Origin, Regulation, and Fitness Effect of Chromosomal Rearrangements in the Yeast Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Origins of Chromosomal Rearrangements
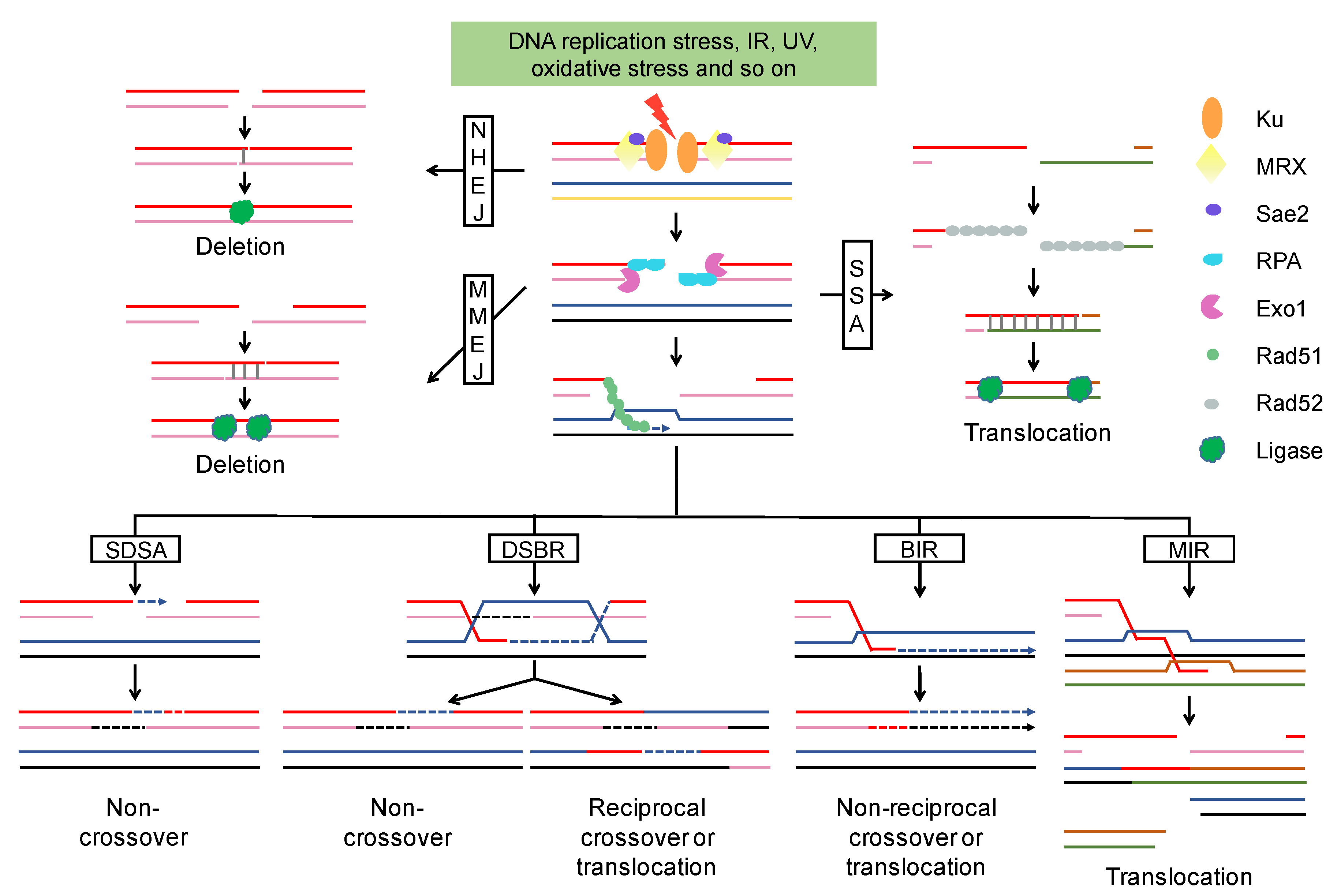
2.1. End Resection of DSBs and Repair Pathway Choice
2.2. Chromosome Rearrangements Result in Poor Outcomes of DSB Repair
2.2.1. NHEJ-Associated Chromosomal Rearrangements
2.2.2. HR-Associated Chromosomal Rearrangements
3. Spontaneous and Genotoxic Factor-Induced Chromosomal Rearrangements in Yeast
3.1. Spontaneous Chromosomal Rearrangements in the Yeast Genome
3.2. Chromosomal Rearrangements under DNA Replication Stress
3.3. DNA Repair Deficiency Contributes to Chromosomal Rearrangements
3.4. Chromosomal Rearrangements Induced by Ion Irradiation (IR), Chemical, and Oxidative Stress
4. Methods to Generate and Map Chromosomal Rearrangements
4.1. Synthetic Chromosome Rearrangement and Modification by loxP-Mediated Evolution (SCRaMbLE)
4.2. Generation of On-Demand Chromosomal Rearrangements
4.3. Detection of Rearrangements by Nanopore Sequencing Technology
5. Phenotypic Effects of Chromosomal Rearrangements
| Strains | Events | Phenotypic Effect | Reference |
|---|---|---|---|
| JSC25-1 (a diploid lab strain) | Duplication of a region that contains catalase-encoding gene CTT1 on chrVII | Improved tolerance to H2O2 | [58] |
| FY834 | Deletion of the region between UBP2 and LSC1 on chrXV | Sensitive to hygromycin B | [131] |
| FY3 | Inversion of DAL cluster on chrIX | Drop in fitness in allantoin-containing medium | [132] |
| Strains isolated from Evolution Canyon, Israel | A rearranged 900-kb chromosome that contains two chrVIII fragments and a rearranged 650-kb chromosome containing a chrVII fragment and a chrVIII fragment. | Enhanced copper tolerance | [130] |
| GN | Translocation between chr XV and chrXVI involving the promoter of ADH1 and the gene SSU1 | Adaptation to sulfite | [134] |
| ScDup | Segmental duplication of region 0–158 kb on chrIII in ScDup (C3-1); 158–317 kb on chrIII in ScDup (C3-2); 398–577 kb on chrV in ScDup (C5-3); 800–1091 kb on chrVII in ScDup (C7-5); 195–403 kb on chr X in ScDup (C10-2); 491-692 kb on chr XII in ScDup (C12-3); 199–401kb on chrXV in ScDup (15-2) | Heat tolerance | [116] |
| ScDup | Segmental duplication of region 400–601 kb on chrII in ScDup (C2-3); 1401–1532 kb on chrIV in ScDup (C4-8) | Acetic acid tolerance | [116] |
| ScDup | Segmental duplication of region 0–201 kb on chrIV in ScDup (C4-1); 0–252 kb on chrXII in ScDup (C12-1); 491–692 kb on chrXII in ScDup (C12-3) | Lactic acid tolerance | [116] |
| ScDup | Segmental duplication of region 400–601 kb on chrII in ScDup (C2-3) | Formic acid tolerance | [116] |
| ScDup | Segmental duplication of region 400–601 kb on chrII in ScDup (C2-3); 0–158 kb on chrIII in ScDup (C3-1); 800–1091 kb on chrVII in ScDup (C7-5); 491–692 kb on chrXII in ScDup (C12-3); 0-205kb on chrXIII in ScDup (C13-1); 596–800 kb on chrXVI in ScDup (C16-4); | Enhanced tolerance to 1.2 M NaCl | [116] |
| ScDup | Segmental duplication of region 398–577 kb on chrV in ScDup (C5-3); 800–1091 kb on chrVII in ScDup (C7-5); 199–401kb on chrXV in ScDup (15-2); 198–399 kb on chrXVI in ScDup (C16-2); 596–800 kb on chrXVI in ScDup (C16-4) | Ethanol tolerance | [116] |
| Haploid strain with synthesized chrV | An inversion of a 7-kb region encoding GCN4, YEL008W, MIT1, and YEA6 genes and deletion of a 785-bp region containing the short MXR1 coding sequence | Improved xylose utilization | [112] |
| Haploid strain with synthesized chrV | Deletion of the YEL013W- containing region on synV | Improvement in carotenoid production | [111] |
| A diploid strain (crossing sake-brewing strain Y12 and a synthesized chrV-bearing strain) | Deletion of the region spanning YJL154C-YJL140W | Improved thermotolerance | [113] |
| Haploid strain with synthesized chrXII | An inversion involves ZRT2 and ACE2 on synXII | Enhanced tolerance to ethanol | [110] |
| A wine yeast strain | An inversion in chrXVI involves SSU1 and GCR1 regulatory regions | Tolerance to sulfite | [135] |
| Haploid strain with synthesized chrV | Deletion of the SPT2-containing region on synV | Enhanced alkali tolerance | [114] |
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rikova, K.; Guo, A.; Zeng, Q.; Possemato, A.; Yu, J.; Haack, H.; Nardone, J.; Lee, K.; Reeves, C.; Li, Y.; et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007, 131, 1190–1203. [Google Scholar] [CrossRef] [Green Version]
- Nebral, K. NUP98 is fused to topoisomerase (DNA) II 180 kDa (TOP2B) in a patient with Acute Myeloid Leukemia with a new t(3;11)(p24;p15). Clin. Cancer Res. 2005, 11, 6489–6494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, T.; Kumar, P.; Koseoglu, M.M.; Dutta, A. Discoveries of extrachromosomal circles of DNA in normal and tumor cells. Trends Genet. 2018, 34, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.M.; Deshpande, V.; Beyter, D.; Koga, T.; Rusert, J.; Lee, C.; Li, B.; Arden, K.; Ren, B.; Nathanson, D.A.; et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 2017, 543, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.R.; Dogan-Artun, N.; Faber, Z.J.; MacLeod, G.; Bartels, C.F.; Piazza, M.S.; Allan, K.C.; Mack, S.C.; Wang, X.X.; Gimple, R.C.; et al. Functional enhancers shape extrachromosomal oncogene amplifications. Cell 2019, 179, 1330–1341. [Google Scholar] [CrossRef]
- Wu, S.; Turner, K.M.; Nguyen, N.; Raviram, R.; Erb, M.; Santini, J.; Luebeck, J.; Rajkumar, U.; Diao, Y.; Li, B.; et al. Circular ecDNA promotes accessible chromatin and high oncogene expression. Nature 2019, 575, 699–703. [Google Scholar] [CrossRef]
- Koche, R.P.; Rodriguez-Fos, E.; Helmsauer, K.; Burkert, M.; MacArthur, I.C.; Maag, J.; Chamorro, R.; Munoz-Perez, N.; Puiggròs, M.; Garcia, H.D. Extrachromosomal circular DNA drives oncogenic genome remodeling in neuroblastoma. Nat. Genet. 2020, 52, 29–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Malone, J.H.; Powell, S.K.; Periwal, V.; Spana, E.; Macalpine, D.M.; Oliver, B. Expression in aneuploid Drosophila S2 cells. PLoS Biol. 2010, 8, e1000320. [Google Scholar] [CrossRef] [Green Version]
- Pologe, L.G.; Ravetch, J.V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. Nature 1986, 322, 474–477. [Google Scholar] [CrossRef]
- Selmecki, A.; Forche, A.; Berman, J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 2006, 313, 367–370. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, L.-J.; Fang, Y.-H.; Jin, X.-N.; Qi, L.; Wu, X.-C.; Zheng, D.-Q. Genomic structural variation contributes to phenotypic change of industrial bioethanol yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2016, 16, fov118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankenberg-Schwager, M.; Frankenberg, D. DNA double-strand breaks: Their repair and relationship to cell killing in yeast. Int. J. Radiat. Biol. 1990, 58, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Mimitou, E.P.; Symington, L.S. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 2008, 455, 770–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Chung, W.-H.; Shim, E.Y.; Lee, S.E.; Ira, G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 2008, 134, 981–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharyevich, K.; Ma, Y.; Tang, S.; Hwang, P.Y.-H.; Boiteux, S.; Hunter, N. Temporally and biochemically distinct activities of Exo1 during meiosis: Double-strand break resection and resolution of double holliday junctions. Mol. Cell 2010, 40, 1001–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichten, M.; Chung, W.-H.; Zhu, Z.; Papusha, A.; Malkova, A.; Ira, G. Defective resection at DNA double-strand breaks leads to de novo telomere formation and enhances gene targeting. PLoS Genet. 2010, 6, e1000948. [Google Scholar] [CrossRef] [Green Version]
- Gravel, S.; Chapman, J.R.; Magill, C.; Jackson, S.P. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008, 22, 2767–2772. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.K.; Yin, Y.; Petes, T.D.; Symington, L.S. Mre11-Sae2 and RPA Collaborate to Prevent Palindromic Gene Amplification. Mol. Cell 2015, 60, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Piazza, A.; Wright, W.D.; Heyer, W.D. Multi-invasions Are Recombination Byproducts that Induce Chromosomal Rearrangements. Cell 2017, 170, 760–773. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Mimitou, E.P.; Symington, L.S. Ku prevents Exo1 and Sgs1-dependent resection of DNA ends in the absence of a functional MRX complex or Sae2. EMBO J. 2010, 29, 3358–3369. [Google Scholar] [CrossRef] [Green Version]
- Pannunzio, N.R.; Watanabe, G.; Lieber, M.R. Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10512–10523. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.; Fu, B.X.H.; Heyer, W.-D. DNA polymerases δ and λ cooperate in repairing double-strand breaks by microhomology-mediated end-joining in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2015, 112, E6907–E6916. [Google Scholar] [CrossRef] [Green Version]
- McVey, M.; Lee, S.E. MMEJ repair of double-strand breaks (director’s cut): Deleted sequences and alternative endings. Trends Genet. 2008, 24, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.C.; Kowalczykowski, S.C. RecA: Regulation and Mechanism of a Molecular Search Engine. Trends Biochem. Sci. 2016, 41, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef]
- Jachimowicz, R.D.; Goergens, J.; Reinhardt, H.C. DNA double-strand break repair pathway choice—from basic biology to clinical exploitation. Cell Cycle 2019, 18, 1423–1434. [Google Scholar] [CrossRef]
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A.D. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016, 26, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Huang, J. DNA double-strand break repair pathway choice: The fork in the road. Genome Instab. Dis. 2020, 1, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Xu, D. Repair pathway choice for double-strand breaks. Essays Biochem. 2020, 64, 765–777. [Google Scholar] [CrossRef]
- Lehner, K.; Mudrak, S.V.; Minesinger, B.K.; Jinks-Robertson, S. Frameshift mutagenesis: The roles of primer-template misalignment and the nonhomologous end-joining pathway in Saccharomyces cerevisiae. Genetics 2012, 190, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.-E.; Jinks-Robertson, S. Deletions associated with stabilization of the Top1 cleavage complex in yeast are products of the nonhomologous end-joining pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 22683–22691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, R.; Carson, C.R.; Lee, G.; Stark, J.M. Contribution of canonical nonhomologous end joining to chromosomal rearrangements is enhanced by ATM kinase deficiency. Proc. Natl. Acad. Sci. USA 2017, 114, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Sunder, S.; Wilson, T.E. Frequency of DNA end joining in trans is not determined by the predamage spatial proximity of double-strand breaks in yeast. Proc. Natl. Acad. Sci. USA 2019, 116, 9481–9490. [Google Scholar] [CrossRef] [Green Version]
- Ahrabi, S.; Sarkar, S.; Pfister, S.X.; Pirovano, G.; Higgins, G.S.; Porter, A.C.; Humphrey, T.C. A role for human homologous recombination factors in suppressing microhomology-mediated end joining. Nucleic Acids Res. 2016, 44, 5743–5757. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, D.W.; Feng, W.; Conlin, M.P.; Yousefzadeh, M.J.; Roberts, S.A.; Mieczkowski, P.; Wood, R.D.; Gupta, G.P.; Ramsden, D.A. Essential Roles for Polymerase θ-Mediated End Joining in the Repair of Chromosome Breaks. Mol. Cell 2016, 63, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Symington, L.S.; Sinha, S.; Li, F.; Villarreal, D.; Shim, J.H.; Yoon, S.; Myung, K.; Shim, E.Y.; Lee, S.E. Microhomology-mediated end joining induces hypermutagenesis at breakpoint junctions. PLoS Genet. 2017, 13, e1006714. [Google Scholar] [CrossRef] [Green Version]
- Vaze, M.B.; Pellicioli, A.; Lee, S.E.; Ira, G.; Haber, J.E. Recovery from checkpoint-mediated arrest after repair of a double-strand break requires Srs2 helicase. Mol. Cell 2002, 10, 373–385. [Google Scholar] [CrossRef]
- Kirkpatrick, D.T.; Manthey, G.M.; Bailis, A.M. Rad51 Inhibits Translocation Formation by Non-Conservative Homologous Recombination in Saccharomyces cerevisiae. PLoS ONE 2010, 5, e11889. [Google Scholar] [CrossRef] [Green Version]
- Argueso, J.L.; Westmoreland, J.; Mieczkowski, P.A.; Gawel, M.; Petes, T.D.; Resnick, M.A. Double-strand breaks associated with repetitive DNA can reshape the genome. Proc. Natl. Acad. Sci. USA 2008, 105, 11845–11850. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, S.; Kockler, Z.; Evans, R.; Downing, B.D.; Malkova, A. Single-strand annealing between inverted DNA repeats: Pathway choice, participating proteins, and genome destabilizing consequences. PLoS Genet. 2018, 14, e1007543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.Z.; Putnam, C.D.; Kolodner, R.D. Mechanisms underlying genome instability mediated by formation of foldback inversions in Saccharomyces cerevisiae. Elife 2020, 9, e58223. [Google Scholar] [CrossRef] [PubMed]
- Symington, L.S.; Rothstein, R.; Lisby, M. Mechanisms and regulation of mitotic recombination in Saccharomyces cerevisiae. Genetics 2014, 198, 795–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansregret, L.; Swanton, C. The role of aneuploidy in cancer evolution. Cold Spring Harb. Persp. Med. 2017, 7, a028373. [Google Scholar] [CrossRef] [Green Version]
- Piazza, A.; Heyer, W.D. Homologous Recombination and the Formation of Complex Genomic Rearrangements. Trends Cell Biol. 2019, 29, 135–149. [Google Scholar] [CrossRef]
- Hastings, P.J.; Lupski, J.R.; Rosenberg, S.M.; Ira, G. Mechanisms of change in gene copy number. Nat. Rev. Genet. 2009, 10, 551–564. [Google Scholar] [CrossRef] [Green Version]
- Kramara, J.; Osia, B.; Malkova, A. Break-induced replication: The where, the why, and the how. Trends Genet. 2018, 34, 518–531. [Google Scholar] [CrossRef]
- Hastings, P.J.; Vasan, S.; Deem, A.; Ramakrishnan, S.; Argueso, J.L.; Malkova, A. Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication. PLoS Genet. 2014, 10, e1004119. [Google Scholar] [CrossRef] [Green Version]
- Piazza, A.; Heyer, W.-D. Multi-Invasion-Induced Rearrangements as a Pathway for Physiological and Pathological Recombination. Bioessays 2018, 40, e1700249. [Google Scholar] [CrossRef]
- Heyer, W.-D. Regulation of Recombination and Genomic Maintenance. Cold Spring Harb. Perspect. Biol. 2015, 7, a016501. [Google Scholar] [CrossRef] [Green Version]
- Myung, K.; Kolodner, R.D. Induction of genome instability by DNA damage in Saccharomyces cerevisiae. DNA Repair 2003, 2, 243–258. [Google Scholar] [CrossRef]
- Fasullo, M.; Dave, P.; Rothstein, R. DNA-damaging agents stimulate the formation of directed reciprocal translocations in Saccharomyces cerevisiae. Mutat. Res. 1994, 314, 121–133. [Google Scholar] [CrossRef]
- Sui, Y.; Qi, L.; Wu, J.K.; Wen, X.P.; Tang, X.X.; Ma, Z.J.; Wu, X.C.; Zhang, K.; Kokoska, R.J.; Zheng, D.Q.; et al. Genome-wide mapping of spontaneous genetic alterations in diploid yeast cells. Proc. Natl. Acad. Sci. USA 2020, 117, 28191–28200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.O.; Siegal, M.L.; Hall, D.W.; Petrov, D.A. Precise estimates of mutation rate and spectrum in yeast. Proc. Natl. Acad. Sci. USA 2014, 111, E2310–E2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio, N.M.V.; Ajith, V.; Watson, R.A.; Heasley, L.R.; Chakraborty, P.; Rodrigues-Prause, A.; Malc, E.P.; Mieczkowski, P.A.; Nishant, K.T.; Argueso, J.L. Characterization of systemic genomic instability in budding yeast. Proc. Natl. Acad. Sci. USA 2020, 117, 28221–28231. [Google Scholar] [CrossRef]
- Loeillet, S.; Herzog, M.; Puddu, F.; Legoix, P.; Baulande, S.; Jackson, S.P.; Nicolas, A.G. Trajectory and uniqueness of mutational signatures in yeast mutators. Proc. Natl. Acad. Sci. USA 2020, 117, 24947–24956. [Google Scholar] [CrossRef]
- St Charles, J.; Petes, T.D. High-resolution mapping of spontaneous mitotic recombination hotspots on the 1.1 Mb arm of yeast chromosome IV. PLoS Genet. 2013, 9, e1003434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zheng, D.Q.; Sui, Y.; Qi, L.; Petes, T.D. Genome-wide analysis of genomic alterations induced by oxidative DNA damage in yeast. Nucleic Acids Res. 2019, 47, 3521–3535. [Google Scholar] [CrossRef]
- Zhou, Z.-X.; Lujan, S.A.; Burkholder, A.B.; Garbacz, M.A.; Kunkel, T.A. Roles for DNA polymerase δ in initiating and terminating leading strand DNA replication. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Garbacz, M.A.; Cox, P.B.; Sharma, S.; Lujan, S.A.; Chabes, A.; Kunkel, T.A. The absence of the catalytic domains of Saccharomyces cerevisiae DNA polymerase ϵ strongly reduces DNA replication fidelity. Nucleic Acids Res. 2019, 47, 3986–3995. [Google Scholar] [CrossRef]
- Zheng, D.-Q.; Petes, T.D. Genome instability induced by low levels of replicative DNA polymerases in yeast. Genes 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ubhi, T.; Brown, G.W. Exploiting DNA replication stress for cancer treatment. Cancer Res. 2019, 79, 1730–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffia, A.; Ranise, C.; Sabbioneda, S. From R-Loops to G-Quadruplexes: Emerging New Threats for the Replication Fork. Int. J. Mol. Sci. 2020, 21, 1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Y.; Qi, L.; Zhang, K.; Saini, N.; Klimczak, L.J.; Sakofsky, C.J.; Gordenin, D.A.; Petes, T.D.; Zheng, D.-Q. Analysis of APOBEC-induced mutations in yeast strains with low levels of replicative DNA polymerases. Proc. Natl. Acad. Sci. USA 2020, 117, 9440–9450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.Q.; Zhang, K.; Wu, X.C.; Mieczkowski, P.A.; Petes, T.D. Global analysis of genomic instability caused by DNA replication stress in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2016, 113, E8114–E8121. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, F.J.; Degtyareva, N.P.; Lobachev, K.; Petes, T.D. Chromosomal translocations in yeast induced by low levels of DNA polymerase: A model for chromosome fragile sites. Cell 2005, 120, 587–598. [Google Scholar] [CrossRef] [Green Version]
- Kokoska, R.J.; Stefanovic, L.; DeMai, J.; Petes, T.D. Increased rates of genomic deletions generated by mutations in the yeast gene encoding DNA polymerase delta or by decreases in the cellular levels of DNA polymerase delta. Mol. Cell. Biol. 2000, 20, 7490–7504. [Google Scholar] [CrossRef] [Green Version]
- Salim, D.; Bradford, W.D.; Freeland, A.; Cady, G.; Wang, J.; Pruitt, S.C.; Gerton, J.L. DNA replication stress restricts ribosomal DNA copy number. PLoS Genet. 2017, 13, e1007006. [Google Scholar] [CrossRef]
- Srivatsan, A.; Li, B.; Sanchez, D.N.; Somach, S.B.; da Silva, V.L.; de Souza, S.J.; Putnam, C.D.; Kolodner, R.D. Essential Saccharomyces cerevisiae genome instability suppressing genes identify potential human tumor suppressors. Proc. Natl. Acad. Sci. USA 2019, 116, 17377–17382. [Google Scholar] [CrossRef] [Green Version]
- Bantele, S.C.S.; Lisby, M.; Pfander, B. Quantitative sensing and signalling of single-stranded DNA during the DNA damage response. Nat. Commun. 2019, 10, 944. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, I.; Seeber, A.; Shimada, K.; Keusch, J.J.; Gut, H.; Gasser, S.M. Structural Basis of Mec1-Ddc2-RPA Assembly and Activation on Single-Stranded DNA at Sites of Damage. Mol. Cell 2017, 68, 431–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menin, L.; Colombo, C.V.; Maestrini, G.; Longhese, M.P.; Clerici, M. Tel1/ATM Signaling to the Checkpoint Contributes to Replicative Senescence in the Absence of Telomerase. Genetics 2019, 213, 411–429. [Google Scholar] [CrossRef]
- McCulley, J.L.; Petes, T.D. Chromosome rearrangements and aneuploidy in yeast strains lacking both Tel1p and Mec1p reflect deficiencies in two different mechanisms. Proc. Natl. Acad. Sci. USA 2010, 107, 11465–11470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myung, K.; Datta, A.; Kolodner, R.D.J.C. Suppression of Spontaneous Chromosomal Rearrangements by S Phase Checkpoint Functions in Saccharomyces cerevisiae. Cell 2001, 104, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Putnam, C.D.; Pallis, K.; Hayes, T.K.; Kolodner, R.D. DNA repair pathway selection caused by defects in TEL1, SAE2, and de novo telomere addition generates specific chromosomal rearrangement signatures. PLoS Genet. 2014, 10, e1004277. [Google Scholar] [CrossRef] [Green Version]
- Putnam, C.D.; Kolodner, R.D. Pathways and Mechanisms that Prevent Genome Instability in Saccharomyces cerevisiae. Genetics 2017, 206, 1187–1225. [Google Scholar] [CrossRef] [Green Version]
- Putnam, C.D.; Hayes, T.K.; Kolodner, R.D. Specific pathways prevent duplication-mediated genome rearrangements. Nature 2009, 460, 984–989. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Kolodner, R.D. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat. Genet. 1999, 23, 81–85. [Google Scholar] [CrossRef]
- Myung, K.; Datta, A.; Chen, C.; Kolodner, R.D. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat. Genet. 2001, 27, 113–116. [Google Scholar] [CrossRef]
- Doerfler, L.; Schmidt, K.H. Exo1 phosphorylation status controls the hydroxyurea sensitivity of cells lacking the Pol32 subunit of DNA polymerases delta and zeta. DNA Repair 2014, 24, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Campos-Doerfler, L.; Syed, S.; Schmidt, K.H. Sgs1 Binding to Rad51 Stimulates Homology-Directed DNA Repair in Saccharomyces cerevisiae. Genetics 2018, 208, 125–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaniecki, K.; De Tullio, L.; Gibb, B.; Kwon, Y.; Sung, P.; Greene, E.C. Dissociation of Rad51 Presynaptic Complexes and Heteroduplex DNA Joints by Tandem Assemblies of Srs2. Cell Rep. 2017, 21, 3166–3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabre, F.; Chan, A.; Heyer, W.D.; Gangloff, S. Alternate pathways involving Sgs1/Top3, Mus81/ Mms4, and Srs2 prevent formation of toxic recombination intermediates from single-stranded gaps created by DNA replication. Proc. Natl. Acad. Sci. USA 2002, 99, 16887–16892. [Google Scholar] [CrossRef] [Green Version]
- Prakash, R.; Satory, D.; Dray, E.; Papusha, A.; Scheller, J.; Kramer, W.; Krejci, L.; Klein, H.; Haber, J.E.; Sung, P.; et al. Yeast Mph1 helicase dissociates Rad51-made D-loops: Implications for crossover control in mitotic recombination. Genes Dev. 2009, 23, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Fasching, C.L.; Cejka, P.; Kowalczykowski, S.C.; Heyer, W.D. Top3-Rmi1 dissolve Rad51-mediated D loops by a topoisomerase-based mechanism. Mol. Cell 2015, 57, 595–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, U.; Alani, E. Understanding how mismatch repair proteins participate in the repair/anti-recombination decision. FEMS Yeast Res. 2016, 16, fow071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hum, Y.F.; Jinks-Robertson, S. Mismatch recognition and subsequent processing have distinct effects on mitotic recombination intermediates and outcomes in yeast. Nucleic Acids Res. 2019, 47, 4554–4568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraki, K.; Han, L.; Miller, D.; Murnane, J.P. Processing by MRE11 is involved in the sensitivity of subtelomeric regions to DNA double-strand breaks. Nucleic Acids Res. 2015, 43, 7911–7930. [Google Scholar] [CrossRef] [Green Version]
- Kupiec, M. Damage-induced recombination in the yeast Saccharomyces cerevisiae. Mutat. Res. 2000, 451, 91–105. [Google Scholar] [CrossRef]
- Boiteux, S.; Jinks-Robertson, S. DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae. Genetics 2013, 193, 1025–1064. [Google Scholar] [CrossRef] [Green Version]
- Arbel, M.; Liefshitz, B.; Kupiec, M. DNA damage bypass pathways and their effect on mutagenesis in yeast. FEMS Microbiol. Rev. 2020. [Google Scholar] [CrossRef]
- Yin, Y.; Petes, T.D. Genome-wide high-resolution mapping of UV-induced mitotic recombination events in Saccharomyces cerevisiae. PLoS Genet. 2013, 9, e1003894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, G.R.; Laterza, A.M.; Sylvia, K.E.; Tartaglione, J.P. Potentiation of the mutagenicity and recombinagenicity of bleomycin in yeast by unconventional intercalating agents. Environ. Mol. Mutagen. 2011, 52, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.M.; Hoffmann, G.R. Frequencies of mutagen-induced coincident mitotic recombination at unlinked loci in Saccharomyces cerevisiae. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2007, 616, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Qi, L.; Sui, Y.; Li, Y.Z.; Yu, L.Z.; Zhang, K.; Xu, J.Z.; Wang, P.M.; Zheng, D.Q. Mapping chromosomal instability induced by small-molecular therapeutics in a yeast model. Appl. Microbiol. Biotechnol. 2019, 103, 4869–4880. [Google Scholar] [CrossRef] [PubMed]
- Gittens, W.H.; Johnson, D.J.; Allison, R.M.; Cooper, T.J.; Thomas, H.; Neale, M.J. A nucleotide resolution map of Top2-linked DNA breaks in the yeast and human genome. Nat. Commun. 2019, 10, 4846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tombline, G.; Millen, J.; Polevoda, B.; Rapaport, M.; Baxter, B.; Van Meter, M.; Gilbertson, M.; Madrey, J.; Piazza, G.A.; Rasmussen, L.J.A. Effects of an unusual poison identify a lifespan role for Topoisomerase 2 in Saccharomyces cerevisiae. Aging 2017, 9, 68–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, S.; Tsuda, M.; Bunch, H.; Sasanuma, H.; Austin, C.; Takeda, S. Type II DNA Topoisomerases Cause Spontaneous Double-Strand Breaks in Genomic DNA. Genes 2019, 10, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloan, R.; Huang, S.-Y.N.; Pommier, Y.; Jinks-Robertson, S. Effects of camptothecin or TOP1 overexpression on genetic stability in Saccharomyces cerevisiae. DNA Repair 2017, 59, 69–75. [Google Scholar] [CrossRef]
- Andersen, S.L.; Sloan, R.S.; Petes, T.D.; Jinks-Robertson, S. Genome-destabilizing effects associated with top1 loss or accumulation of top1 cleavage complexes in yeast. PLoS Genet. 2015, 11, e1005098. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Wang, Y.-T.; Tang, X.-X.; Zhang, K.; Wang, P.-M.; Sui, Y.; Zheng, D.-Q. Heat shock drives genomic instability and phenotypic variations in yeast. AMB Express 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.Q.; Jin, X.N.; Zhang, K.; Fang, Y.H.; Wu, X.C. Novel strategy to improve vanillin tolerance and ethanol fermentation performances of Saccharomycere cerevisiae strains. Bioresour. Technol. 2017, 231, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tong, M.; Gao, K.; Di, Y.; Wang, P.; Zhang, C.; Wu, X.; Zheng, D. Genomic reconstruction to improve bioethanol and ergosterol production of industrial yeast Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2015, 42, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.-Q.; Chen, J.; Zhang, K.; Gao, K.-H.; Li, O.; Wang, P.-M.; Zhang, X.-Y.; Du, F.-G.; Sun, P.-Y.; Qu, A.-M.; et al. Genomic structural variations contribute to trait improvement during whole-genome shuffling of yeast. Appl. Microbiol. Biotechnol. 2013, 98, 3059–3070. [Google Scholar] [CrossRef]
- Qi, L.; Wu, X.C.; Zheng, D.Q. Hydrogen peroxide, a potent inducer of global genomic instability. Curr. Genet. 2019, 65, 913–917. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J. Oxidative DNA damage & repair: An introduction. Free Radic. Biol. Med. 2017, 107, 2–12. [Google Scholar]
- Huang, M.-E.; Kolodner, R.D. A biological network in Saccharomyces cerevisiae prevents the deleterious effects of endogenous oxidative DNA damage. Mol. Cell 2005, 17, 709–720. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, K.; Wang, Y.T.; Wu, J.K.; Sui, Y.; Liang, X.Z.; Yu, L.Z.; Wu, X.C.; Wang, P.M.; Xu, J.Z.; et al. Global Analysis of Furfural-Induced Genomic Instability Using a Yeast Model. Appl. Environ. Microbiol. 2019, 85, e01237–e01319. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.M.; Mitchell, L.A.; Stracquadanio, G.; Yang, K.; Dymond, J.S.; DiCarlo, J.E.; Lee, D.; Huang, C.L.V.; Chandrasegaran, S.; Cai, Y. Design of a synthetic yeast genome. Science 2017, 355, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Wang, L.; Wang, Y.; Zhang, W.; Guo, Y.; Shen, Y.; Jiang, L.; Wu, Q.; Zhang, C.; Cai, Y.; et al. Identifying and characterizing SCRaMbLEd synthetic yeast using ReSCuES. Nat. Commun. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Jia, B.; Wu, Y.; Li, B.Z.; Mitchell, L.A.; Liu, H.; Pan, S.; Wang, J.; Zhang, H.R.; Jia, N.; Li, B.; et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast. Nat. Commun. 2018, 9, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blount, B.A.; Gowers, G.F.; Ho, J.C.H.; Ledesma-Amaro, R.; Jovicevic, D.; McKiernan, R.M.; Xie, Z.X.; Li, B.Z.; Yuan, Y.J.; Ellis, T. Rapid host strain improvement by in vivo rearrangement of a synthetic yeast chromosome. Nat. Commun. 2018, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Wu, Y.; Yang, K.; Li, Y.; Xu, H.; Zhang, H.; Li, B.Z.; Li, X.; Xiao, W.H.; Zhou, X.; et al. Heterozygous diploid and interspecies SCRaMbLEing. Nat. Commun. 2018, 9, 1934. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Li, Y.; Chen, X.; Ding, M.; Wu, Y.; Yuan, Y.J. SCRaMbLE generates evolved yeasts with increased alkali tolerance. Microb. Cell Fact. 2019, 18, 52. [Google Scholar] [CrossRef] [Green Version]
- Fleiss, A.; O’Donnell, S.; Fournier, T.; Lu, W.; Agier, N.; Delmas, S.; Schacherer, J.; Fischer, G. Reshuffling yeast chromosomes with CRISPR/Cas9. PLoS Genet. 2019, 15, e1008332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natesuntorn, W.; Iwami, K.; Matsubara, Y.; Sasano, Y.; Sugiyama, M.; Kaneko, Y.; Harashima, S. Genome-wide construction of a series of designed segmental aneuploids in Saccharomyces cerevisiae. Sci. Rep. 2015, 5, 12510. [Google Scholar] [CrossRef] [Green Version]
- Charles, J.S.; Hazkani-Covo, E.; Yin, Y.; Andersen, S.L.; Dietrich, F.S.; Greenwell, P.W.; Malc, E.; Mieczkowski, P.; Petes, T.D. High-resolution Genome-wide Analysis of Irradiated (UV and gamma rays) Diploid Yeast Cells Reveals a High Frequency of Genomic Loss of Heterozygosity (LOH) Events. Genetics 2012, 190, 1267–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGinty, R.J.; Rubinstein, R.G.; Neil, A.J.; Dominska, M.; Kiktev, D.; Petes, T.D.; Mirkin, S.M. Nanopore sequencing of complex genomic rearrangements in yeast reveals mechanisms of repeat-mediated double-strand break repair. Genome Res. 2017, 27, 2072–2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, A.; Holmes, N.; Rakyan, V.; Loose, M.; Birol, I. BulkVis: A graphical viewer for Oxford nanopore bulk FAST5 files. Bioinformatics 2019, 35, 2193–2198. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Holt, K.E. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol. 2019, 20, 129. [Google Scholar] [CrossRef] [Green Version]
- Sedlazeck, F.J.; Rescheneder, P.; Smolka, M.; Fang, H.; Nattestad, M.; Von Haeseler, A.; Schatz, M.C.J.N.M. Accurate detection of complex structural variations using single-molecule sequencing. Nat. Methods 2018, 15, 461–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nattestad, M.; Aboukhalil, R.; Chin, C.-S.; Schatz, M.C. Ribbon: Intuitive visualization for complex genomic variation. Bioinformatics 2020. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Urban, A.E.; Mills, R.E. Structural variation in the sequencing era. Nat. Rev. Genet. 2020, 21, 171–189. [Google Scholar] [CrossRef]
- Qin, L.; Dong, S.; Yu, J.; Ning, X.; Xu, K.; Zhang, S.J.; Xu, L.; Li, B.Z.; Li, J.; Yuan, Y.J.; et al. Stress-driven dynamic regulation of multiple tolerance genes improves robustness and productive capacity of Saccharomyces cerevisiae in industrial lignocellulose fermentation. Metab. Eng. 2020, 61, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Auesukaree, C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. J. Biosci. Bioeng. 2017, 124, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.; Stelkens, R. Aneuploidy in yeast: Segregation error or adaptation mechanism? Yeast 2019, 36, 525–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Jia, B.; Yuan, Y.-J. Yeast chromosomal engineering to improve industrially-relevant phenotypes. Curr. Opin. Biotechnol. 2020, 66, 165–170. [Google Scholar] [CrossRef]
- Hose, J.; Escalante, L.E.; Clowers, K.J.; Dutcher, H.A.; Robinson, D.; Bouriakov, V.; Coon, J.J.; Shishkova, E.; Gasch, A.P. The genetic basis of aneuploidy tolerance in wild yeast. Elife 2020, 9, e52063. [Google Scholar] [CrossRef]
- Sun, X.; Liu, L.; Zhao, Y.; Ma, T.; Zhao, F.; Huang, W.; Zhan, J. Effect of copper stress on growth characteristics and fermentation properties of Saccharomyces cerevisiae and the pathway of copper adsorption during wine fermentation. Food Chem. 2016, 192, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Lai, H.Y.; Tung, S.Y.; Leu, J.Y. Dynamic large-scale chromosomal rearrangements fuel rapid adaptation in yeast populations. PLoS Genet. 2013, 9, e1003232. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, M.; Nakazawa, T.; Murakami, K.; Sumiya, T.; Nakamura, A.; Kaneko, Y.; Nishizawa, M.; Harashima, S. PCR-mediated one-step deletion of targeted chromosomal regions in haploid Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2008, 80, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Naseeb, S.; Delneri, D. Impact of chromosomal inversions on the yeast DAL cluster. PLoS ONE 2012, 7, e42022. [Google Scholar] [CrossRef] [Green Version]
- Nadai, C.; Treu, L.; Campanaro, S.; Giacomini, A.; Corich, V. Different mechanisms of resistance modulate sulfite tolerance in wine yeasts. Appl. Microbiol. Biotechnol. 2016, 100, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Durand, C.; Loira, N.; Durrens, P.; Sherman, D.J.; Marullo, P. QTL dissection of Lag phase in wine fermentation reveals a new translocation responsible for Saccharomyces cerevisiae adaptation to sulfite. PLoS ONE 2014, 9, e86298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ríos, E.; Nuévalos, M.; Barrio, E.; Puig, S.; Guillamón, J.M. A new chromosomal rearrangement improves the adaptation of wine yeasts to sulfite. Environ. Microbiol. 2019, 21, 1771–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunham, M.J.; Badrane, H.; Ferea, T.; Adams, J.; Brown, P.O.; Rosenzweig, F.; Botstein, D. Characteristic genome rearrangements in experimental evolution of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2002, 99, 16144–16149. [Google Scholar] [CrossRef] [Green Version]
- Gresham, D.; Desai, M.M.; Tucker, C.M.; Jenq, H.T.; Pai, D.A.; Ward, A.; DeSevo, C.G.; Botstein, D.; Dunham, M.J. The repertoire and dynamics of evolutionary adaptations to controlled nutrient-limited environments in yeast. PLoS Genet. 2008, 4, e1000303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, C.R.; Sim, J.E.; Sullivan, S.J.; Featherstone, T.; Golden, W.; Von Kapp-Herr, C.; Hock, R.A.; Gomez, R.A.; Parsian, A.J.; Spitz, D.R. Genomic instability and catalase gene amplification induced by chronic exposure to oxidative stress. Cancer Res. 1998, 58, 3986–3992. [Google Scholar]
- Zhu, J.; Tsai, H.J.; Gordon, M.R.; Li, R. Cellular Stress Associated with Aneuploidy. Dev. Cell 2018, 44, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Jilderda, L.J.; Foijer, F. Exploiting aneuploidy-imposed stresses and coping mechanisms to battle cancer. Open Biol. 2020, 10, 200148. [Google Scholar] [CrossRef]
- Hull, R.M.; King, M.; Pizza, G.; Krueger, F.; Vergara, X.; Houseley, J. Transcription-induced formation of extrachromosomal DNA during yeast ageing. PLoS Biol. 2019, 17, e3000471. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.-X.; Wen, X.-P.; Qi, L.; Sui, Y.; Zhu, Y.-X.; Zheng, D.-Q. Origin, Regulation, and Fitness Effect of Chromosomal Rearrangements in the Yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 786. https://doi.org/10.3390/ijms22020786
Tang X-X, Wen X-P, Qi L, Sui Y, Zhu Y-X, Zheng D-Q. Origin, Regulation, and Fitness Effect of Chromosomal Rearrangements in the Yeast Saccharomyces cerevisiae. International Journal of Molecular Sciences. 2021; 22(2):786. https://doi.org/10.3390/ijms22020786
Chicago/Turabian StyleTang, Xing-Xing, Xue-Ping Wen, Lei Qi, Yang Sui, Ying-Xuan Zhu, and Dao-Qiong Zheng. 2021. "Origin, Regulation, and Fitness Effect of Chromosomal Rearrangements in the Yeast Saccharomyces cerevisiae" International Journal of Molecular Sciences 22, no. 2: 786. https://doi.org/10.3390/ijms22020786
APA StyleTang, X.-X., Wen, X.-P., Qi, L., Sui, Y., Zhu, Y.-X., & Zheng, D.-Q. (2021). Origin, Regulation, and Fitness Effect of Chromosomal Rearrangements in the Yeast Saccharomyces cerevisiae. International Journal of Molecular Sciences, 22(2), 786. https://doi.org/10.3390/ijms22020786





