Dynamic Changes in the Ability to Release Neutrophil ExtraCellular Traps in the Course of Childhood Acute Leukemias
Abstract
1. Introduction
2. Results
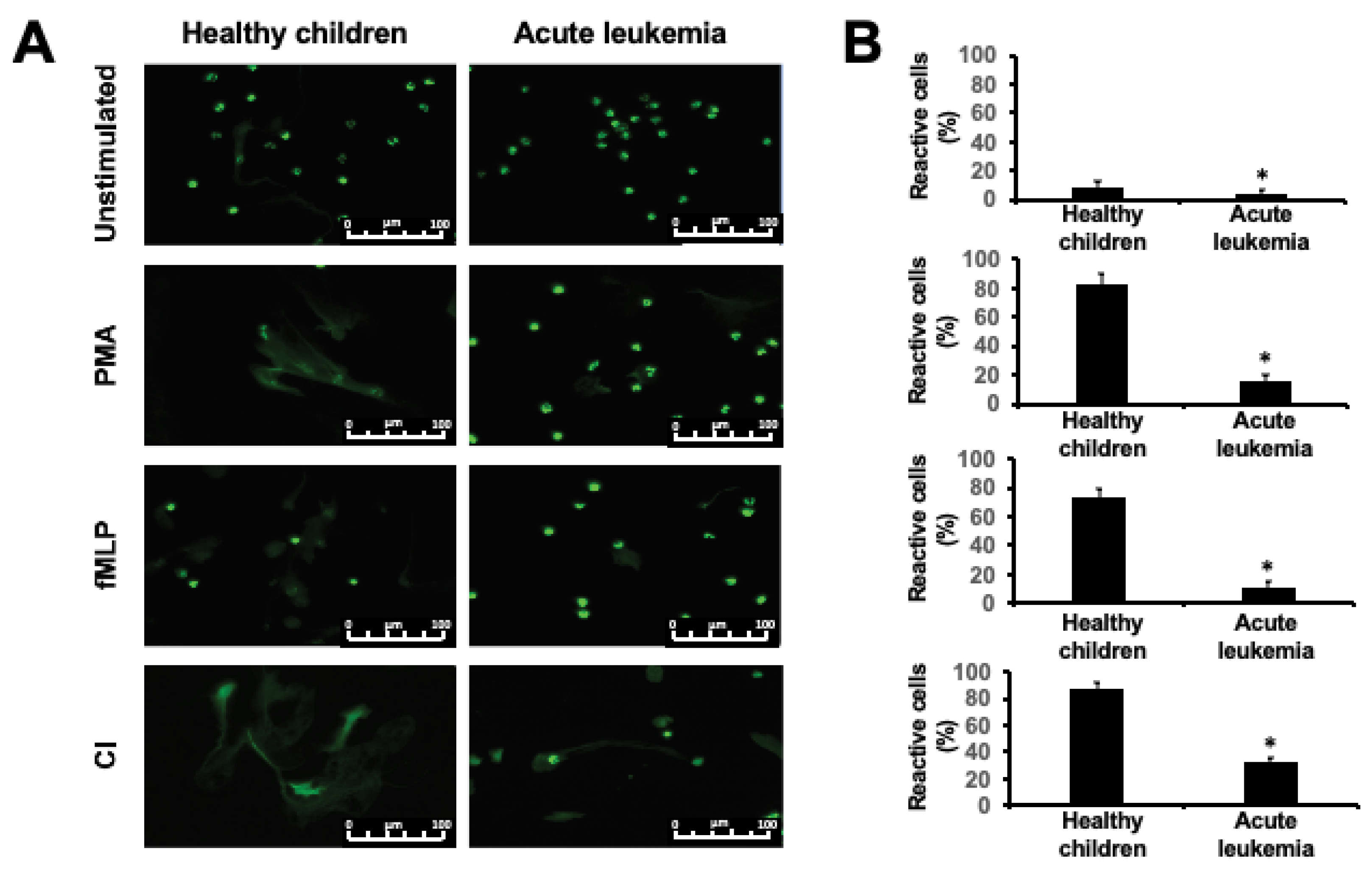
2.1. NET Release is Impaired in Children with Acute Leukemia
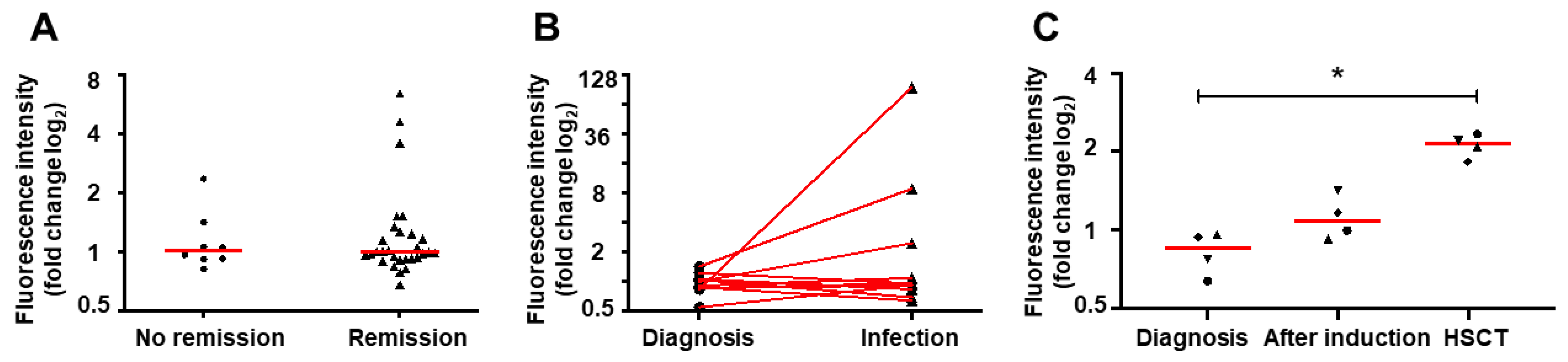
2.2. NET Formation Impairment is Retained During Infections
2.3. Induction Therapy Does Not Restore NET Release In Vitro
2.4. Hematopoietic Stem Cell Transplantation Restores NETs Formation
2.5. NETs Release Changes Dynamically During the Treatment of Acute Leukemia
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Isolation of Neutrophils from Peripheral Blood
4.3. Quantitative NET Formation Measurement
4.4. Microscopic Evaluation of NET Formation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extra-cellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet tlr4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Mihalache, C.C.; Kozlowski, E.O.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.-J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Manda-Handzlik, A.; Pruchniak, M.P.; Arazna, M.; Demkow, U.A. Neutrophil extracellular traps in physiology and pathology. Central Eur. J. Immunol. 2014, 39, 116–121. [Google Scholar] [CrossRef]
- Hazeldine, J.; Harris, P.; Chapple, I.L.; Grant, M.; Greenwood, H.; Livesey, A.; Sapey, E.; Lord, J.M. Impaired neutrophil extracellular trap formation: A novel defect in the innate immune system of aged individuals. Aging Cell 2014, 13, 690–698. [Google Scholar] [CrossRef]
- Hansen, B.-A.; Wendelbo, Ø.; Bruserud, Ø.; Hemsing, A.L.; Mosevoll, K.A.; Reikvam, H. Febrile neutropenia in acute leukemia. Epidemiology, etiology, pathophysiology and treatment. Mediterr. J. Hematol. Infect. Dis. 2019, 12, e2020009. [Google Scholar] [CrossRef]
- Levy-Mendelovich, S.; Barg, A.A.; Kenet, G. Thrombosis in pediatric patients with leukemia. Thromb. Res. 2018, 164, S94–S97. [Google Scholar] [CrossRef]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular dna traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.W.; Devidas, M.; Wang, C.; Mattano, L.A.; Friedmann, A.M.; Buckley, P.; Borowitz, M.J.; Carroll, A.J.; Gastier-Foster, J.M.; Heerema, N.A.; et al. Outcome in Children With Standard-Risk B-Cell Acute Lymphoblastic Leukemia: Results of Children’s Oncology Group Trial AALL0331. J. Clin. Oncol. 2020, 38, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Zawitkowska, J.; Drabko, K.; Szmydki-Baran, A.; Zaucha-Prażmo, A.; Lejman, M.; Czyżewski, K.; Zalas-Więcek, P.; Gryniewicz–Kwiatkowska, O.; Czajńska-Deptuła, A.; Kulicka, E.; et al. Infectious profile in children with ALL during chemotherapy: A report of study group for infections. J. Infect. Chemother. 2019, 25, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Zając-Spychała, O.; Skalska-Sadowska, J.; Wachowiak, J.; Szmydki-Baran, A.; Hutnik, Ł.; Matysiak, M.; Pierlejewski, F.; Młynarski, W.; Czyżewski, K.; Dziedzic, M.; et al. Infections in children with acute myeloid leukemia: Increased mortality in re-lapsed/refractory patients. Leuk. Lymphoma 2019, 60, 3028–3035. [Google Scholar]
- Caniza, M.; Odio, C.; Mukkada, S.; González, M.; Ceppi, F.; Chaisavaneeyakorn, S.; Apiwattanakul, N.; Howard, S.C.; Conter, V.; Bonilla, M. Infectious complications in children with acute lymphoblastic leukemia treated in low-middle-income countries. Expert Rev. Hematol. 2015, 8, 627–645. [Google Scholar] [CrossRef]
- Schmidt, T.; Brodesser, A.; Schnitzler, N.; Grüger, T.; Brandenburg, K.; Zinserling, J.; Zündorf, J. CD66b Overexpression and Loss of C5a Receptors as Surface Markers for Staphylococcus aureus-Induced Neutrophil Dysfunction. PLoS ONE 2015, 10, e0132703. [Google Scholar] [CrossRef]
- Cox, L.E.; Walstein, K.; Völlger, L.; Reuner, F.; Bick, A.; Dötsch, A.; Engler, A.; Peters, J.; von Köckritz-Blickwede, M.; Schäfer, S.T. Neu-trophil extracellular trap formation and nuclease activity in septic patients. BMC Anesthesiol. 2020, 20, 15. [Google Scholar]
- Agraz-Cibrián, J.M.; Delgado-Rizo, V.; Segura-Ortega, J.E.; Maldonado-Gómez, H.A.; Zambrano-Zaragoza, J.F.; Durán-Avelar, M.D.J.; Vibanco-Perez, N.; Morris, M.F. Impaired neutrophil extracellular traps and inflammatory responses in the peritoneal fluid of patients with liver cirrhosis. Scand. J. Immunol. 2018, 88, e12714. [Google Scholar] [CrossRef]
- Hashiba, M.; Huq, A.; Tomino, A.; Hirakawa, A.; Hattori, T.; Miyabe, H.; Tsuda, M.; Takeyama, N. Neutrophil extracellular traps in patients with sepsis. J. Surg. Res. 2015, 194, 248–254. [Google Scholar] [CrossRef]
- Chechlinska, M.; Kowalewska, M.; Nowak, R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat. Rev. Cancer 2010, 10, 2–3. [Google Scholar] [CrossRef]
- Kim, T.Y.; Gu, J.-Y.; Jung, H.S.; Koh, Y.; Kim, I.; Kim, H.K. Elevated extracellular trap formation and contact system activation in acute leukemia. J. Thromb. Thrombolysis 2018, 46, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Podaza, E.; Sabbione, F.; Risnik, D.; Borge, M.; Almejún, M.B.; Colado, A.; Fernández-Grecco, H.; Cabrejo, M.; Bezares, R.F.; Trevani, A.; et al. Neutrophils from chronic lymphocytic leukemia patients exhibit an increased capacity to release extracellular traps (NETs). Cancer Immunol. Immunother. 2016, 66, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Erpenbeck, L.; Schön, M.P. Neutrophil extracellular traps: Protagonists of cancer progression? Oncogene 2017, 36, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Bystrzycka, W.; Moskalik, A.; Sieczkowska, S.; Manda-Handzlik, A.; Demkow, U.; Ciepiela, O. The effect of clindamycin and amoxicillin on neutrophil extracellular trap (NET) release. Central Eur. J. Immunol. 2016, 41, 1–5. [Google Scholar] [CrossRef]
- Fomenko, Y.; Kolesnikova, Y.; Beynikova, I.; Muravlyova, L.Y.; Sirota, V.; Bakirova, R. Influence of Combined Therapy on Generation of Neutrophil Extracellular Traps in Patients with Cervical Cancer. Open Access Maced. J. Med. Sci. 2018, 6, 2097–2100. [Google Scholar] [CrossRef]
- Hamblin, A.D.; Hamblin, T.J. The immunodeficiency of chronic lymphocytic leukaemia. Br. Med. Bull. 2008, 87, 49–62. [Google Scholar] [CrossRef]
- Pietarinen, P.O.; Eide, C.A.; Ayuda-Durán, P.; Potdar, S.; Kuusanmäki, H.; Andersson, E.I.; Mpindi, J.P.; Pemovska, T.; Kontro, M.; Heckman, C.A.; et al. Differentiation status of primary chronic myeloid leukemia cells affects sensitivity to bcr-abl1 inhibitors. Oncotarget 2017, 8, 22606–22615. [Google Scholar] [CrossRef]
- Berger-Achituv, S.; Elhasid, R. Reduced Neutrophil Elastase Activity and Neutrophil Extracellular Traps in Pediatric Acute Myeloid Leukemia May Increase the Rate of Infections. J. Pediatr. Hematol. 2018, 40, e248–e252. [Google Scholar] [CrossRef]
- Lukášová, E.; Kořistek, Z.; Klabusay, M.; Ondřej, V.; Grigoryev, S.; Bačíková, A.; Řezáčová, M.; Falk, M.; Vávrová, J.; Kohútová, V.; et al. Granulocyte maturation determines ability to release chromatin nets and loss of dna damage response; these properties are absent in immature aml granulocytes. Biochim. Biophys. Acta 2013, 1833, 767–779. [Google Scholar]
- Tanaka, F.; Goto, H.; Yokosuka, T.; Yanagimachi, M.; Kajiwara, R.; Naruto, T.; Nishimaki, S.; Yokota, S. Suppressed neutrophil function in children with acute lymphoblastic leukemia. Int. J. Hematol. 2009, 90, 311–317. [Google Scholar] [CrossRef]
- Boeltz, S.; Amini, P.; Anders, H.-J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Radic, M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front. Immunol. 2013, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Manda-Handzlik, A.; Bystrzycka, W.; Cieloch, A.; Glodkowska-Mrowka, E.; Jankowska-Steifer, E.; Heropolitanska-Pliszka, E.; Skrobot, A.; Muchowicz, A.; Ciepiela, O.; Wachowska, M.; et al. Nitric oxide and peroxynitrite trigger and enhance release of neu-trophil extracellular traps. Cell. Mol. Life Sci. 2020, 77, 3059–3075. [Google Scholar]
- de Buhr, N.; von Köckritz-Blickwede, M. Detection, visualization, and quantification of neutrophil extracellular traps (nets) and net markers. Methods Mol. Biol. 2020, 2087, 425–442. [Google Scholar] [PubMed]
- Kamoshida, G.; Kikuchi-Ueda, T.; Nishida, S.; Tansho-Nagakawa, S.; Kikuchi, H.; Ubagai, T.; Ono, Y. Spontaneous formation of neutrophil extracellular traps in serum-free culture conditions. FEBS Open Bio. 2017, 7, 877–886. [Google Scholar] [CrossRef] [PubMed]


| Patient ID | Age at Diagnosis | Sex | WBC at Diagnosis [G/L] | Type of Leukemia | Response to 1st Induction Therapy at Day 30 | BMT Donor | Time of Analysis (Days after BMT) |
|---|---|---|---|---|---|---|---|
| 8 | 14 | F | 1.1 | Common ALL | Remission | Sibling | 197 |
| 14 | 14 | M | 12.4 | Common ALL | No remission | Unrelated | 209 |
| 15 | 5 | M | 7.6 | Common ALL | Remission | Sibling | 242 |
| 16 | 5 | F | 3.9 | Common ALL | Remission | Sibling | 213 |
| Diagnosis | Number of Patients (n) | Sex (Females/ Males) | Age (Years) Median (min–max) | WBC at Diagnosis (Mean +/− SD) (G/L) | Neutrophil at Diagnosis (Mean +/− SD) (G/L) | PLT at Diagnosis (Mean +/− SD) (G/L) | |
|---|---|---|---|---|---|---|---|
| ALL | common B | 24 | 13/11 | 4 (1–14) | 12.85 ± 20.7 | 1.7 ± 3.35 | 133.9 ± 112.2 |
| pre-B | 6 | 4/2 | 9.5 (1–16) | 13.36 ± 8.4 | 3.47 ± 3.7 | 66.2 ± 23.5 | |
| pro-B | 3 | 2/1 | 7 (1–8) | 235.5 ± 402.2 | 0.3 ± 0.36 | 50.33 ± 26.63 | |
| T-cell | 4 | 1/3 | 7.5 (6–9) | 101.02 ± 104.23 | 34.87 ± 44.44 | 84.5 ± 20.2 | |
| AML | M0 | 1 | 1/0 | 11 | 1 | 0.2 | 26 |
| M1 | 2 | 2/0 | 10 (8–12) | 3.95 ± 63.3 | 17.37 ± 16.74 | 29.48 ± 44.74 | |
| M4 | 1 | 1/0 | 16 | 145.6 | 123 | 59 | |
| M0/M1 | 1 | 1/0 | 1 | 6.9 | n/d | 62 | |
| M1/M2 | 2 | 2/0 | 8.5 (8–9) | 7.05 ± 8.99 | 1.7 +/− 1.84 | 331.5 ± 412.24 | |
| M4/M5 | 1 | 0/1 | 2 | 8.1 | 1.6 | 79 | |
| Control group | 28 | 13/15 | 9 (1–16) | 8.7 ± 2.4 | 3.5 ± 1.2 | 283.6 ± 65 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostafin, M.; Ciepiela, O.; Pruchniak, M.; Wachowska, M.; Ulińska, E.; Mrówka, P.; Głodkowska-Mrówka, E.; Demkow, U. Dynamic Changes in the Ability to Release Neutrophil ExtraCellular Traps in the Course of Childhood Acute Leukemias. Int. J. Mol. Sci. 2021, 22, 821. https://doi.org/10.3390/ijms22020821
Ostafin M, Ciepiela O, Pruchniak M, Wachowska M, Ulińska E, Mrówka P, Głodkowska-Mrówka E, Demkow U. Dynamic Changes in the Ability to Release Neutrophil ExtraCellular Traps in the Course of Childhood Acute Leukemias. International Journal of Molecular Sciences. 2021; 22(2):821. https://doi.org/10.3390/ijms22020821
Chicago/Turabian StyleOstafin, Magdalena, Olga Ciepiela, Michał Pruchniak, Małgorzata Wachowska, Edyta Ulińska, Piotr Mrówka, Eliza Głodkowska-Mrówka, and Urszula Demkow. 2021. "Dynamic Changes in the Ability to Release Neutrophil ExtraCellular Traps in the Course of Childhood Acute Leukemias" International Journal of Molecular Sciences 22, no. 2: 821. https://doi.org/10.3390/ijms22020821
APA StyleOstafin, M., Ciepiela, O., Pruchniak, M., Wachowska, M., Ulińska, E., Mrówka, P., Głodkowska-Mrówka, E., & Demkow, U. (2021). Dynamic Changes in the Ability to Release Neutrophil ExtraCellular Traps in the Course of Childhood Acute Leukemias. International Journal of Molecular Sciences, 22(2), 821. https://doi.org/10.3390/ijms22020821






