698–701. [Google Scholar] [CrossRef]
- Stelzer, A.; Simon, G.; Kovacs, G.; Rai, R. Synaptic disinhibition during maintenance of long-term potentiation in the CA1 hippocampal subfield. Proc Natl Acad Sci USA 1994, 91, 3058–3062. [Google Scholar] [CrossRef]
- McLean, A.H.; Caillard, O.; Ben-Ari, Y.; Gaiarsa, J.-L. Bidirectional plasticity expressed by GABAergic synapses in the neonatal rat hippocampus. J. Physiol. 1996, 496, 471–477. [Google Scholar] [CrossRef]
- Mei, L.; Nave, K.-A. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 2014, 83, 27–49. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Zhang, M.; Yin, D.-M.; Wen, L.; Ting, A.; Wang, P.; Lu, Y.-S.; Zhu, X.-H.; Li, S.-J.; Wu, C.-Y.; et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 21818–21823. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Won, S.; Ali, D.W.; Wang, Q.; Tanowitz, M.; Du, Q.S.; Pelkey, K.A.; Yang, D.J.; Xiong, W.C.; Salter, M.W.; et al. Regulation of Neuregulin Signaling by PSD-95 Interacting with ErbB4 at CNS Synapses. Neuron 2000, 26, 443–455. [Google Scholar] [CrossRef]
- Pitcher, G.M.; Beggs, S.; Woo, R.-S.; Mei, L.; Salter, M.W. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. NeuroReport 2008, 19, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.-B.; Longart, M.; Vullhorst, D.; Hoffman, D.A.; Buonanno, A. Neuregulin-1 Reverses Long-Term Potentiation at CA1 Hippocampal Synapses. J. Neurosci. 2005, 25, 9378–9383. [Google Scholar] [CrossRef]
- Bezaire, M.J.; Soltesz, I. Quantitative assessment of CA1 local circuits: Knowledge base for interneuron-pyramidal cell connectivity. Hippocampus 2013, 23, 751–785. [Google Scholar] [CrossRef]
- Takács, V.T.; Szőnyi, A.; Freund, T.F.; Nyiri, G.; Gulyás, A.I. Quantitative ultrastructural analysis of basket and axo-axonic cell terminals in the mouse hippocampus. Brain Struct. Funct. 2015, 220, 919–940. [Google Scholar] [CrossRef]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal GABAergic inhibitory interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Neuman, K.M.; Molina-Campos, E.; Musial, T.F.; Price, A.L.; Oh, K.-J.; Wolke, M.L.; Buss, E.W.; Scheff, S.W.; Mufson, E.J.; Nicholson, D.A. Evidence for Alzheimer’s disease-linked synapse loss and compensation in mouse and human hippocampal CA1 pyramidal neurons. Brain Struct. Funct. 2015, 220, 3143–3165. [Google Scholar] [CrossRef]
- Huh, S.; Baek, S.-J.; Lee, K.-H.; Whitcomb, D.; Jo, J.; Choi, S.-M.; Kim, N.H.; Park, M.-S.; Lee, K.H.; Kim, B.C. The reemergence of long-term potentiation in aged Alzheimer’s disease mouse model. Sci. Rep. 2016, 6, 29152. [Google Scholar] [CrossRef]
- Shamir, A.; Kwon, O.-B.; Karavanova, I.; Vullhorst, D.; Leiva-Salcedo, E.; Janssen, M.J.; Buonanno, A.; Karavanov, I. The Importance of the NRG-1/ErbB4 Pathway for Synaptic Plasticity and Behaviors Associated with Psychiatric Disorders. J. Neurosci. 2012, 32, 2988–2997. [Google Scholar] [CrossRef] [PubMed]
- Bin Kwon, O.; Paredes, D.; Gonzalez, C.M.; Neddens, J.; Hernandez, L.; Vullhorst, D.; Buonanno, A. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 15587–15592. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Zorumski, C.F. Neuregulin and Dopamine D4 Receptors Contribute Independently to Depotentiation of Schaffer Collateral LTP by Temperoammonic Path Stimulation. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C.; Anwyl, R.; Rowan, M.J. Stimulation on the Positive Phase of Hippocampal Theta Rhythm Induces Long-Term Potentiation That Can Be Depotentiated by Stimulation on the Negative Phase in Area CA1In Vivo. J. Neurosci. 1997, 17, 6470–6477. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Hsu, K.-S.; Hsu, C.-C.H.K.-S. Progress in Understanding the Factors Regulating Reversibility of Long-term Potentiation. Rev. Neurosci. 2001, 12, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Staubli, U.; Chun, D. Factors regulating the reversibility of long-term potentiation. J. Neurosci. 1996, 16, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; De Winter, F.; Farrokhi, C.; Rockenstein, E.; Mante, M.; Adame, A.; Cook, J.; Jin, X.; Masliah, E.; Lee, K.-F. Neuregulin 1 improves cognitive deficits and neuropathology in an Alzheimer’s disease model. Sci. Rep. 2016, 6, 31692. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Yoo, J.-Y.; Kim, O.-S.; Kim, H.-B.; Ryu, J.; Kim, H.-S.; Lee, J.-H.; Yoo, H.-I.; Song, D.-Y.; Baik, T.-K.; et al. Neuregulin 1 regulates amyloid precursor protein cell surface expression and non-amyloidogenic processing. J. Pharmacol. Sci. 2018, 137, 146–153. [Google Scholar] [CrossRef]
- Kimura, R.; Ohno, M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol. Dis. 2009, 33, 229–235. [Google Scholar] [CrossRef]
- Eimer, A.W.; Vassar, R. Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Aβ42 accumulation and Caspase-3 activation. Mol. Neurodegener. 2013, 8, 2. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Zhou, D.; He, X.; Wang, D.; Pan, H.; Zhang, X.; Mei, Y.; Qian, Q.; Zheng, T.; et al. Ablating ErbB4 in PV neurons attenuates synaptic and cognitive deficits in an animal model of Alzheimer’s disease. Neurobiol. Dis. 2017, 106, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Liu, J.Y.; Chen, X.W.; Zou, W.J.; Wu, J.L.; Rodriguez, G.; Peng, C.; Tian, J.; Lu, G.F. ErbB4 Receptors in the Medial Habenula Regulate Contextual Fear Memory. Pharmacology 2019, 103, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Geng, F.; Gao, F.; Chen, Y.-H.; Liu, J.-H.; Wu, J.-L.; Lan, Y.-J.; Zeng, Y.-N.; Li, X.-W.; Yang, J.-M.; et al. Down-Regulation of Neuregulin1/ErbB4 Signaling in the Hippocampus Is Critical for Learning and Memory. Mol. Neurobiol. 2017, 54, 3976–3987. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, X.-D.; Hou, F.-Q.; Bi, L.-L.; Yin, N.-M.; Liu, F.; Chen, Y.-J.; Bean, J.C.; Jiao, H.-F.; Liu, X.; et al. Maintenance of GABAergic Activity by Neuregulin 1-ErbB4 in Amygdala for Fear Memory. Neuron 2014, 84, 835–846. [Google Scholar] [CrossRef]
- Wagner, J.J.; Alger, B.E. Homosynaptic LTD and depotentiation: Do they differ in name only? Hippocampus 1996, 6, 24–29. [Google Scholar] [CrossRef]
- Sachser, R.M.; Santana, F.; Crestani, A.P.; Lunardi, P.; Pedraza, L.K.; Quillfeldt, J.A.; Hardt, O.; Alvares, L.D.O. Forgetting of long-term memory requires activation of NMDA receptors, L-type voltage-dependent Ca2+ channels, and calcineurin. Sci. Rep. 2016, 6, 22771. [Google Scholar] [CrossRef]
- Chaudhury, A.R.; Gerecke, K.M.; Wyss, J.M.; Morgan, D.G.; Gordon, M.N.; Carroll, S.L. Neuregulin-1 and ErbB4 Immunoreactivity Is Associated with Neuritic Plaques in Alzheimer Disease Brain and in a Transgenic Model of Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2003, 62, 42–54. [Google Scholar] [CrossRef]
- Woo, R.-S.; Lee, J.-H.; Yu, H.-N.; Song, D.-Y.; Baik, T.-K. Expression of ErbB4 in the neurons of Alzheimer’s disease brain and APP/PS1 mice, a model of Alzheimer’s disease. Anat. Cell Biol. 2011, 44, 116–127. [Google Scholar] [CrossRef]
- Li, B.; Woo, R.-S.; Mei, L.; Malinow, R. The Neuregulin-1 Receptor ErbB4 Controls Glutamatergic Synapse Maturation and Plasticity. Neuron 2007, 54, 583–597. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J.; Moryś, J.; Kusiak, A. Computer Model of Synapse Loss During an Alzheimer’s Disease-like Pathology in Hippocampal Subregions DG, CA3 and CA1—the Way to Chaos and Information Transfer. Entropy 2019, 21, 408. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.J.; Park, J.E.; Choi, S.-M.; Kim, T.; Cho, S.H.; Lee, K.-H.; Song, W.K.; Song, J.; Jeong, H.-S.; Kim, D.H.; et al. Inhibitory Neural Network’s Impairments at Hippocampal CA1 LTP in an Aged Transgenic Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 698. https://doi.org/10.3390/ijms22020698
Seo HJ, Park JE, Choi S-M, Kim T, Cho SH, Lee K-H, Song WK, Song J, Jeong H-S, Kim DH, et al. Inhibitory Neural Network’s Impairments at Hippocampal CA1 LTP in an Aged Transgenic Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2021; 22(2):698. https://doi.org/10.3390/ijms22020698
Chicago/Turabian StyleSeo, Hyeon Jeong, Jung Eun Park, Seong-Min Choi, Taekyoung Kim, Soo Hyun Cho, Kyung-Hwa Lee, Woo Keun Song, Juhyun Song, Han-Seong Jeong, Dong Hyun Kim, and et al. 2021. "Inhibitory Neural Network’s Impairments at Hippocampal CA1 LTP in an Aged Transgenic Mouse Model of Alzheimer’s Disease" International Journal of Molecular Sciences 22, no. 2: 698. https://doi.org/10.3390/ijms22020698
APA StyleSeo, H. J., Park, J. E., Choi, S.-M., Kim, T., Cho, S. H., Lee, K.-H., Song, W. K., Song, J., Jeong, H.-S., Kim, D. H., & Kim, B. C. (2021). Inhibitory Neural Network’s Impairments at Hippocampal CA1 LTP in an Aged Transgenic Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences, 22(2), 698. https://doi.org/10.3390/ijms22020698