Full-Length Transcriptome Analysis of Four Different Tissues of Cephalotaxus oliveri
Abstract
:1. Introduction
2. Results
2.1. The Full-Length Sequences of Pacbio Iso-Seq
2.2. De Novo Assembly of Illumina RNA-Seq Data
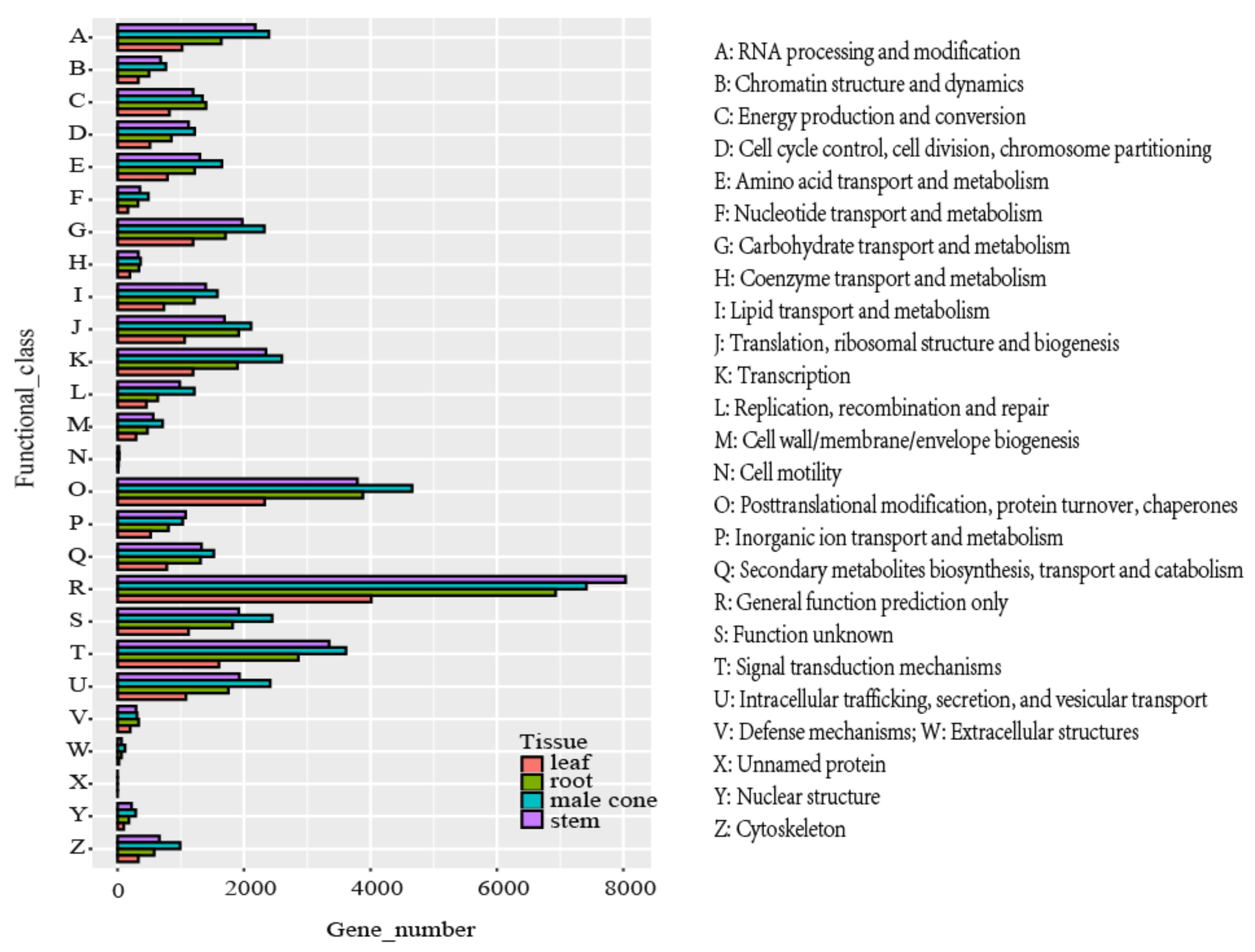
2.3. Functional Annotation
2.4. Identification of TFs, SSRs and LncRNAs
2.5. Gene Expression Level Analysis
2.6. GO Enrichment of Tissue-Specific Expressed Genes
2.7. KEGG Enrichment of Tissue-Specific Expressed Unigenes
2.8. Gene Families
2.9. Pathway Related to Environmental Adaptation
3. Discussion
3.1. Transcriptome Sequencing
3.2. Funtional Annotation
3.3. Potential Aoles of Transcription Factors, LncRNAs and SSRs
3.4. Tissue-Specific Expressed Genes
3.5. Gene Families
3.6. Characterization of the Unigenes in Plant Hormone Signal Transduction
3.7. Characterization of the Unigenes in Circadian Rhythm-Plant
4. Materials and Methods
4.1. Plant Materials and RNA Extraction
4.2. Illumina Library Preparation, Sequencing and de Novo Assembly
4.3. PacBio Library Preparation, Sequencing and Preprocessing
4.4. Functional Annotation of Transcripts
4.5. Prediction of CDSs, TFs, LncRNAs
4.6. Simple Sequence Repeat (SSR) Detection
4.7. Gene Expression Quantification and DEG analysis
4.8. Enrichment Analysis of Tissue-Specific Expressed Genes
4.9. Gene Family Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, S.; Mu, Z.Q.; Cheng, C.R.; Ding, J. Three new biflavonoids from the branches and leaves of Cephalotaxus oliveri and their antioxidant activity. Nat. Prod. Res. 2019, 33, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.G.; Li, N.; Mill, R.R. Cephalotaxaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press and Beijing and Missouri Botanical Garden Press: Beijing, China, 1999; pp. 85–88. [Google Scholar]
- Wang, T.; Wang, Z.; Xia, F.; Su, Y. Local adaptation to temperature and precipitation in naturally fragmented populations of Cephalotaxus oliveri, an endangered conifer endemic to China. Sci. Rep. 2016, 6, 25031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.; Lu, J.; Li, Z.Y.; Liu, R.L. Analysis of the Characteristics of Geographical Distribution and Main Community of Species Cephalotaxus Oliveri in Jiangxi. Jiangxi Sci. 2017, 35, 16–22. [Google Scholar] [CrossRef]
- Miao, Y.; Lang, X.; Li, S.; Su, J.; Wang, Y. Characterization of 15 Polymorphic Microsatellite Loci for Cephalotaxus oliveri (Cephalotaxaceae), a Conifer of Medicinal Importance. Int. J. Mol. Sci. 2012, 13, 11165–11172. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.W.; Guo, Y.R.; Su, Y.J.; Wang, T. Development of microsatellite loci for Cephalotaxus oliveri (Cephalotaxaceae) and cross-amplification in Cephalotaxus. Am. J. Bot. 2011, 98, e229–e232. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.B.; Wang, T.; Su, Y.J. Phylogeography of Cephalotaxus oliveri (Cephalotaxaceae) in relation to habitat heterogeneity, physical barriers and the uplift of the Yungui Plateau. Mol. Phylogenet. Evol. 2014, 80, 205–216. [Google Scholar] [CrossRef]
- De Miguel, M.; Guevara, M.A.; Sanchez-Gomez, D.; de Maria, N.; Diaz, L.M.; Mancha, J.A.; Fernandez de Simon, B.; Cadahia, E.; Desai, N.; Aranda, I.; et al. Organ-specific metabolic responses to drought in Pinus pinaster Ait. Plant. Physiol. Biochem. 2016, 102, 17–26. [Google Scholar] [CrossRef]
- Zhou, S.S.; Xing, Z.; Liu, H.; Hu, X.G.; Gao, Q.; Xu, J.; Jiao, S.Q.; Jia, K.H.; Jin, Y.Q.; Zhao, W.; et al. In-depth transcriptome characterization uncovers distinct gene family expansions for Cupressus gigantea important to this long-lived species’ adaptability to environmental cues. BMC Genom. 2019, 20, 213. [Google Scholar] [CrossRef]
- Meng, D.; Yu, X.; Ma, L.; Hu, J.; Liang, Y.; Liu, X.; Yin, H.; Liu, H.; He, X.; Li, D. Transcriptomic Response of Chinese Yew (Taxus chinensis) to Cold Stress. Front. Plant Sci. 2017, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Doron-Faigenboim, A.; Kelly, G.; Bourstein, R.; Attia, Z.; Zhou, J.; Moshe, Y.; Moshelion, M.; David-Schwartz, R. Transcriptome analysis of Pinus halepensis under drought stress and during recovery. Tree Physiol. 2018, 38, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Perdiguero, P.; Soto, Á.; Collada, C. Comparative analysis of Pinus pinea and Pinus pinaster dehydrins under drought stress. Tree Genet. Genomes 2015, 11. [Google Scholar] [CrossRef]
- Velasco-Conde, T.; Yakovlev, I.; Majada, J.P.; Aranda, I.; Johnsen, Ø. Dehydrins in maritime pine (Pinus pinaster) and their expression related to drought stress response. Tree Genet. Genomes 2012, 8, 957–973. [Google Scholar] [CrossRef] [Green Version]
- Takata, N.; Kasuga, J.; Takezawa, D.; Arakawa, K.; Fujikawa, S. Gene expression associated with increased supercooling capability in xylem parenchyma cells of larch (Larix kaempferi). J. Exp. Bot. 2007, 58, 3731–3742. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ma, J.; Niu, Z.; Bai, X.; Lei, W.; Shao, X.; Chen, N.; Zhou, F.; Wan, D. Tissue-Specific Transcriptome Analysis Reveals Multiple Responses to Salt Stress in Populus euphratica Seedlings. Genes 2017, 8, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barat, A.; Kumar, R.; Goel, C.; Singh, A.K.; Sahoo, P.K. De novo assembly and characterization of tissue-specific transcriptome in the endangered golden mahseer, Tor putitora. Meta Gene 2016, 7, 28–33. [Google Scholar] [CrossRef]
- Garg, R.; Patel, R.K.; Jhanwar, S.; Priya, P.; Bhattacharjee, A.; Yadav, G.; Bhatia, S.; Chattopadhyay, D.; Tyagi, A.K.; Jain, M. Gene discovery and tissue-specific transcriptome analysis in chickpea with massively parallel pyrosequencing and web resource development. Plant. Physiol. 2011, 156, 1661–1678. [Google Scholar] [CrossRef] [Green Version]
- Minio, A.; Massonnet, M.; Figueroa-Balderas, R.; Vondras, A.M.; Blanco-Ulate, B.; Cantu, D. Iso-Seq Allows Genome-Independent Transcriptome Profiling of Grape Berry Development. G3 Genes Genomes Genet. 2019, 9, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Kuang, X.; Sun, S.; Wei, J.; Li, Y.; Sun, C. Iso-Seq analysis of the Taxus cuspidata transcriptome reveals the complexity of Taxol biosynthesis. BMC Plant Biol. 2019, 19, 210. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.J.; Carneiro, M.O.; Schatz, M.C. The advantages of SMRT sequencing. Genome Biol. 2013, 14, 405. [Google Scholar] [CrossRef]
- Rhoads, A.; Au, K.F. PacBio Sequencing and Its Applications. Genom. Proteom. Bioinform. 2015, 13, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plan Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Batistič, O.; Kudla, J. Analysis of calcium signaling pathways in plants. Biochim. Biophys. Acta 2012, 1820, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q. Study on the Mechanism of Calcium Signaling in Cephalotaxus fortunei under Cold Stress. Master’s Thesis, Fujian Agriculture and Forestry University, Fujian, China, 2006. [Google Scholar]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Creux, N.; Harmer, S. Circadian Rhythms in Plants. Cold Spring Harb. Perspect. Biol. 2019, 11, a034611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurepin, L.V.; Dahal, K.P.; Savitch, L.V.; Singh, J.; Bode, R.; Ivanov, A.G.; Hurry, V.; Huener, N. Role of CBFs as integrators of chloroplast redox, phytochrome and plant hormone signaling during cold acclimation. Int. J. Mol. Sci. 2013, 14, 12729–12763. [Google Scholar] [CrossRef]
- Zhang, G.; Ryyppö, A.; Vapaavuori, E.; Repo, T. Quantification of additive response and stationarity of frost hardiness by photoperiod and temperature in Scots pine. Can. J. For. Res. 2003, 33, 1772–1784. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zhao, Z.; Li, Y.; Zhou, K.; Su, L.; Zhou, Q. Root transcriptome sequencing and differentially expressed drought-responsive genes in the Platycladus orientalis (L.). Tree Genet. Genomes 2016, 12. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, X.; Guo, Y.; Luo, C.; Zhang, L. Picea wilsonii transcription factor NAC2 enhanced plant tolerance to abiotic stress and participated in RFCP1-regulated flowering time. Plant Mol. Biol. 2018, 98, 471–493. [Google Scholar] [CrossRef]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 Zinc Finger Proteins: Master Regulators of Abiotic Stress Responses in Plants. Front. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xia, X.; Jiang, H.; Lu, Z.; Cui, J.; Cao, F.; Jin, B. Genome-wide identification and characterization of novel lncRNAs in Ginkgo biloba. Trees 2018, 32, 1429–1442. [Google Scholar] [CrossRef]
- Silva-Sanchez, C.; Li, H.; Chen, S. Recent advances and challenges in plant phosphoproteomics. Proteomics 2015, 15, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Velten, J.; Oliver, M.J. Tr288, a rehydrin with a dehydrin twist. Plant Mol. Biol. 2001, 45, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Close, T.J. Dehydrins: A commonalty in the response of plants to dehydration and low temperature. Physiol. Plant. 1997, 100, 291–296. [Google Scholar] [CrossRef]
- Ingram, J.; Bartels, D. The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Biol. 1996, 47, 377–403. [Google Scholar] [CrossRef] [Green Version]
- Drira, M.; Saibi, W.; Brini, F.; Gargouri, A.; Masmoudi, K.; Hanin, M. The K-segments of the wheat dehydrin DHN-5 are essential for the protection of lactate dehydrogenase and β-glucosidase activities in vitro. Mol. Biotechnol. 2013, 54, 643–650. [Google Scholar] [CrossRef]
- Rorat, T. Plant dehydrins-tissue location, structure and function. Cell. Mol. Biol. Lett. 2006, 11, 536–556. [Google Scholar] [CrossRef]
- Kosová, K.; Prášil, I.T.; Vítámvás, P. 10 Role of Dehydrins in Plant Stress Response. In Handbook of Plant and Crop Stress, 3rd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Hara, M. The multifunctionality of dehydrins: An overview. Plant Signal. Behav. 2010, 5, 503–508. [Google Scholar] [CrossRef]
- Cao, F.; Cheng, H.; Cheng, S.; Li, L.; Xu, F.; Yu, W.; Yuan, H. Expression of selected Ginkgo biloba heat shock protein genes after cold treatment could be induced by other abiotic stress. Int. J. Mol. Sci. 2012, 13, 5768–5788. [Google Scholar] [CrossRef]
- Lee, G.J.; Vierling, E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 2000, 122, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Ehrnsperger, M.; Gräber, S.; Gaestel, M.; Buchner, J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997, 16, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.-L.; Wang, J.-S.; Liu, H.-C.; Chen, R.-W.; Meyer, Y.; Barakat, A.; Delseny, M. Genomic analysis of the Hsp70 superfamily in Arabidopsis thaliana. Cell Stress Chaperones 2001, 6, 201. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Moarefi, I.; Hartl, F.U. Hsp90: A specialized but essential protein-folding tool. J. Cell Biol. 2001, 154, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Queitsch, C.; Sangster, T.A.; Lindquist, S. Hsp90 as a capacitor of phenotypic variation. Nature 2002, 417, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Werck-Reichhart, D. A P450-centric view of plant evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, S.; Zhang, M.; Dong, K.; Chen, Y.; Fu, C.; Yu, L. Transcriptome Assembly and Systematic Identification of Novel Cytochrome P450s in Taxus chinensis. Front. Plant Sci. 2017, 8, 1468. [Google Scholar] [CrossRef] [Green Version]
- Geisler, K.; Hughes, R.K.; Sainsbury, F.; Lomonossoff, G.P.; Rejzek, M.; Fairhurst, S.; Olsen, C.-E.; Motawia, M.S.; Melton, R.E.; Hemmings, A.M. Biochemical analysis of a multifunctional cytochrome P450 (CYP51) enzyme required for synthesis of antimicrobial triterpenes in plants. Proc. Natl. Acad. Sci. USA 2013, 110, E3360–E3367. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.K.; Duan, C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2019, 62, 563–580. [Google Scholar] [CrossRef]
- Kumar, M.; Kesawat, M.S.; Ali, A.; Lee, S.C.; Gill, S.S.; Kim, A.H.U. Integration of Abscisic Acid Signaling with Other Signaling Pathways in Plant Stress Responses and Development. Plants 2019, 8, 592. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. Omics approaches toward defining the comprehensive abscisic acid signaling network in plants. Plant Cell Physiol. 2015, 56, 1043–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goossens, J.; Fernandez-Calvo, P.; Schweizer, F.; Goossens, A. Jasmonates: Signal transduction components and their roles in environmental stress responses. Plant Mol. Biol. 2016, 91, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Palmer, I.A.; Shang, Z.; Fu, Z.Q. Salicylic acid-mediated plant defense: Recent developments, missing links, and future outlook. Front. Biol. 2017, 12, 258–270. [Google Scholar] [CrossRef]
- Herrera-Vasquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef] [Green Version]
- Yerushalmi, S.; Green, R.M. Evidence for the adaptive significance of circadian rhythms. Ecol. Lett. 2009, 12, 970–981. [Google Scholar] [CrossRef]
- Pruneda-Paz, J.L.; Kay, S.A. An expanding universe of circadian networks in higher plants. Trends Plant Sci. 2010, 15, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Harmer, S.L.; Hogenesch, J.B.; Straume, M.; Chang, H.-S.; Han, B.; Zhu, T.; Wang, X.; Kreps, J.A.; Kay, S.A. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 2000, 290, 2110–2113. [Google Scholar] [CrossRef]
- Fornara, F.; De Montaigu, A.; Sánchez-Villarreal, A.; Takahashi, Y.; Ver Loren van Themaat, E.; Huettel, B.; Davis, S.J.; Coupland, G. The GI–CDF module of Arabidopsis affects freezing tolerance and growth as well as flowering. Plant J. 2015, 81, 695–706. [Google Scholar] [CrossRef] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–U130. [Google Scholar] [CrossRef] [Green Version]
- Wenger, A.M.; Peluso, P.; Rowell, W.J.; Chang, P.-C.; Hall, R.J.; Concepcion, G.T.; Ebler, J.; Fungtammasan, A.; Kolesnikov, A.; Olson, N.D.; et al. Accurate circular consensus long-read sequencing improves variant detection and assembly of a human genome. Nat. Biotechnol. 2019, 37, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Salmela, L.; Rivals, E. LoRDEC: Accurate and efficient long read error correction. Bioinformatics 2014, 30, 3506–3514. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Simao, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [Green Version]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [Green Version]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Adachi, J.; Muraoka, Y. ANGLE: A sequencing errors resistant program for predicting protein coding regions in unfinished cDNA. J. Bioinform. Comput. Biol. 2006, 4, 649–664. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, C.; Sun, H.; Rosli, H.G.; Pombo, M.A.; Zhang, P.; Banf, M.; Dai, X.; Martin, G.B.; Giovannoni, J.J. iTAK: A program for genome-wide prediction and classification of plant transcription factors, transcriptional regulators, and protein kinases. Mol. Plant 2016, 9, 1667–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Ge, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Zhou, Z. PLEK: A tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinform. 2014, 15, 311. [Google Scholar]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Goseq: Gene Ontology testing for RNA-seq datasets. R Bioconduct. 2012, 8, 1–25. [Google Scholar]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Bailey, T.L.; Gribskov, M. Combining evidence using p-values: Application to sequence homology searches. Bioinformatics 1998, 14, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozewicki, J.; Li, S.; Amada, K.M.; Standley, D.M.; Katoh, K. MAFFT-DASH: Integrated protein sequence and structural alignment. Nucleic Acids Res. 2019, 47, W5–W10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]









| Database | Male Cone | Stem | Leaf | Root |
|---|---|---|---|---|
| Nr | 57,787 | 51,624 | 28,649 | 48,379 |
| Swiss-Prot | 50,366 | 44,725 | 24,361 | 39,525 |
| KEGG | 57,094 | 50,652 | 27,952 | 46,851 |
| KOG | 38,768 | 34,613 | 18,671 | 31,056 |
| GO | 40,828 | 35,387 | 19,395 | 29,940 |
| Nt | 40,691 | 32,627 | 18,436 | 29,751 |
| Pfam | 40,828 | 35,387 | 19,395 | 29,940 |
| At least one database | 58,601 | 52,990 | 29,411 | 51,054 |
| All databases | 23,857 | 18,801 | 10,302 | 14,577 |
| GO_ID | GO_Term | Male Cone | Leaf | Root | Stem |
|---|---|---|---|---|---|
| GO: 0006950 | Response to stress | 218 | 124 | 207 | 190 |
| GO: 0009733 | Response to auxin stimulus | 9 | 8 | 15 | 5 |
| GO: 0009415 | Response to water | 28 | 53 | 43 | 63 |
| GO: 0006979 | Response to oxidative stress | 68 | 39 | 105 | 61 |
| GO: 0009725 | Response to hormone stimulus | 78 | 24 | 39 | 68 |
| Degs Set Name | All Degs Number | Up-Regulated Degs Number | Down-Regulated Degs Number |
|---|---|---|---|
| Male cone vs. leaf | 10,343 | 5371 | 4972 |
| Male cone vs. root | 12,021 | 5779 | 6242 |
| male cone vs. stem | 9465 | 4897 | 4568 |
| Leaf vs. root | 9704 | 4262 | 5442 |
| Leaf vs. stem | 1634 | 1076 | 558 |
| Root vs. stem | 8385 | 5046 | 3339 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Su, Y.; Wang, T. Full-Length Transcriptome Analysis of Four Different Tissues of Cephalotaxus oliveri. Int. J. Mol. Sci. 2021, 22, 787. https://doi.org/10.3390/ijms22020787
He Z, Su Y, Wang T. Full-Length Transcriptome Analysis of Four Different Tissues of Cephalotaxus oliveri. International Journal of Molecular Sciences. 2021; 22(2):787. https://doi.org/10.3390/ijms22020787
Chicago/Turabian StyleHe, Ziqing, Yingjuan Su, and Ting Wang. 2021. "Full-Length Transcriptome Analysis of Four Different Tissues of Cephalotaxus oliveri" International Journal of Molecular Sciences 22, no. 2: 787. https://doi.org/10.3390/ijms22020787
APA StyleHe, Z., Su, Y., & Wang, T. (2021). Full-Length Transcriptome Analysis of Four Different Tissues of Cephalotaxus oliveri. International Journal of Molecular Sciences, 22(2), 787. https://doi.org/10.3390/ijms22020787





