Why Is the Invasive Plant Sphagneticola trilobata More Resistant to High Temperature Than Its Native Congener?
Abstract
1. Introduction
2. Results
2.1. Changes in Plant Appearance and Photosynthetic Pigments under HT Treatment
2.2. Changes in Photosynthetic Capacity under HT Treatment
2.3. Changes in Reactive Oxygen Species (ROS) and Cellular Membrane Stability under HT Treatment
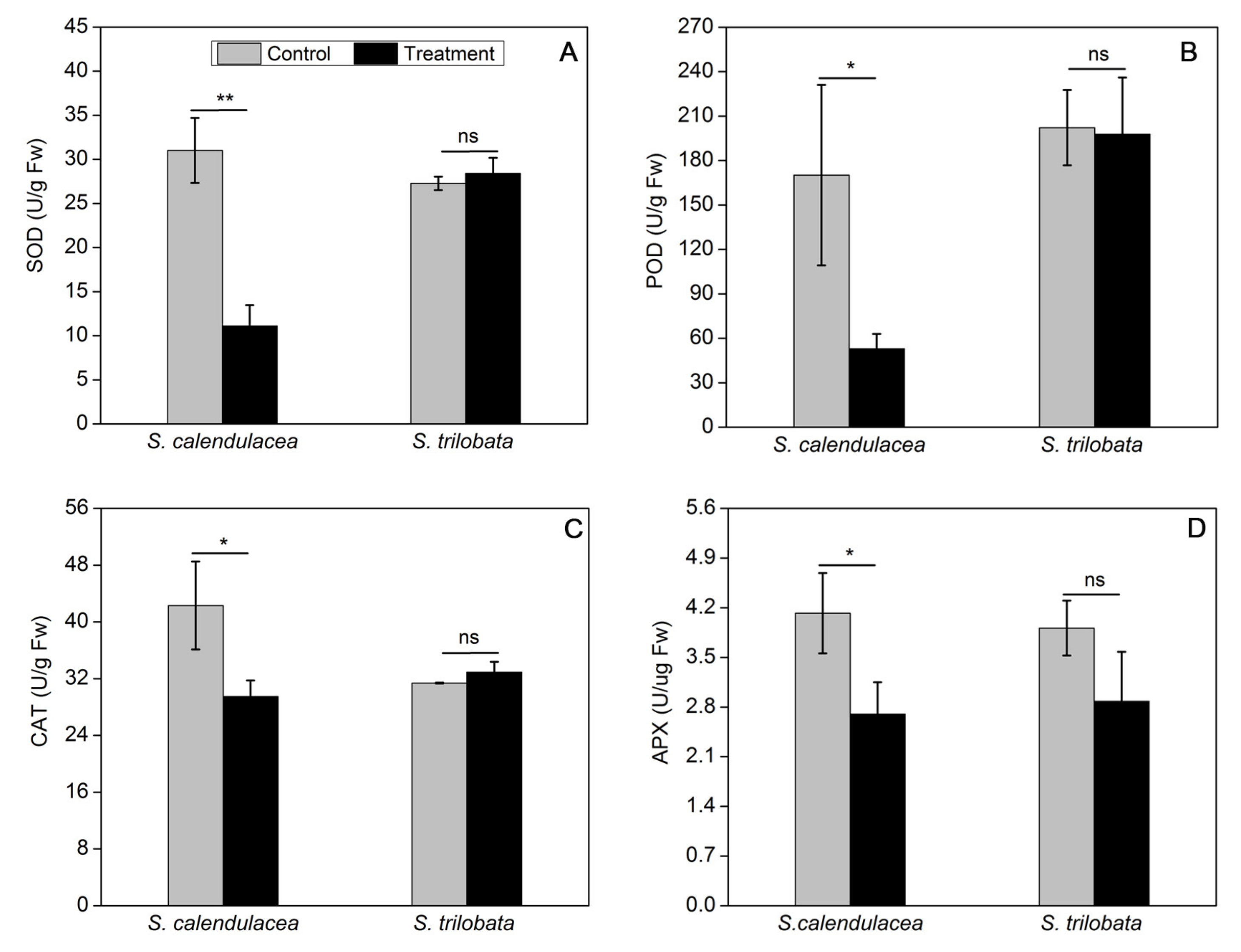
2.4. Changes in Non-Enzymatic Antioxidant Content and Antioxidant Enzyme Activity under HT Treatment
2.5. Changes in the Expression of Genes Encoding Photosynthesis and Antioxidant Enzymes under HT Treatment
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Cultivation, and HT Treatment
4.2. Gas Exchange and Chlorophyll Fluorescence Measurements
4.3. Chlorophyll Content Determination
4.4. Hydrogen Peroxide (H2O2) Histochemical Staining and H2O2 Content Measurements
4.5. Cell Membrane Leakage Rate and Malondialdehyde (MDA) Content Analysis
4.6. Enzymatic and Non-Enzymatic Antioxidants Determination
4.7. Gene Expression Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Schellnhuber, H.J. Global warming: Stop worrying, start panicking? Proc. Natl. Acad. Sci. USA 2008, 105, 14239–14240. [Google Scholar] [CrossRef]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Sintayehu, D.W. Impact of climate change on biodiversity and associated key ecosystem services in Africa: A systematic review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Ibáñez, I.; Blumenthal, D.M.; Molinari, N.A.; Miller, L.P.; Grosholz, E.D.; Diez, J.M.; D’Antonio, C.M.; Olden, J.D.; Jones, S.J.; et al. Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol. Lett. 2018, 16, 261–270. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Peng, S.; Zobel, K. Climate Warming May Facilitate Invasion of the Exotic Shrub Lantana camara. PLoS ONE 2014, 9, e105500. [Google Scholar] [CrossRef]
- IUCN. 100 of the World’s Worst Invasive Alien Species; Invasive Species Specialist Group: Auckland, New Zealand, 2001. [Google Scholar]
- Xie, Y.; Li, Z.; William, P.; Dianmo, L. Invasive species in China an overview. Biodivers. Conserv. 2001, 10, 1317–1341. [Google Scholar]
- Song, L.; Chow, W.S.; Sun, L.; Li, C.; Peng, C. Acclimation of photosystem II to high temperature in two Wedelia species from different geographical origins: Implications for biological invasions upon global warming. J. Exp. Bot. 2010, 61, 4087–4096. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Salvucci, M.; Crafts-Brandner, S. Relationship between the Heat Tolerance of Photosynthesis and the Thermal Stability of Rubisco Activase in Plants from Contrasting Thermal Environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Boyle, D.L.; Welti, R.; Jagadish, S.V.K.; Prasad, P.V.V. Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 2018, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Zhang, X.; Li, Z.; Peng, Y. Effects of Heat Acclimation on Photosynthesis, Antioxidant Enzyme Activities, and Gene Expression in Orchardgrass under Heat Stress. Molecules 2014, 19, 13564–13576. [Google Scholar] [CrossRef]
- Song, L.; Sun, L.; Shu, Z.; Zeng, W.; Li, W.; Peng, C. Effects of drought stress and rehydration on chlorophyll fluorescence characteristics in leaves of invasive Wedelia trìlobata. Acta Ecol. Sin. 2009, 29, 3713–3721. [Google Scholar]
- Cai, M.; Ding, W.; Zhai, J.; Zheng, X.; Yu, Z.; Zhang, Q.; Lin, X.; Chow, W.S.; Peng, C. Photosynthetic compensation of non-leaf organ stems of the invasive species Sphagneticola trilobata (L.) Pruski at low temperature. Photosynth. Res. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Li, G.F.; Sun, H.Q.; Ma, L.; Guo, Y.P.; Zhao, Z.Y.; Gao, H.; Mei, L.X. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Huo, Y.J.; Wang, M.P.; Wei, Y.Y.; Xia, Z.L. Overexpression of the Maize psbA Gene Enhances Drought Tolerance Through Regulating Antioxidant System, Photosynthetic Capability, and Stress Defense Gene Expression in Tobacco. Front. Plant Sci. 2016, 6, 1223. [Google Scholar] [CrossRef]
- Enami, I.; Kitamura, M.; Tomo, T.; Isokawa, Y.; Ohta, H.; Katoh, S. Is the primary cause of thermal inactivation of oxygen evolution in spinach PS II membranes release of the extrinsic 33 kDa protein or of Mn? Biochim. Et Biophys. Acta (Bba) Bioenerg. 1994, 1186, 52–58. [Google Scholar] [CrossRef]
- Li, H.; Ahammed, G.J.; Zhou, G.N.; Xia, X.J.; Zhou, J.; Shi, K.; Yu, J.Q.; Zhou, Y.H. Unraveling Main Limiting Sites of Photosynthesis under Below- and Above-Ground Heat Stress in Cucumber and the Alleviatory Role of Luffa Rootstock. Front. Plant Sci. 2016, 7, 746. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Lu, J.; Gao, Z. Effects of Photosynthetic, PSⅡ Electron Transport and Reactive Oxygen Species on Short-term High Temperature Stress in Tomato Seedlings. North. Hortic. 2019, 43, 1–11. [Google Scholar]
- Crafts-Brandner, S.; Law, R. Effect of heat stress on the inhibition and recovery of the ribulose-1,5-bisphosphate carboxylase/oxygenase activation state. Planta 2000, 212, 67–174. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.; Crafts-Brandner, S. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 2004, 120, 179–1186. [Google Scholar] [CrossRef]
- Liu, M.; Gong, J.; Yang, B.; Ding, Y.; Zhang, Z.; Wang, B.; Zhu, C.; Hou, X. Differences in the photosynthetic and physiological responses of Leymus chinensis to different levels of grazing intensity. BMC Plant Biol. 2019, 19, 558. [Google Scholar] [CrossRef]
- Tardy, F.; Creach, A.; Havaux, M. Photosynthetic pigment concentration, organization and interconversions in a pale green Syrian landrace of barley (Hordeum vulgare L., Tadmor) adapted to harsh climatic conditions. Plant Cell Environ. 1998, 21, 479–489. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Q.; Ci, D.; Shao, X.; Zhang, D. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 2014, 14, 111. [Google Scholar] [CrossRef]
- Liu, A.; Hu, Z.; Bi, A.; Fan, J.; Gitau, M.; Amombo, E.; Chen, L.; Fu, J. Photosynthesis, antioxidant system and gene expression of bermudagrass in response to low temperature and salt stress. Ecotoxicology 2016, 25, 1445–1457. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y. Degradation effects of active oxygen damage on garden plant seedling chlorophyll. J. Cent. South Univ. For. Technol. 2012, 32, 73–77. (In Chinese) [Google Scholar]
- Takeuchi, Y.; Kubo, H.; Kasahara, H.; Sakaki, T. Adaptive Alterations in the Activities of Scavengers of Active Oxygen in Cucumber Cotyledons Irradiated with UV-B. J. Plant Physiol. 1996, 147, 589–592. [Google Scholar] [CrossRef]
- Wang, W.B.; Kim, Y.H.; Lee, H.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhou, P.; Huang, B. Antioxidant enzymatic activities and gene expression associated with heat tolerance in a cool-season perennial grass species. Environ. Exp. Bot. 2013, 87, 159–166. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Evers, D.; Dommes, J. Ascorbic Acid, Phenolic Acid, Flavonoid, and Carotenoid Profiles of Selected Extracts from Ribes nigrum. J. Agric. Food Chem. 2011, 59, 4763–4770. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, Y.; Zhang, X.; Cai, M.; Yu, Z.; Peng, C. ANS-deficient Arabidopsis is sensitive to high light due to impaired anthocyanin photoprotection. Funct. Plant Biol. 2019, 46, 756–765. [Google Scholar] [CrossRef]
- Wang, H.; Jin, J.Y. Photosynthetic rate, chlorophyll fluorescence parameters, and lipid peroxidation of maize leaves as affected by zinc deficiency. Photosynthetica 2005, 43, 591–596. [Google Scholar] [CrossRef]
- Park, Y.; Jung, S.; Kang, S.; Kang, S.; Heo, B.; Arancibia-Avila, P.; Toledo, F.; Drzewiecki, J.; Namiesnik, J.; Gorinstein, S. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008, 107, 640–648. [Google Scholar] [CrossRef]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, T.; Zheng, J.; Huang, X.; Yu, Z.-C.; Peng, C.-L.; Chow, W.S. Anthocyanins function as a light attenuator to compensate for insufficient photoprotection mediated by nonphotochemical quenching in young leaves of Acmena acuminatissima in winter. Photosynthetica 2018, 56, 445–454. [Google Scholar] [CrossRef]
- Tan, W.; Liu, J.; Dai, T.; Jing, Q.; Cao, W.; Jiang, D. Alterations in photosynthesis and antioxidant enzyme activity in winter wheat subjected to post-anthesis water-logging. Photosynthetica 2008, 46, 21–27. [Google Scholar] [CrossRef]
- Du, H.; Wang, Z.; Huang, B. Differential responses of warm-season and cool season turfgrass species to heat stress associated with antioxidant enzyme activity. J. Am. Soc. Hortic. Sci. 2009, 134, 417–422. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Drought and Heat Stress Injury to Two Cool-Season Turfgrasses in Relation to Antioxidant Metabolism and Lipid Peroxidation. Crop Sci. 2001, 41, 436. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Livak, K.; Schmittgen, T. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





| Gene Name | Primer Sequence |
|---|---|
| GAPDH | Forward: 5′-CTGCTTCATTCAACATC-3′ |
| Reverse: 5′-CTCACGGTCAGATCAACA-3′ | |
| PsbP | Forward: 5′-TGCAGCAAGGGATAAGGATGT-3′ |
| Reverse: 5′-ACAAATGAAAGAGCATGAACAAAGA-3′ | |
| PsbA | Forward: 5′-TGGAGGAGCAGCAATGA-3′ |
| Reverse: 5′-GCGAAAGCGAAAGCCTA-3′ | |
| RubiscoL | Forward: 5′-CGGTCTCTCCAGCGCATAAA-3′ |
| Reverse: 5′-CGCCTCACGGTATCCAAGTT-3′ | |
| SOD | Forward: 5′-TGGTTTGAAAGCGGTGG-3′ |
| Reverse: 5′-CTGGTTTAAGCCCTGTGAT-3′ | |
| POD | Forward: 5′-CAACACCGCAGAGAAAGACT-3′ |
| Reverse: 5′-CTGGGAGGTAAAGAGAAC-3′ | |
| CAT | Forward: 5′-CAAGACCTGGCCTGAG-3′ |
| Reverse: 5′-TGTCTCTGAGTGTCCG-3′ | |
| APX | Forward: 5′-CTAAGGAAGGCAGACTC-3′ |
| Reverse: 5′-CCTGATCTATCTGCATGTG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Lin, X.; Peng, J.; Zhang, J.; Chen, M.; Huang, J.; Chen, L.; Sun, F.; Ding, W.; Peng, C. Why Is the Invasive Plant Sphagneticola trilobata More Resistant to High Temperature Than Its Native Congener? Int. J. Mol. Sci. 2021, 22, 748. https://doi.org/10.3390/ijms22020748
Cai M, Lin X, Peng J, Zhang J, Chen M, Huang J, Chen L, Sun F, Ding W, Peng C. Why Is the Invasive Plant Sphagneticola trilobata More Resistant to High Temperature Than Its Native Congener? International Journal of Molecular Sciences. 2021; 22(2):748. https://doi.org/10.3390/ijms22020748
Chicago/Turabian StyleCai, Minling, Xiaohua Lin, Jindi Peng, Junjie Zhang, Minghao Chen, Jundong Huang, Lihua Chen, Feng Sun, Wenqiao Ding, and Changlian Peng. 2021. "Why Is the Invasive Plant Sphagneticola trilobata More Resistant to High Temperature Than Its Native Congener?" International Journal of Molecular Sciences 22, no. 2: 748. https://doi.org/10.3390/ijms22020748
APA StyleCai, M., Lin, X., Peng, J., Zhang, J., Chen, M., Huang, J., Chen, L., Sun, F., Ding, W., & Peng, C. (2021). Why Is the Invasive Plant Sphagneticola trilobata More Resistant to High Temperature Than Its Native Congener? International Journal of Molecular Sciences, 22(2), 748. https://doi.org/10.3390/ijms22020748




