Stress-Induced Hyperglycaemia in Non-Diabetic Patients with Acute Coronary Syndrome: From Molecular Mechanisms to New Therapeutic Perspectives
Abstract
:1. Introduction
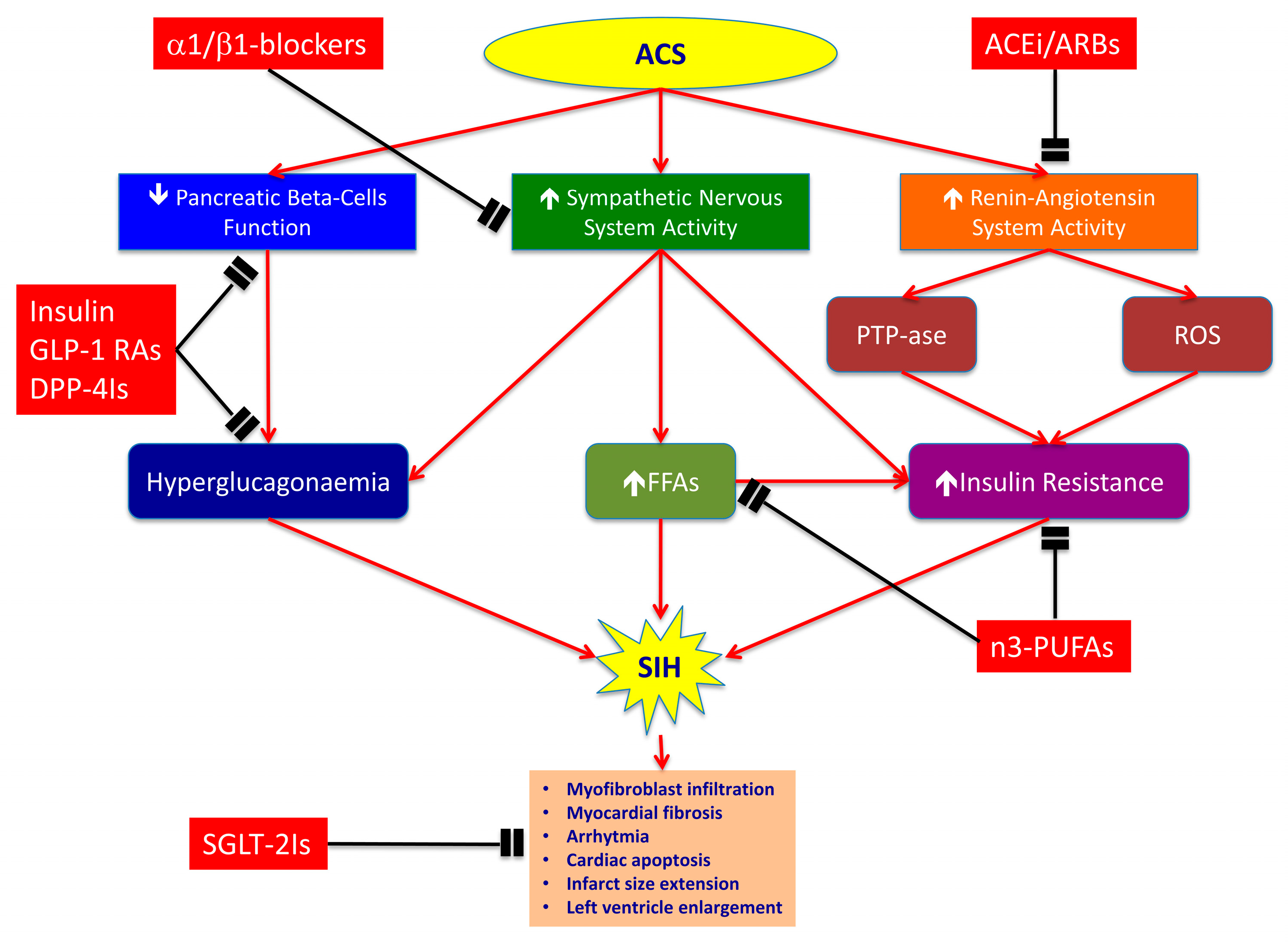
2. SIH in the Context of Acute Coronary Syndromes
2.1. Pancreatic Beta-Cell Dysfunction and Increased Glucagon Levels
2.2. Sympathetic Nervous System
2.3. Renin–Angiotensin System (RAAS)
3. Detrimental Effects of SIH in Acute Coronary Syndrome
4. Results of Insulin Therapy for SIH in Acute Coronary Syndrome
4.1. Tighter Glycaemic Control Versus Standard Therapy
4.2. GIK Versus Standard Therapy
4.3. Clinical Remarks of RCTs with Insulin
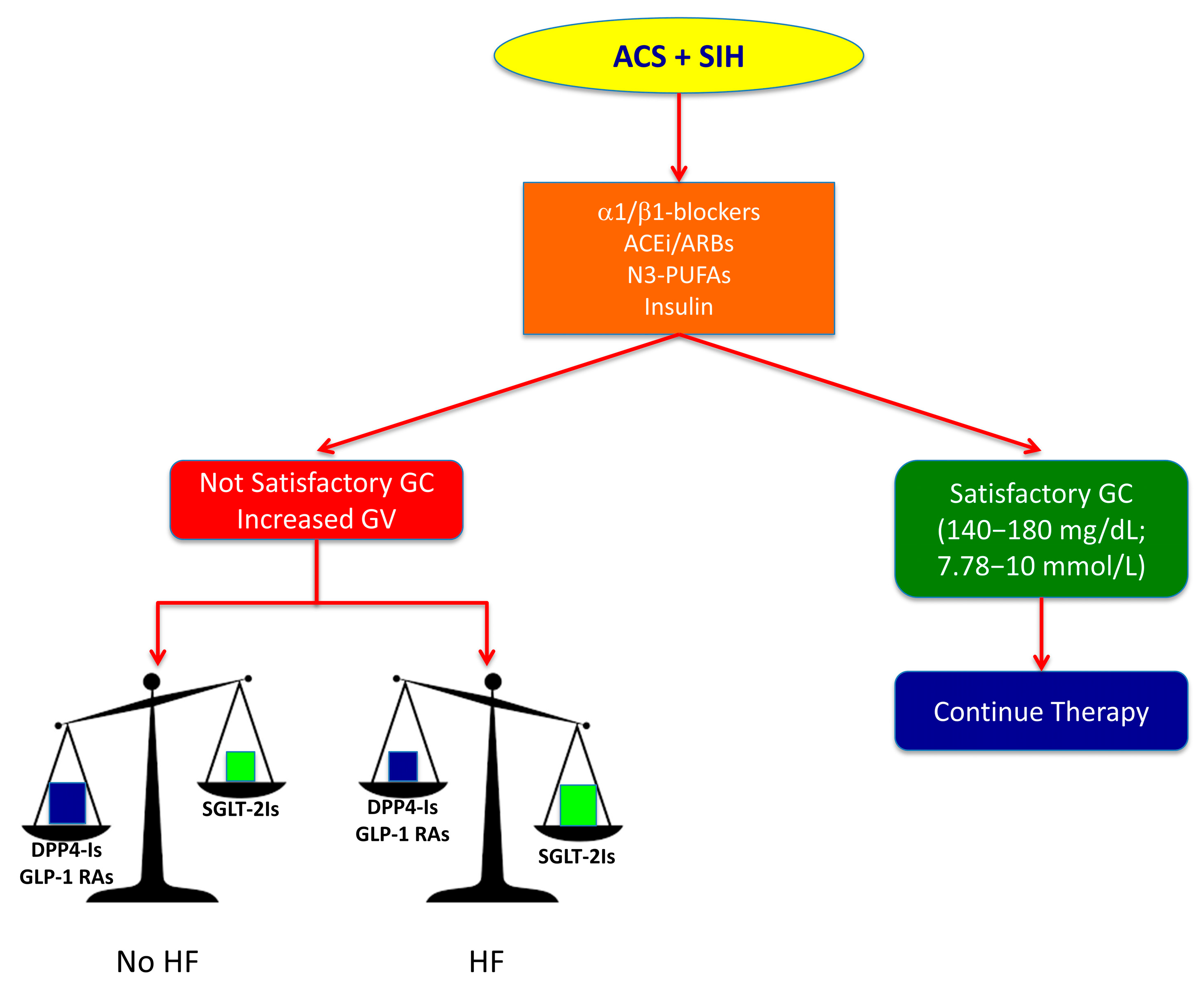
5. Therapeutic Strategies for Improving Prognosis
5.1. β-Adrenergic Blockers
5.2. ACE Inhibitors and ARBs
5.3. Mono- and Poly-Unsaturated Fatty Acids
5.4. Glucagon-Like Peptide 1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors
5.5. Sodium-Glucose Co-Transporter 2 Inhibitors (SGLT-2Is)
6. Multitargeted Therapeutic Strategy for SIH in Non-Diabetic patients with ACS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef]
- Norhammar, A.M.; Ryden, L.; Malmberg, K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care 1999, 22, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Wahab, N.N.; Cowden, E.A.; Pearce, N.J.; Gardner, M.J.; Merry, H.; Cox, J.L. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J. Am. Coll. Cardiol. 2002, 40, 1748–1754. [Google Scholar] [CrossRef] [Green Version]
- Capes, S.E.; Hunt, D.; Malmberg, K.; Gerstein, H.C. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet 2000, 355, 773–778. [Google Scholar] [CrossRef]
- Kosiborod, M.; Rathore, S.S.; Inzucchi, S.E.; Masoudi, F.A.; Wang, Y.; Havranek, E.P.; Krumholz, H.M. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: Implications for patients with and without recognized diabetes. Circulation 2005, 111, 3078–3086. [Google Scholar] [CrossRef] [Green Version]
- Sinnaeve, P.R.; Steg, P.G.; Fox, K.A.; Van de Werf, F.; Montalescot, G.; Granger, C.B.; Knobel, E.; Anderson, F.A.; Dabbous, O.H.; Avezum, A. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: The Global Registry of Acute Coronary Events. Arch. Intern. Med. 2009, 169, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartnik, M.; Malmberg, K.; Hamsten, A.; Efendic, S.; Norhammar, A.; Silveira, A.; Tenerz, A.; Ohrvik, J.; Ryden, L. Abnormal glucose tolerance—A common risk factor in patients with acute myocardial infarction in comparison with population-based controls. J. Intern. Med. 2004, 256, 288–297. [Google Scholar] [CrossRef]
- Lenzen, M.; Ryden, L.; Ohrvik, J.; Bartnik, M.; Malmberg, K.; Scholte Op Reimer, W.; Simoons, M.L. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: A report from the Euro Heart Survey on diabetes and the heart. Eur. Heart J. 2006, 27, 2969–2974. [Google Scholar] [CrossRef] [Green Version]
- Guha, M.; Bai, W.; Nadler, J.L.; Natarajan, R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J. Biol. Chem. 2000, 275, 17728–17739. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, P.; Hamouda, W.; Garg, R.; Aljada, A.; Ghanim, H.; Dandona, P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J. Clin. Endocrinol. Metab. 2000, 85, 2970–2973. [Google Scholar] [CrossRef]
- Ceriello, A. Acute hyperglycaemia: A ‘new’ risk factor during myocardial infarction. Eur. Heart J. 2005, 26, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Quagliaro, L.; D’Amico, M.; Di Filippo, C.; Marfella, R.; Nappo, F.; Berrino, L.; Rossi, F.; Giugliano, D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes 2002, 51, 1076–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriello, A.; dello Russo, P.; Amstad, P.; Cerutti, P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes 1996, 45, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A.; Ceriello, A.; Griffin, C.A.; Catena, C.; Amstad, P.; Schambelan, M.; Bartoli, E. Renal antioxidant enzyme mRNA levels are increased in rats with experimental diabetes mellitus. Diabetologia 1997, 40, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, T.; Hikoso, S.; Nakatani, D.; Suna, S.; Dohi, T.; Mizuno, H.; Okada, K.; Kitamura, T.; Kida, H.; Oeun, B.; et al. Impact of Hyperglycemia on Long-Term Outcome in Patients with ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2020, 125, 851–859. [Google Scholar] [CrossRef]
- Wallander, M.; Bartnik, M.; Efendic, S.; Hamsten, A.; Malmberg, K.; Ohrvik, J.; Ryden, L.; Silveira, A.; Norhammar, A. Beta cell dysfunction in patients with acute myocardial infarction but without previously known type 2 diabetes: A report from the GAMI study. Diabetologia 2005, 48, 2229–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luyckx, A. The role of glucagon in hyperglycemia. A review (author’s transl). Diabete Metab. 1975, 1, 201–208. [Google Scholar]
- Willerson, J.T.; Hutcheson, D.R.; Leshin, S.J.; Faloona, G.R.; Unger, R.H. Serum glucagon and insulin levels and their relationship to blood glucose values in patients with acute myocardial infarction and acute coronary insufficiency. Am. J. Med. 1974, 57, 747–752. [Google Scholar] [CrossRef]
- Christensen, N.J.; Videbaek, J. Plasma catecholamines and carbohydrate metabolism in patients with acute myocardial infarction. J. Clin. Investig. 1974, 54, 278–286. [Google Scholar] [CrossRef]
- Shamoon, H.; Hendler, R.; Sherwin, R.S. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J. Clin. Endocrinol. Metab. 1981, 52, 1235–1241. [Google Scholar] [CrossRef]
- Cohn, J.N. Neuroendocrine activation after acute myocardial infarction. Am. J. Cardiol. 1990, 65, 28I–31I. [Google Scholar] [CrossRef]
- Gromada, J.; Franklin, I.; Wollheim, C.B. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr. Rev. 2007, 28, 84–116. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, B.A.; Cryer, P.E. Insulin reciprocally regulates glucagon secretion in humans. Diabetes 2010, 59, 2936–2940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, A. Inhibition of glucagon secretion. Adv. Pharmacol. 2005, 52, 151–171. [Google Scholar] [CrossRef]
- Banarer, S.; McGregor, V.P.; Cryer, P.E. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 2002, 51, 958–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, C.K.; Zinman, B.; Choi, H.; Retnakaran, R. Effect of Short-term Intensive Insulin Therapy on Post-challenge Hyperglucagonemia in Early Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2015, 100, 2987–2995. [Google Scholar] [CrossRef] [Green Version]
- Taborsky, G.J., Jr. The physiology of glucagon. J. Diabetes Sci. Technol. 2010, 4, 1338–1344. [Google Scholar] [CrossRef] [Green Version]
- Unger, R.H.; Orci, L. Glucagon and the A cell: Physiology and pathophysiology (second of two parts). N. Engl. J. Med. 1981, 304, 1575–1580. [Google Scholar] [CrossRef]
- Unger, R.H.; Orci, L. Glucagon and the A cell: Physiology and pathophysiology (first two parts). N. Engl. J. Med. 1981, 304, 1518–1524. [Google Scholar] [CrossRef]
- Dünser, M.W.; Hasibeder, W.R. Sympathetic overstimulation during critical illness: Adverse effects of adrenergic stress. J. Intensive Care Med. 2009, 24, 293–316. [Google Scholar] [CrossRef]
- Schömig, A. Catecholamines in myocardial ischemia. Systemic and cardiac release. Circulation 1990, 82, Ii13–Ii22. [Google Scholar] [PubMed]
- Klein, J.; Fasshauer, M.; Ito, M.; Lowell, B.B.; Benito, M.; Kahn, C.R. beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J. Biol. Chem. 1999, 274, 34795–34802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morisco, C.; Condorelli, G.; Trimarco, V.; Bellis, A.; Marrone, C.; Sadoshima, J.; Trimarco, B. Akt mediates the cross-talk between beta-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ. Res. 2005, 96, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morisco, C.; Marrone, C.; Trimarco, V.; Crispo, S.; Monti, M.G.; Sadoshima, J.; Trimarco, B. Insulin resistance affects the cytoprotective effect of insulin in cardiomyocytes through an impairment of MAPK phosphatase-1 expression. Cardiovasc. Res. 2007, 76, 453–464. [Google Scholar] [CrossRef]
- Vetter, N.J.; Strange, R.C.; Adams, W.; Oliver, M.F. Initial metabolic and hormonal response to acute myocardial infarction. Lancet 1974, 1, 284–288. [Google Scholar] [CrossRef]
- Brehm, A.; Krssak, M.; Schmid, A.I.; Nowotny, P.; Waldhausl, W.; Roden, M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 2006, 55, 37. [Google Scholar] [CrossRef]
- Witteles, R.M.; Fowler, M.B. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J. Am. Coll. Cardiol. 2008, 51, 93–102. [Google Scholar] [CrossRef]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Yusuf, S.; Gerstein, H.; Hoogwerf, B.; Pogue, J.; Bosch, J.; Wolffenbuttel, B.H.; Zinman, B. Ramipril and the development of diabetes. JAMA 2001, 286, 1882–1885. [Google Scholar] [CrossRef] [Green Version]
- Julius, S.; Kjeldsen, S.E.; Weber, M.; Brunner, H.R.; Ekman, S.; Hansson, L.; Hua, T.; Laragh, J.; McInnes, G.T.; Mitchell, L.; et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet 2004, 363, 2022–2031. [Google Scholar] [CrossRef]
- Sowers, J.R. Insulin resistance and hypertension. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1597–H1602. [Google Scholar] [CrossRef]
- Byon, J.C.; Kusari, A.B.; Kusari, J. Protein-tyrosine phosphatase-1B acts as a negative regulator of insulin signal transduction. Mol. Cell. Biochem. 1998, 182, 101–108. [Google Scholar] [CrossRef]
- Taniyama, Y.; Hitomi, H.; Shah, A.; Alexander, R.W.; Griendling, K.K. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Morisco, C.; Lembo, G.; Trimarco, B. Insulin resistance and cardiovascular risk: New insights from molecular and cellular biology. Trends Cardiovasc. Med. 2006, 16, 183–188. [Google Scholar] [CrossRef]
- Ogihara, T.; Asano, T.; Ando, K.; Chiba, Y.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Onishi, Y.; Fujishiro, M.; et al. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension 2002, 40, 872–879. [Google Scholar] [CrossRef] [Green Version]
- Andreozzi, F.; Laratta, E.; Sciacqua, A.; Perticone, F.; Sesti, G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ. Res. 2004, 94, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Kersten, J.R.; Toller, W.G.; Tessmer, J.P.; Pagel, P.S.; Warltier, D.C. Hyperglycemia reduces coronary collateral blood flow through a nitric oxide-mediated mechanism. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2097–H2104. [Google Scholar] [CrossRef] [Green Version]
- Kersten, J.R.; Schmeling, T.J.; Orth, K.G.; Pagel, P.S.; Warltier, D.C. Acute hyperglycemia abolishes ischemic preconditioning in vivo. Am. J. Physiol. 1998, 275, H721–H725. [Google Scholar] [CrossRef]
- Timmer, J.R.; Ottervanger, J.P.; de Boer, M.J.; Dambrink, J.H.; Hoorntje, J.C.; Gosselink, A.T.; Suryapranata, H.; Zijlstra, F.; van’t Hof, A.W. Hyperglycemia is an important predictor of impaired coronary flow before reperfusion therapy in ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 999–1002. [Google Scholar] [CrossRef] [Green Version]
- Iwakura, K.; Ito, H.; Ikushima, M.; Kawano, S.; Okamura, A.; Asano, K.; Kuroda, T.; Tanaka, K.; Masuyama, T.; Hori, M.; et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2003, 41, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sardu, C.; Barbieri, M.; Balestrieri, M.L.; Siniscalchi, M.; Paolisso, P.; Calabrò, P.; Minicucci, F.; Signoriello, G.; Portoghese, M.; Mone, P.; et al. Thrombus aspiration in hyperglycemic ST-elevation myocardial infarction (STEMI) patients: Clinical outcomes at 1-year follow-up. Cardiovasc. Diabetol. 2018, 17, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, H.; Motoyama, T.; Hirashima, O.; Hirai, N.; Miyao, Y.; Sakamoto, T.; Kugiyama, K.; Ogawa, H.; Yasue, H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J. Am. Coll. Cardiol. 1999, 34, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Pandolfi, A.; Giaccari, A.; Cilli, C.; Alberta, M.M.; Morviducci, L.; De Filippis, E.A.; Buongiorno, A.; Pellegrini, G.; Capani, F.; Consoli, A. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol. 2001, 38, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Giacomello, R.; Stel, G.; Motz, E.; Taboga, C.; Tonutti, L.; Pirisi, M.; Falleti, E.; Bartoli, E. Hyperglycemia-induced thrombin formation in diabetes. The possible role of oxidative stress. Diabetes 1995, 44, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Giugliano, D.; Quatraro, A.; Dello Russo, P.; Marchi, E.; Torella, R. Hyperglycemia may determine fibrinopeptide A plasma level increase in humans. Metabolism 1989, 38, 1162–1163. [Google Scholar] [CrossRef]
- Ceriello, A.; Giugliano, D.; Quatraro, A.; Dello Russo, P.; Torella, R. Blood glucose may condition factor VII levels in diabetic and normal subjects. Diabetologia 1988, 31, 889–891. [Google Scholar] [CrossRef] [Green Version]
- Gigante, B.; Bellis, A.; Visconti, R.; Marino, M.; Morisco, C.; Trimarco, V.; Galasso, G.; Piscione, F.; De Luca, N.; Prince, J.A.; et al. Retrospective analysis of coagulation factor II receptor (F2R) sequence variation and coronary heart disease in hypertensive patients. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Morohoshi, M.; Fujisawa, K.; Uchimura, I.; Numano, F. Glucose-dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes 1996, 45, 954–959. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhao, L.; Liu, M.; Du, X.; Ding, W.; Zhang, J.; Mehta, J.L. Kinetics of tumor necrosis factor alpha in plasma and the cardioprotective effect of a monoclonal antibody to tumor necrosis factor alpha in acute myocardial infarction. Am. Heart J. 1999, 137, 1145–1152. [Google Scholar] [CrossRef]
- Das, U.N. Free radicals, cytokines and nitric oxide in cardiac failure and myocardial infarction. Mol. Cell. Biochem. 2000, 215, 145–152. [Google Scholar] [CrossRef]
- Morigi, M.; Angioletti, S.; Imberti, B.; Donadelli, R.; Micheletti, G.; Figliuzzi, M.; Remuzzi, A.; Zoja, C.; Remuzzi, G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J. Clin. Investig. 1998, 101, 1905–1915. [Google Scholar] [CrossRef] [Green Version]
- Aljada, A.; Friedman, J.; Ghanim, H.; Mohanty, P.; Hofmeyer, D.; Chaudhuri, A.; Dandona, P. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism 2006, 55, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, A.; Iaccarino, G. NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599. [Google Scholar] [CrossRef] [Green Version]
- Aljada, A.; Ghanim, H.; Mohanty, P.; Syed, T.; Bandyopadhyay, A.; Dandona, P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am. J. Clin. Nutr. 2004, 80, 51–57. [Google Scholar]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [Green Version]
- Wright, R.J.; Frier, B.M. Vascular disease and diabetes: Is hypoglycaemia an aggravating factor? Diabetes/Metab. Res. Rev. 2008, 24, 353–363. [Google Scholar] [CrossRef]
- Singh, P.; Jain, A.; Kaur, G. Impact of hypoglycemia and diabetes on CNS: Correlation of mitochondrial oxidative stress with DNA damage. Mol. Cell. Biochem. 2004, 260, 153–159. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Suh, S.W.; Gum, E.T.; Hamby, A.M.; Chan, P.H.; Swanson, R.A. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J. Clin. Investig. 2007, 117, 910–918. [Google Scholar] [CrossRef]
- Ceriello, A.; Novials, A.; Ortega, E.; La Sala, L.; Pujadas, G.; Testa, R.; Bonfigli, A.R.; Esposito, K.; Giugliano, D. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes 2012, 61, 2993–2997. [Google Scholar] [CrossRef] [Green Version]
- Tansey, M.J.; Opie, L.H. Relation between plasma free fatty acids and arrhythmias within the first twelve hours of acute myocardial infarction. Lancet 1983, 2, 419–422. [Google Scholar] [CrossRef]
- Oliver, M.F. Metabolic causes and prevention of ventricular fibrillation during acute coronary syndromes. Am. J. Med. 2002, 112, 305–311. [Google Scholar] [CrossRef]
- De Leiris, J.; Opie, L.H.; Lubbe, W.F. Effects of free fatty acid and enzyme release in experimental glucose on myocardial infarction. Nature 1975, 253, 746–747. [Google Scholar] [CrossRef]
- Gupta, D.K.; Jewitt, D.E.; Young, R.; Hartog, M.; Opie, L.H. Increased plasma-free-fatty-acid concentrations and their significance in patients with acute myocardial infarction. Lancet 1969, 2, 1209–1213. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Janicke, D.; Wilson, M.; Ghanim, H.; Wilding, G.E.; Aljada, A.; Dandona, P. Effect of modified glucose-insulin-potassium on free fatty acids, matrix metalloproteinase, and myoglobin in ST-elevation myocardial infarction. Am. J. Cardiol. 2007, 100, 1614–1618. [Google Scholar] [CrossRef]
- Oliver, M.F.; Opie, L.H. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet 1994, 343, 155–158. [Google Scholar] [CrossRef]
- Mjos, O.D.; Kjekshus, J. Increased local metabolic rate by free fatty acids in the intact dog heart. Scand. J. Clin. Lab. Investig. 1971, 28, 389–393. [Google Scholar] [CrossRef]
- McGavock, J.M.; Lingvay, I.; Zib, I.; Tillery, T.; Salas, N.; Unger, R.; Levine, B.D.; Raskin, P.; Victor, R.G.; Szczepaniak, L.S. Cardiac steatosis in diabetes mellitus: A 1H-magnetic resonance spectroscopy study. Circulation 2007, 116, 1170–1175. [Google Scholar] [CrossRef] [Green Version]
- Dandona, P.; Chaudhuri, A.; Ghanim, H.; Mohanty, P. Effect of hyperglycemia and insulin in acute coronary syndromes. Am. J. Cardiol. 2007, 99, 12H–18H. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ 1997, 314, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K.; Norhammar, A.; Wedel, H.; Ryden, L. Glycometabolic state at admission: Important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: Long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999, 99, 2626–2632. [Google Scholar] [PubMed]
- Ritsinger, V.; Malmberg, K.; Martensson, A.; Ryden, L.; Wedel, H.; Norhammar, A. Intensified insulin-based glycaemic control after myocardial infarction: Mortality during 20 year follow-up of the randomised Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI 1) trial. Lancet Diabetes Endocrinol. 2014, 2, 627–633. [Google Scholar] [CrossRef]
- Malmberg, K.; Ryden, L.; Wedel, H.; Birkeland, K.; Bootsma, A.; Dickstein, K.; Efendic, S.; Fisher, M.; Hamsten, A.; Herlitz, J.; et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): Effects on mortality and morbidity. Eur. Heart J. 2005, 26, 650–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, N.W.; Wong, V.W.; McLean, M. The Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study: A randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care 2006, 29, 765–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buono, F.; Spinelli, L.; Giallauria, F.; Assante di Panzillo, E.; Di Marino, S.; Ferrara, F.; Vigorito, C.; Trimarco, B.; Morisco, C. Usefulness of satisfactory control of low-density lipoprotein cholesterol to predict left ventricular remodeling after a first ST-elevation myocardial infarction successfully reperfused. Am. J. Cardiol. 2011, 107, 1772–1778. [Google Scholar] [CrossRef]
- Marfella, R.; Di Filippo, C.; Portoghese, M.; Ferraraccio, F.; Rizzo, M.R.; Siniscalchi, M.; Musacchio, E.; D’Amico, M.; Rossi, F.; Paolisso, G. Tight glycemic control reduces heart inflammation and remodeling during acute myocardial infarction in hyperglycemic patients. J. Am. Coll. Cardiol. 2009, 53, 1425–1436. [Google Scholar] [CrossRef]
- Marfella, R.; Sasso, F.C.; Cacciapuoti, F.; Portoghese, M.; Rizzo, M.R.; Siniscalchi, M.; Carbonara, O.; Ferraraccio, F.; Torella, M.; Petrella, A.; et al. Tight glycemic control may increase regenerative potential of myocardium during acute infarction. J. Clin. Endocrinol. Metab. 2012, 97, 933–942. [Google Scholar] [CrossRef] [Green Version]
- Marfella, R.; Rizzo, M.R.; Siniscalchi, M.; Paolisso, P.; Barbieri, M.; Sardu, C.; Savinelli, A.; Angelico, N.; Del Gaudio, S.; Esposito, N.; et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: Effects on myocardial salvage. Int. J. Cardiol. 2013, 168, 3954–3962. [Google Scholar] [CrossRef] [Green Version]
- Marfella, R.; Sasso, F.C.; Siniscalchi, M.; Paolisso, P.; Rizzo, M.R.; Ferraro, F.; Stabile, E.; Sorropago, G.; Calabrò, P.; Carbonara, O.; et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention is associated with a lower rate of in-stent restenosis in patients with acute ST-elevation myocardial infarction. J. Clin. Endocrinol. Metab. 2012, 97, 2862–2871. [Google Scholar] [CrossRef] [Green Version]
- De Mulder, M.; Umans, V.A.; Cornel, J.H.; van der Zant, F.M.; Stam, F.; Oemrawsingh, R.M.; Akkerhuis, K.M.; Boersma, E. Intensive glucose regulation in hyperglycemic acute coronary syndrome: Results of the randomized BIOMarker study to identify the acute risk of a coronary syndrome-2 (BIOMArCS-2) glucose trial. JAMA Intern. Med. 2013, 173, 1896–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerenberg, K.A.; Goyal, A.; Xavier, D.; Sigamani, A.; Ng, J.; Mehta, S.R.; Diaz, R.; Kosiborod, M.; Yusuf, S.; Gerstein, H.C.; et al. Piloting a novel algorithm for glucose control in the coronary care unit: The RECREATE (REsearching Coronary REduction by Appropriately Targeting Euglycemia) trial. Diabetes Care 2012, 35, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, R.; Paolasso, E.A.; Piegas, L.S.; Tajer, C.D.; Moreno, M.G.; Corvalan, R.; Isea, J.E.; Romero, G. Metabolic modulation of acute myocardial infarction. The ECLA (Estudios Cardiologicos Latinoamerica) Collaborative Group. Circulation 1998, 98, 2227–2234. [Google Scholar] [PubMed] [Green Version]
- Van der Horst, I.C.; Zijlstra, F.; van’t Hof, A.W.; Doggen, C.J.; de Boer, M.J.; Suryapranata, H.; Hoorntje, J.C.; Dambrink, J.H.; Gans, R.O.; Bilo, H.J. Glucose-insulin-potassium infusion inpatients treated with primary angioplasty for acute myocardial infarction: The glucose-insulin-potassium study: A randomized trial. J. Am. Coll. Cardiol. 2003, 42, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Timmer, J.R.; Svilaas, T.; Ottervanger, J.P.; Henriques, J.P.; Dambrink, J.H.; van den Broek, S.A.; van der Horst, I.C.; Zijlstra, F. Glucose-insulin-potassium infusion in patients with acute myocardial infarction without signs of heart failure: The Glucose-Insulin-Potassium Study (GIPS)-II. J. Am. Coll. Cardiol. 2006, 47, 1730–1731. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.R.; Yusuf, S.; Diaz, R.; Zhu, J.; Pais, P.; Xavier, D.; Paolasso, E.; Ahmed, R.; Xie, C.; Kazmi, K.; et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: The CREATE-ECLA randomized controlled trial. JAMA 2005, 293, 437–446. [Google Scholar] [PubMed]
- Diaz, R.; Goyal, A.; Mehta, S.R.; Afzal, R.; Xavier, D.; Pais, P.; Chrolavicius, S.; Zhu, J.; Kazmi, K.; Liu, L.; et al. Glucose-insulin-potassium therapy in patients with ST-segment elevation myocardial infarction. JAMA 2007, 298, 2399–2405. [Google Scholar] [CrossRef]
- Selker, H.P.; Beshansky, J.R.; Sheehan, P.R.; Massaro, J.M.; Griffith, J.L.; D’Agostino, R.B.; Ruthazer, R.; Atkins, J.M.; Sayah, A.J.; Levy, M.K.; et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: The IMMEDIATE randomized controlled trial. JAMA 2012, 307, 1925–1933. [Google Scholar] [CrossRef] [Green Version]
- De Caterina, R.; Madonna, R.; Sourij, H.; Wascher, T. Glycaemic control in acute coronary syndromes: Prognostic value and therapeutic options. Eur. Heart J. 2010, 31, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- Kosiborod, M.; Inzucchi, S.E.; Goyal, A.; Krumholz, H.M.; Masoudi, F.A.; Xiao, L.; Spertus, J.A. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 2009, 301, 1556–1564. [Google Scholar] [CrossRef]
- Svensson, A.M.; McGuire, D.K.; Abrahamsson, P.; Dellborg, M. Association between hyper- and hypoglycaemia and 2 year all-cause mortality risk in diabetic patients with acute coronary events. Eur. Heart J. 2005, 26, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Goyal, A.; Mahaffey, K.W.; Garg, J.; Nicolau, J.C.; Hochman, J.S.; Weaver, W.D.; Theroux, P.; Oliveira, G.B.; Todaro, T.G.; Mojcik, C.F.; et al. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: Results from the CARDINAL study. Eur. Heart J. 2006, 27, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Wallhaus, T.R.; Taylor, M.; DeGrado, T.R.; Russell, D.C.; Stanko, P.; Nickles, R.J.; Stone, C.K. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation 2001, 103, 2441–2446. [Google Scholar] [CrossRef]
- Igarashi, N.; Nozawa, T.; Fujii, N.; Suzuki, T.; Matsuki, A.; Nakadate, T.; Igawa, A.; Inoue, H. Influence of beta-adrenoceptor blockade on the myocardial accumulation of fatty acid tracer and its intracellular metabolism in the heart after ischemia-reperfusion injury. Circ. J. 2006, 70, 1509–1514. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.M.; Pan, H.C.; Chen, Y.P.; Peto, R.; Collins, R.; Jiang, L.X.; Xie, J.X.; Liu, L.S. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet 2005, 366, 1622–1632. [Google Scholar]
- Gersh, B.J.; Stone, G.W.; White, H.D.; Holmes, D.R., Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: Is the slope of the curve the shape of the future? JAMA 2005, 293, 979–986. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Ma, J.Z.; Kalil, R.S.; Louis, T.A. Effects of antihypertensive therapy on serum lipids. Ann. Intern. Med. 1995, 122, 133–141. [Google Scholar] [CrossRef]
- Elliott, W.J.; Meyer, P.M. Incident diabetes in clinical trials of antihypertensive drugs: A network meta-analysis. Lancet 2007, 369, 201–207. [Google Scholar] [CrossRef]
- Brixius, K.; Bundkirchen, A.; Bolck, B.; Mehlhorn, U.; Schwinger, R.H. Nebivolol, bucindolol, metoprolol and carvedilol are devoid of intrinsic sympathomimetic activity in human myocardium. Br. J. Pharmacol. 2001, 133, 1330–1338. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.A. The role of the new beta-blockers in treating cardiovascular disease. Am. J. Hypertens. 2005, 18, 169S–176S. [Google Scholar] [CrossRef] [Green Version]
- Bakris, G.L.; Fonseca, V.; Katholi, R.E.; McGill, J.B.; Messerli, F.H.; Phillips, R.A.; Raskin, P.; Wright, J.T., Jr.; Oakes, R.; Lukas, M.A.; et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: A randomized controlled trial. JAMA 2004, 292, 2227–2236. [Google Scholar] [CrossRef] [Green Version]
- Messerli, F.H.; Bangalore, S.; Julius, S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation 2008, 117, 2706–2715. [Google Scholar] [CrossRef]
- Mason, R.P.; Kubant, R.; Jacob, R.F.; Walter, M.F.; Boychuk, B.; Malinski, T. Effect of nebivolol on endothelial nitric oxide and peroxynitrite release in hypertensive animals: Role of antioxidant activity. J. Cardiovasc. Pharmacol. 2006, 48, 862–869. [Google Scholar] [CrossRef]
- Cockcroft, J.R.; Chowienczyk, P.J.; Brett, S.E.; Chen, C.P.; Dupont, A.G.; Van Nueten, L.; Wooding, S.J.; Ritter, J.M. Nebivolol vasodilates human forearm vasculature: Evidence for an L-arginine/NO-dependent mechanism. J. Pharmacol. Exp. Ther. 1995, 274, 1067–1071. [Google Scholar]
- Poirier, L.; Cleroux, J.; Nadeau, A.; Lacourciere, Y. Effects of nebivolol and atenolol on insulin sensitivity and haemodynamics in hypertensive patients. J. Hypertens. 2001, 19, 1429–1435. [Google Scholar] [CrossRef]
- Celik, T.; Iyisoy, A.; Kursaklioglu, H.; Kardesoglu, E.; Kilic, S.; Turhan, H.; Yilmaz, M.I.; Ozcan, O.; Yaman, H.; Isik, E.; et al. Comparative effects of nebivolol and metoprolol on oxidative stress, insulin resistance, plasma adiponectin and soluble P-selectin levels in hypertensive patients. J. Hypertens. 2006, 24, 591–596. [Google Scholar] [CrossRef]
- Tikellis, C.; Wookey, P.J.; Candido, R.; Andrikopoulos, S.; Thomas, M.C.; Cooper, M.E. Improved islet morphology after blockade of the renin- angiotensin system in the ZDF rat. Diabetes 2004, 53, 989–997. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Jacob, S. Angiotensin converting enzyme inhibitors and modulation of skeletal muscle insulin resistance. Diabetes Obes. Metab. 2003, 5, 214–222. [Google Scholar] [CrossRef]
- Gillespie, E.L.; White, C.M.; Kardas, M.; Lindberg, M.; Coleman, C.I. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care 2005, 28, 2261–2266. [Google Scholar] [CrossRef] [Green Version]
- Abuissa, H.; Jones, P.G.; Marso, S.P.; O’Keefe, J.H., Jr. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: A meta-analysis of randomized clinical trials. J. Am. Coll. Cardiol. 2005, 46, 821–826. [Google Scholar] [CrossRef] [Green Version]
- Andraws, R.; Brown, D.L. Effect of inhibition of the renin-angiotensin system on development of type 2 diabetes mellitus (meta-analysis of randomized trials). Am. J. Cardiol. 2007, 99, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, J.C.; Maia, L.N.; Vitola, J.V.; Mahaffey, K.W.; Machado, M.N.; Ramires, J.A. Baseline glucose and left ventricular remodeling after acute myocardial infarction. J. Diabetes Complicat. 2007, 21, 294–299. [Google Scholar] [CrossRef]
- Usami, M.; Sakata, Y.; Nakatani, D.; Suna, S.; Matsumoto, S.; Hara, M.; Kitamura, T.; Ueda, Y.; Iwakura, K.; Sato, H.; et al. Clinical impact of acute hyperglycemia on development of diabetes mellitus in non-diabetic patients with acute myocardial infarction. J. Cardiol. 2014, 63, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Colaiori, I.; Izzo, R. Severity of Coronary Atherosclerosis and Risk of Diabetes Mellitus. J. Clin. Med. 2019, 8, 1069. [Google Scholar] [CrossRef] [Green Version]
- Strisciuglio, T.; Izzo, R. Insulin Resistance Predicts Severity of Coronary Atherosclerotic Disease in Non-Diabetic Patients. J. Clin. Med. 2020, 9, 2144. [Google Scholar] [CrossRef]
- Mjos, O.D. Effect of inhibition of lipolysis on myocardial oxygen consumption in the presence of isoproterenol. J. Clin. Investig. 1971, 50, 1869–1873. [Google Scholar] [CrossRef]
- Russell, D.C.; Oliver, M.F. Effect of antilipolytic therapy on ST segment elevation during myocardial ischaemia in man. Br. Heart J. 1978, 40, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Rackley, C.E.; Russell, R.O., Jr.; Rogers, W.J.; Mantle, J.A.; McDaniel, H.G.; Papapietro, S.E. Clinical experience with glucose-insulin-potassium therapy in acute myocardial infarction. Am. Heart J. 1981, 102, 1038–1049. [Google Scholar] [CrossRef]
- Degirolamo, C.; Rudel, L.L. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr. Atheroscler. Rep. 2010, 12, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef]
- Abeywardena, M.Y.; Head, R.J. Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovasc. Res. 2001, 52, 361–371. [Google Scholar] [CrossRef]
- Wan, J.B.; Huang, L.L.; Rong, R.; Tan, R.; Wang, J.; Kang, J.X. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2487–2494. [Google Scholar] [CrossRef] [Green Version]
- Lamping, K.G.; Nuno, D.W.; Coppey, L.J.; Holmes, A.J.; Hu, S.; Oltman, C.L.; Norris, A.W.; Yorek, M.A. Modification of high saturated fat diet with n-3 polyunsaturated fat improves glucose intolerance and vascular dysfunction. Diabetes Obes. Metab. 2013, 15, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [Green Version]
- Wettergren, A.; Schjoldager, B.; Mortensen, P.E.; Myhre, J.; Christiansen, J.; Holst, J.J. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig. Dis. Sci. 1993, 38, 665–673. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J.; Cavender, M.A.; Abd El Aziz, M.; Drucker, D.J. Cardiovascular Actions and Clinical Outcomes with Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation 2017, 136, 849–870. [Google Scholar] [CrossRef]
- Xu, G.; Stoffers, D.A.; Habener, J.F.; Bonner-Weir, S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999, 48, 2270–2276. [Google Scholar] [CrossRef]
- Farilla, L.; Bulotta, A.; Hirshberg, B.; Li Calzi, S.; Khoury, N.; Noushmehr, H.; Bertolotto, C.; Di Mario, U.; Harlan, D.M.; Perfetti, R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003, 144, 5149–5158. [Google Scholar] [CrossRef] [Green Version]
- Mu, J.; Woods, J.; Zhou, Y.P.; Roy, R.S.; Li, Z.; Zycband, E.; Feng, Y.; Zhu, L.; Li, C.; Howard, A.D.; et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 2006, 55, 1695–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.J.; Choi, S.E.; Kang, Y.; Jung, J.G.; Yi, S.A.; Kim, H.J.; Lee, K.W.; Kim, D.J. Effect of sitagliptin plus metformin on beta-cell function, islet integrity and islet gene expression in Zucker diabetic fatty rats. Diabetes Res. Clin. Pract. 2011, 92, 213–222. [Google Scholar] [CrossRef]
- Kahles, F.; Rückbeil, M.V.; Mertens, R.W.; Foldenauer, A.C.; Arrivas, M.C.; Moellmann, J.; Lebherz, C.; Biener, M.; Giannitsis, E.; Katus, H.A.; et al. Glucagon-like peptide 1 levels predict cardiovascular risk in patients with acute myocardial infarction. Eur. Heart J. 2020, 41, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.; Moellmann, J.; Kahles, F.; Haj-Yehia, E.; Liehn, E.A.; Nickel, A.; Lebherz, C.; Maack, C.; Marx, N.; Lehrke, M. Myocardial infarction is sufficient to increase GLP-1 secretion, leading to improved left ventricular contractility and mitochondrial respiratory capacity. Diabetes Obes. Metab. 2018, 20, 2911–2918. [Google Scholar] [CrossRef] [Green Version]
- Cavusoglu, E.; Marmur, J.D.; Hojjati, M.R.; Chopra, V.; Butala, M.; Subnani, R.; Huda, M.S.; Yanamadala, S.; Ruwende, C.; Eng, C.; et al. Plasma interleukin-10 levels and adverse outcomes in acute coronary syndrome. Diabetes Obes. Metab. 2011, 124, 724–730. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Omland, T.; Aakvaag, A.; Bonarjee, V.V.; Caidahl, K.; Lie, R.T.; Nilsen, D.W.; Sundsfjord, J.A.; Dickstein, K. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation 1996, 93, 1963–1969. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef]
- Nikolaidis, L.A.; Mankad, S.; Sokos, G.G.; Miske, G.; Shah, A.; Elahi, D.; Shannon, R.P. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004, 109, 962–965. [Google Scholar] [CrossRef] [Green Version]
- Lonborg, J.; Vejlstrup, N.; Kelbaek, H.; Botker, H.E.; Kim, W.Y.; Mathiasen, A.B.; Jorgensen, E.; Helqvist, S.; Saunamaki, K.; Clemmensen, P.; et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Heart J. 2012, 33, 1491–1499. [Google Scholar] [CrossRef]
- Woo, J.S.; Kim, W.; Ha, S.J.; Kim, J.B.; Kim, S.J.; Kim, W.S.; Seon, H.J.; Kim, K.S. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: Results of exenatide myocardial protection in revascularization study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2252–2260. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.R.; Chen, Y.D.; Tian, F.; Yang, N.; Cheng, L.Q.; Hu, S.Y.; Wang, J.; Yang, J.J.; Wang, S.F.; Gu, X.F. Effects of Liraglutide on Reperfusion Injury in Patients with ST-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.R.; Hu, S.Y.; Chen, Y.D.; Zhang, Y.; Qian, G.; Wang, J.; Yang, J.J.; Wang, Z.F.; Tian, F.; Ning, Q.X. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am. Heart J. 2015, 170, 845–854. [Google Scholar] [CrossRef]
- Chen, W.R.; Shen, X.Q.; Zhang, Y.; Chen, Y.D.; Hu, S.Y.; Qian, G.; Wang, J.; Yang, J.J.; Wang, Z.F.; Tian, F. Effects of liraglutide on left ventricular function in patients with non-ST-segment elevation myocardial infarction. Endocrine 2016, 52, 516–526. [Google Scholar] [CrossRef]
- Verma, S.; Poulter, N.R.; Bhatt, D.L.; Bain, S.C.; Buse, J.B.; Leiter, L.A.; Nauck, M.A.; Pratley, R.E.; Zinman, B.; Ørsted, D.D.; et al. Effects of Liraglutide on Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus with or without History of Myocardial Infarction or Stroke. Circulation 2018, 138, 2884–2894. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Brismar, K.; Efendic, S.; Lundman, P.; Ryden, L.; Mellbin, L. Sitagliptin improves beta-cell function in patients with acute coronary syndromes and newly diagnosed glucose abnormalities—The BEGAMI study. J. Intern. Med. 2013, 273, 410–421. [Google Scholar] [CrossRef] [PubMed]
- McCormick, L.M.; Kydd, A.C.; Read, P.A.; Ring, L.S.; Bond, S.J.; Hoole, S.P.; Dutka, D.P. Chronic dipeptidyl peptidase-4 inhibition with sitagliptin is associated with sustained protection against ischemic left ventricular dysfunction in a pilot study of patients with type 2 diabetes mellitus and coronary artery disease. Circ. Cardiovasc. Imaging 2014, 7, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuire, D.K.; Van de Werf, F.; Armstrong, P.W.; Standl, E.; Koglin, J.; Green, J.B.; Bethel, M.A.; Cornel, J.H.; Lopes, R.D.; Halvorsen, S.; et al. Association Between Sitagliptin Use and Heart Failure Hospitalization and Related Outcomes in Type 2 Diabetes Mellitus: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2016, 1, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Scirica, B.M.; Braunwald, E.; Raz, I.; Cavender, M.A.; Morrow, D.A.; Jarolim, P.; Udell, J.A.; Mosenzon, O.; Im, K.; Umez-Eronini, A.A.; et al. Heart failure, saxagliptin, and diabetes mellitus: Observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014, 130, 1579–1588. [Google Scholar] [CrossRef] [Green Version]
- Zannad, F.; Cannon, C.P.; Cushman, W.C.; Bakris, G.L.; Menon, V.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; Wilson, C.; et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: A multicentre, randomised, double-blind trial. Lancet 2015, 385, 2067–2076. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B., Sr.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Fudim, M.; White, J.; Pagidipati, N.J.; Lokhnygina, Y.; Wainstein, J.; Murin, J.; Iqbal, N.; Öhman, P.; Lopes, R.D.; Reicher, B.; et al. Effect of Once-Weekly Exenatide in Patients with Type 2 Diabetes Mellitus with and without Heart Failure and Heart Failure-Related Outcomes: Insights From the EXSCEL Trial. Circulation 2019, 140, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Bain, S.C. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes. Metab. 2020, 22, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Baeres, F.M.M.; Bain, S.C.; Goldman, B.; Husain, M.; Nauck, M.A.; Poulter, N.R.; Pratley, R.E.; Thomsen, A.B.; Buse, J.B. Effects of Liraglutide on Cardiovascular Outcomes in Patients with Diabetes with or without Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H. Role of SGLT2 Inhibitors in Patients with Diabetes Mellitus and Heart Failure. Curr. Heart Fail. Rep. 2017, 14, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Lahnwong, S.; Palee, S.; Apaijai, N.; Sriwichaiin, S.; Kerdphoo, S.; Jaiwongkam, T.; Chattipakorn, S.C.; Chattipakorn, N. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc. Diabetol. 2020, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Chang, N.C.; Lin, S.Z. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic. Biol. Med. 2017, 104, 298–310. [Google Scholar] [CrossRef]
- Tanajak, P.; Sa-Nguanmoo, P.; Sivasinprasasn, S.; Thummasorn, S.; Siri-Angkul, N.; Chattipakorn, S.C.; Chattipakorn, N. Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. J. Endocrinol. 2018, 236, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Cardiovasc. Diabetol. 2019, 140, 1693–1702. [Google Scholar] [CrossRef]
- Furtado, R.H.M.; Bonaca, M.P.; Raz, I.; Zelniker, T.A.; Mosenzon, O.; Cahn, A.; Kuder, J.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus and Previous Myocardial Infarction. Circulation 2019, 139, 2516–2527. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Circulation 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Fitchett, D.; Inzucchi, S.E.; Cannon, C.P.; McGuire, D.K.; Scirica, B.M.; Johansen, O.E.; Sambevski, S.; Kaspers, S.; Pfarr, E.; George, J.T.; et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation 2019, 139, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Tripolt, N.J.; Kolesnik, E.; Pferschy, P.N.; Verheyen, N.; Ablasser, K.; Sailer, S.; Alber, H.; Berger, R.; Kaulfersch, C.; Leitner, K.; et al. Impact of EMpagliflozin on cardiac function and biomarkers of heart failure in patients with acute MYocardial infarction-The EMMY trial. Am. Heart J. 2020, 221, 39–47. [Google Scholar] [CrossRef]
- Vergès, B.; Avignon, A.; Bonnet, F.; Catargi, B.; Cattan, S.; Cosson, E.; Ducrocq, G.; Elbaz, M.; Fredenrich, A.; Gourdy, P.; et al. Consensus statement on the care of the hyperglycaemic/diabetic patient during and in the immediate follow-up of acute coronary syndrome. Diabetes Metab. 2012, 38, 113–127. [Google Scholar] [CrossRef]
- Jacobi, J.; Bircher, N.; Krinsley, J.; Agus, M.; Braithwaite, S.S.; Deutschman, C.; Freire, A.X.; Geehan, D.; Kohl, B.; Nasraway, S.A.; et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit. Care Med. 2012, 40, 3251–3276. [Google Scholar] [CrossRef] [PubMed]
- Corbett, S.J. NICE recommendations for the management of hyperglycaemia in acute coronary syndrome. Heart Br. Card. Soc. 2012, 98, 1189–1191. [Google Scholar] [CrossRef]
- Buturlin, K.; Minha, S.; Rozenbaum, Z.; Neuman, Y.; Shlezinger, M.; Goldenberg, I.; Mosseri, M.; Pereg, D. Admission plasma glucose levels within the normal to mildly impaired range and the outcome of patients with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Finfer, S.; Liu, B.; Chittock, D.R.; Norton, R.; Myburgh, J.A.; McArthur, C.; Mitchell, I.; Foster, D.; Dhingra, V.; Henderson, W.R.; et al. Hypoglycemia and risk of death in critically ill patients. N. Engl. J. Med. 2012, 367, 1108–1118. [Google Scholar] [CrossRef]
- Krinsley, J.S. Glycemic variability and mortality in critically ill patients: The impact of diabetes. J. Diabetes Sci. Technol. 2009, 3, 1292–1301. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S111–S134. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Diabetes Care 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes-state-of-the-art. Mol. Metab. 2020, 101102. [Google Scholar] [CrossRef]
- Ray, K.K.; Seshasai, S.R.; Wijesuriya, S.; Sivakumaran, R.; Nethercott, S.; Preiss, D.; Erqou, S.; Sattar, N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials. Lancet 2009, 373, 1765–1772. [Google Scholar] [CrossRef]
- Turnbull, F.M.; Abraira, C.; Anderson, R.J.; Byington, R.P.; Chalmers, J.P.; Duckworth, W.C.; Evans, G.W.; Gerstein, H.C.; Holman, R.R.; Moritz, T.E.; et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009, 52, 2288–2298. [Google Scholar] [CrossRef]
- Zhao, T.; Parikh, P.; Bhashyam, S.; Bolukoglu, H.; Poornima, I.; Shen, Y.T.; Shannon, R.P. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J. Pharmacol. Exp. Ther. 2006, 317, 1106–1113. [Google Scholar] [CrossRef]
- Nystrom, T.; Gonon, A.T.; Sjoholm, A.; Pernow, J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul. Pept. 2005, 125, 173–177. [Google Scholar] [CrossRef]
- Timmers, L.; Henriques, J.P.; de Kleijn, D.P.; Devries, J.H.; Kemperman, H.; Steendijk, P.; Verlaan, C.W.; Kerver, M.; Piek, J.J.; Doevendans, P.A.; et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J. Am. Coll. Cardiol. 2009, 53, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem. Biophys. Res. Commun. 2010, 391, 1405–1408. [Google Scholar] [CrossRef]
- Anagnostis, P.; Athyros, V.G.; Adamidou, F.; Panagiotou, A.; Kita, M.; Karagiannis, A.; Mikhailidis, D.P. Glucagon-like peptide-1-based therapies and cardiovascular disease: Looking beyond glycaemic control. Diabetes Obes. Metab. 2011, 13, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; La Sala, L.; Pujadas, G.; Esposito, K.; Giugliano, D.; Genovese, S. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care 2013, 36, 2346–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, M.; Marfella, R.; Esposito, A.; Rizzo, M.R.; Angellotti, E.; Mauro, C.; Siniscalchi, M.; Chirico, F.; Caiazzo, P.; Furbatto, F.; et al. Incretin treatment and atherosclerotic plaque stability: Role of adiponectin/APPL1 signaling pathway. J. Clin. Med. 2017, 31, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Honigberg, M.C.; Chang, L.S.; McGuire, D.K.; Plutzky, J.; Aroda, V.R.; Vaduganathan, M. Use of Glucagon-Like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes and Cardiovascular Disease: A Review. JAMA Cardiol. 2020, 5, 1182–1190. [Google Scholar] [CrossRef]
- Huthmacher, J.A.; Meier, J.J.; Nauck, M.A. Efficacy and Safety of Short- and Long-Acting Glucagon-Like Peptide 1 Receptor Agonists on a Background of Basal Insulin in Type 2 Diabetes: A Meta-analysis. Diabetes Care 2020, 43, 2303–2312. [Google Scholar] [CrossRef]
- Diamant, M.; Nauck, M.A.; Shaginian, R.; Malone, J.K.; Cleall, S.; Reaney, M.; de Vries, D.; Hoogwerf, B.J.; MacConell, L.; Wolffenbuttel, B.H. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Obes. Metab. 2014, 37, 2763–2773. [Google Scholar] [CrossRef] [Green Version]
- Fitchett, D.; Zinman, B.; Wanner, C.; Lachin, J.M.; Hantel, S.; Salsali, A.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Inzucchi, S.E. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: Results of the EMPA-REG OUTCOME® trial. Eur. Heart J. 2016, 37, 1526–1534. [Google Scholar] [CrossRef] [Green Version]
- Marx, N.; McGuire, D.K. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Eur. Heart J. 2016, 37, 3192–3200. [Google Scholar] [CrossRef] [Green Version]
- Sattar, N.; McLaren, J.; Kristensen, S.L.; Preiss, D.; McMurray, J.J. SGLT2 Inhibition and cardiovascular events: Why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia 2016, 59, 1333–1339. [Google Scholar] [CrossRef] [Green Version]
- Vallon, V.; Thomson, S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V.; Cherney, D.Z.I. The Metabolodiuretic Promise of Sodium-Dependent Glucose Cotransporter 2 Inhibition: The Search for the Sweet Spot in Heart Failure. JAMA Cardiol. 2017, 2, 939–940. [Google Scholar] [CrossRef]
- Matsumura, M.; Nakatani, Y.; Tanka, S.; Aoki, C.; Sagara, M.; Yanagi, K.; Suzuki, K.; Aso, Y. Efficacy of Additional Canagliflozin Administration to Type 2 Diabetes Patients Receiving Insulin Therapy: Examination of Diurnal Glycemic Patterns Using Continuous Glucose Monitoring (CGM). Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2017, 8, 821–827. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, K.W.; Lee, B.W.; Jeong, I.K.; Yoo, S.J.; Kwon, H.S.; Choi, Y.H.; Yoon, K.H. Effect of Dapagliflozin as an Add-on Therapy to Insulin on the Glycemic Variability in Subjects with Type 2 Diabetes Mellitus (DIVE): A Multicenter, Placebo-Controlled, Double-Blind, Randomized Study. Diabetes Metab. J. 2020. [Google Scholar] [CrossRef]
- Nomoto, H.; Miyoshi, H.; Sugawara, H.; Ono, K.; Yanagiya, S.; Oita, M.; Nakamura, A.; Atsumi, T. A randomized controlled trial comparing the effects of dapagliflozin and DPP-4 inhibitors on glucose variability and metabolic parameters in patients with type 2 diabetes mellitus on insulin. Diabetes Metab. J. 2017, 9, 54. [Google Scholar] [CrossRef] [Green Version]
| Clinical Trial (Year) | Number of Patients | Study Population | Admission Glycaemia | Specific Glycaemic Target | Reached Glycaemic Target (Intervention vs. Control) | Primary Endpoint | Result |
|---|---|---|---|---|---|---|---|
| DIGAMI-1 (1995) | 620 | ACS | ≈280 mg/dL (15.56 mmol/L) | 126–180 mg/dL (7–10 mmol/L) in acute phase 90–126 mg/dL (5–7 mmol/L) post-recovery | 173 mg/dL (9.61 mmol/L) vs. 211 mg/dL (11.72 mmol/L) during first 24 h | Intra-hospital Mortality | Neutral at 3 months |
| DIGAMI-2 (2005) | 1253 | ACS | ≈229 mg/dL (12.72 mmol/L) | 126–180 mg/dL (7–10 mmol/L) in acute phase 90–126 mg/dL (5–7 mmol/L) post-recovery | 164 mg/dL (9.11 mmol/L) vs. 180 mg/dL (10 mmol/L) during first 24 h | Intra-hospital Mortality | Neutral at 3 months |
| HI-5 (2006) | 244 | ACS | ≈198 mg/dL (11 mmol/L) | ≥140 mg/dL (7.78 mmol/L) | 149 mg/dL (8.28 mmol/L) vs. 162 mg/dL (9 mmol/L) during first 24 h | Intra-hospital Mortality | Neutral at 3 months |
| Marfella (2009) | 50 | ACS (CABG) | ≥140 mg/dL (7.78 mmol/L) | 80–140 vs. 180–200 mg/dL (4.44–7.78 vs. 10–11.11 mmol/L) | 163 mg/dL (9.06 mmol/L) vs. 192 mg/dL (10.67 mmol/L) | LVEF, Oxidative Stress, Apoptosis | ↑LVEF↓Oxidative Stress and Apoptosis |
| Marfella (2012) | 50 | STEMI (CABG) | ≥140 mg/dL (7.78 mmol/L) | 80–140 vs. 180–200 mg/dL (4.44–7.78 vs. 10–11.11 mmol/L) | 161 mg/dL (8.94 mmol/L) vs. 194 mg/dL (10.78 mmol/L) vs. 182 mg/dL (10.11 mmol/L) | Myocardial Regeneration | ↑Myocardial Regeneration |
| Marfella (2013) | 194 | STEMI(pPCI) | ≥140 mg/dL (7.78 mmol/L) | 80–140 vs. 180–200 mg/dL (4.44–7.78 vs. 10–11.11 mmol/L) | 144 mg/dL (8 mmol/L) vs. 201 mg/dL (11.17 mmol/L) | Myocardial Salvage | ↑Myocardial Salvage |
| Marfella (2012) | 165 | STEMI(pPCI) | ≥140 mg/dL (7.78 mmol/L) | 80–140 vs. 180–200 mg/dL (4.44–7.78 vs. 10–11.11 mmol/L) | 145 mg/dL (8.06 mmol/L) vs. 191 mg/dL (10.61 mmol/L) | ISR | ↓ISR |
| RECREATE (2012) | 287 | STEMI (pPCI) | ≥144 mg/dL (8 mmol/L) | 90–117 mg/dL (5–6.5 mmol/L) vs. standard therapy | 117 mg/dL (6.5 mmol/L) vs. 143 mg/dL (7.94 mmol/L) | Glycaemia, Intra-hospital Mortality | ↓Glycaemia, Intra-hospital Mortality |
| BIOMArKS2 (2013) | 280 | ACS | ≥140 mg/dL (7.78 mmol/L) | 85–110 mg/dL (4.72–6.11 mmol/L; day), 85–139 mg/dL (4.72–7.72 mmol/L; night) vs. <288 mg/dL (16 mmol/L) | 112 mg/dL (6.22 mmol/L) vs. ≈130 mg/dL (7.22 mmol/L) | Intra-hospital Mortality, Re-Infarction | ↑Intra-hospital Mortality, Re-Infarction |
| Clinical Trial (Year) | Number of Patients | Study Population | Admission Glycaemia | Specific Glycaemic Target | Reached Glycaemic Target (Intervention vs. Control) | Primary Endpoint | Result |
|---|---|---|---|---|---|---|---|
| ECLA-GIK (1998) | 407 | ACS | 140 ± 15 mg/dL (7.78 ± 0.83 mmol/L; both GIK groups) vs. 143 ± 15 mg/dL (7.94 ± 0.83 mmol/L) | - | 122 ± 7 mg/dL (6.78 ± 0.39 mmol/L; both GIK groups) vs. 135 ± 5 mg/dL (7.5 ± 0.28 mmol/L) | In-hospital mortality | Similar In-hospital mortality |
| GIPS (2003) | 940 | STEMI | 153 mg/dL (8.5 mmol/L) in both groups | - | 139 ± 10 mg/dL (7.72 ± 0.56 mmol/L) vs. 146 ± 10 mg/dL (8.11 ± 0.56 mmol/L) | 30 day-Mortality | Similar 30 day-Mortality |
| GIPS-2 (2006) | 889 | STEMI (Killip Class I) | 153 ± 50.4 mg/dL (8.5 ± 2.8 mmol/L) vs. 149.4 ± 45 mg/dL (8.28 ± 2.5 mmol/L) | - | - | 30 day-Mortality | Similar 30 day-Mortality |
| CREATE-ECLA (2005) | 20,201 | STEMI | 162 mg/dL (9 mmol/L) in both groups | - | 187 mg/dL (10.39 mmol/L) vs. 148 mg/dL (8.22 mmol/L) | 30 day-Mortality | Similar 30 day-Mortality |
| OASIS-6 GIK (2007) | 2748 | STEMI(14.9% vs. 14%) | - | - | - | 30 day-Mortality | Similar 30 day-Mortality |
| IMMEDIATE (2012) | 911 | ACS | - | - | - | Progression to AMI, 30 day-Mortality | Similar Progression to AMI and 30 day-Mortality |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellis, A.; Mauro, C.; Barbato, E.; Ceriello, A.; Cittadini, A.; Morisco, C. Stress-Induced Hyperglycaemia in Non-Diabetic Patients with Acute Coronary Syndrome: From Molecular Mechanisms to New Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 775. https://doi.org/10.3390/ijms22020775
Bellis A, Mauro C, Barbato E, Ceriello A, Cittadini A, Morisco C. Stress-Induced Hyperglycaemia in Non-Diabetic Patients with Acute Coronary Syndrome: From Molecular Mechanisms to New Therapeutic Perspectives. International Journal of Molecular Sciences. 2021; 22(2):775. https://doi.org/10.3390/ijms22020775
Chicago/Turabian StyleBellis, Alessandro, Ciro Mauro, Emanuele Barbato, Antonio Ceriello, Antonio Cittadini, and Carmine Morisco. 2021. "Stress-Induced Hyperglycaemia in Non-Diabetic Patients with Acute Coronary Syndrome: From Molecular Mechanisms to New Therapeutic Perspectives" International Journal of Molecular Sciences 22, no. 2: 775. https://doi.org/10.3390/ijms22020775
APA StyleBellis, A., Mauro, C., Barbato, E., Ceriello, A., Cittadini, A., & Morisco, C. (2021). Stress-Induced Hyperglycaemia in Non-Diabetic Patients with Acute Coronary Syndrome: From Molecular Mechanisms to New Therapeutic Perspectives. International Journal of Molecular Sciences, 22(2), 775. https://doi.org/10.3390/ijms22020775






