BAFF Inhibition Effectively Suppresses the Development of Anti-HLA.A2 Antibody in the Highly Sensitized Mouse Model
Abstract
1. Introduction
2. Results
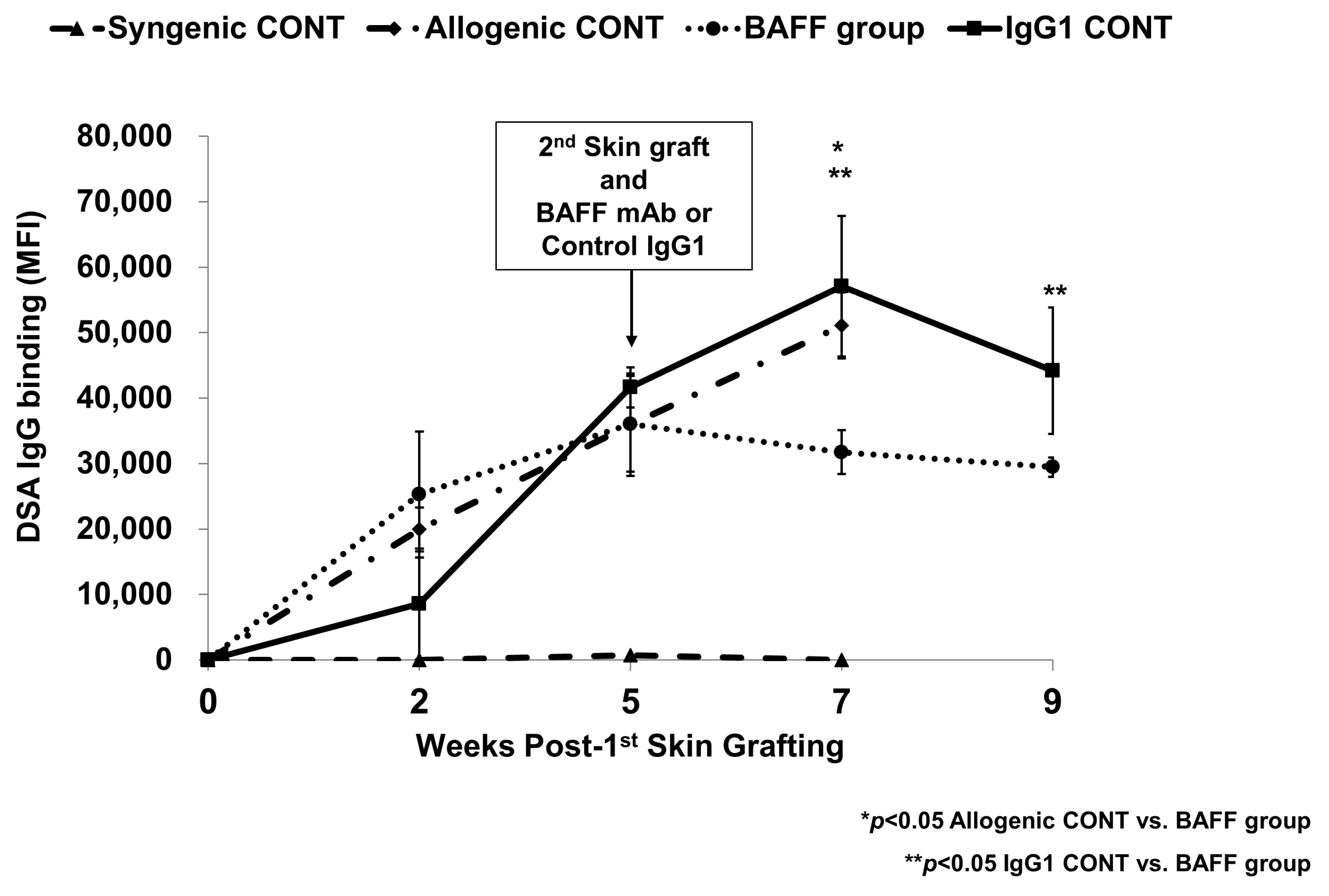
2.1. Comparison of Specific IgG Responses to Skin Allograft in Each Group
2.2. Comparison of B Cell Fractions in the Bone Marrow
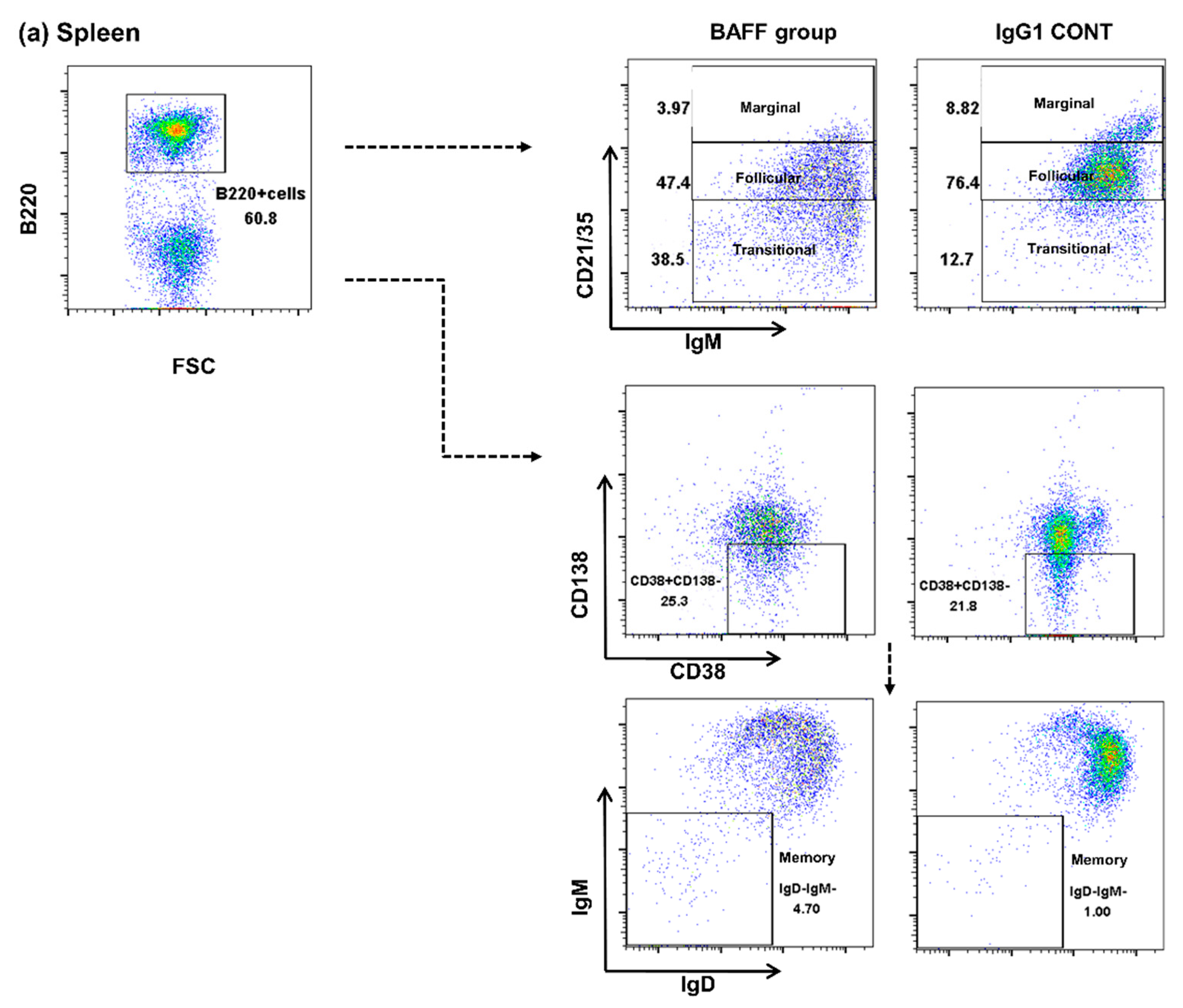
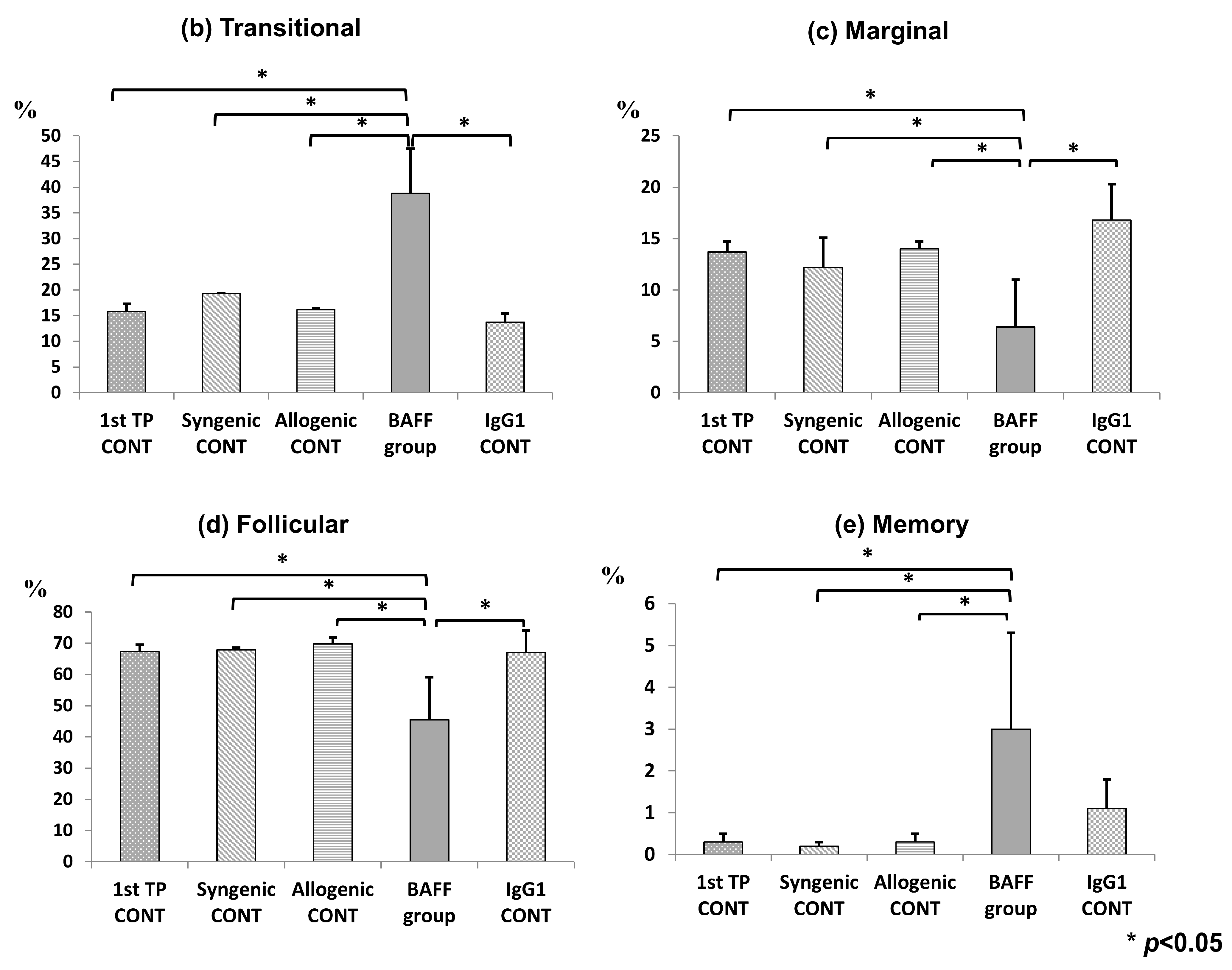
2.3. Comparison of B Cell Fractions in the Spleen
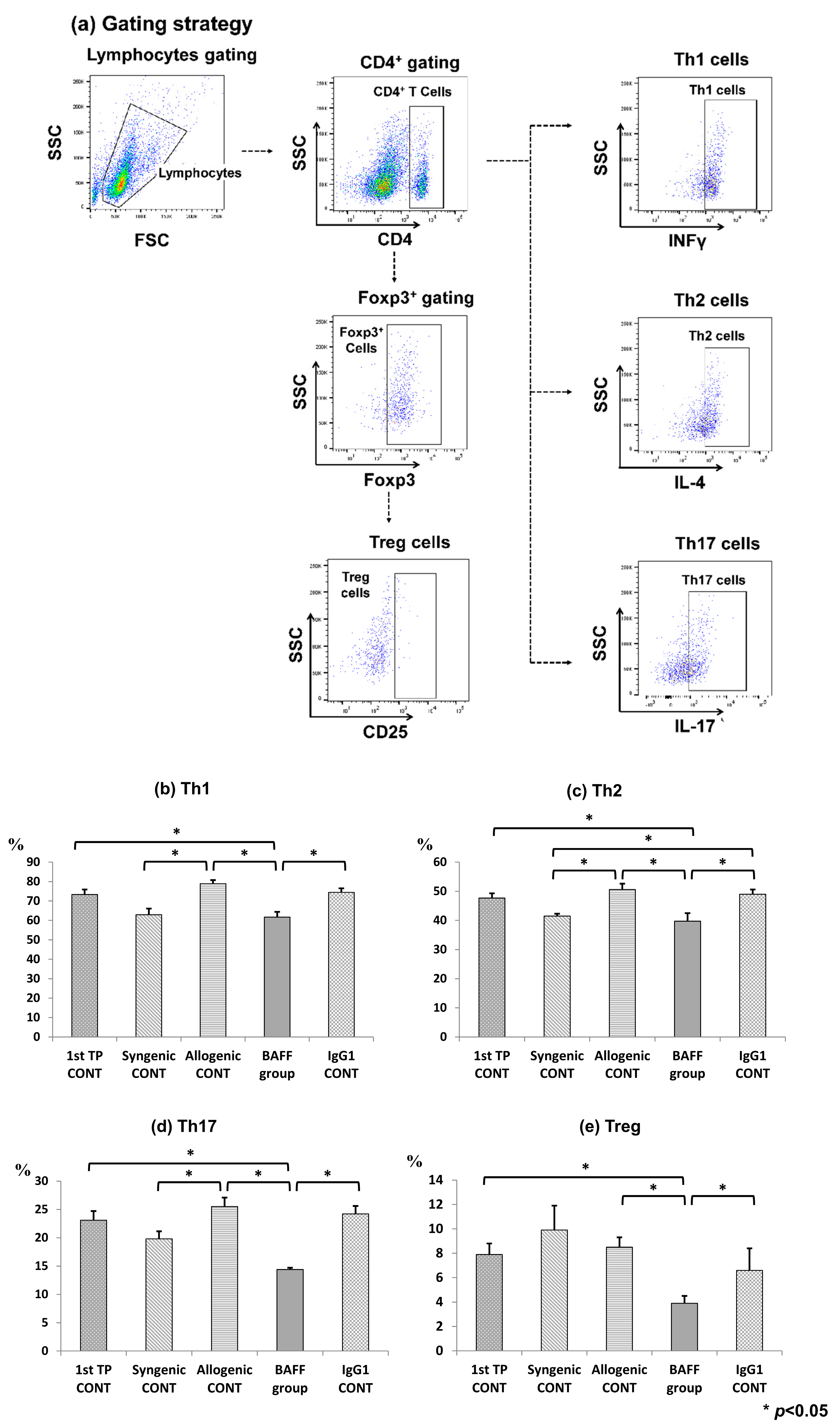
2.4. Comparison of T Cell Fractions in the Spleen
2.5. Cell Surface BAFFR Expression on B Cells of the Bone Marrow and Spleen
2.6. Microarray Analysis of the Sensitized Mouse and BAFF Inhibition Models
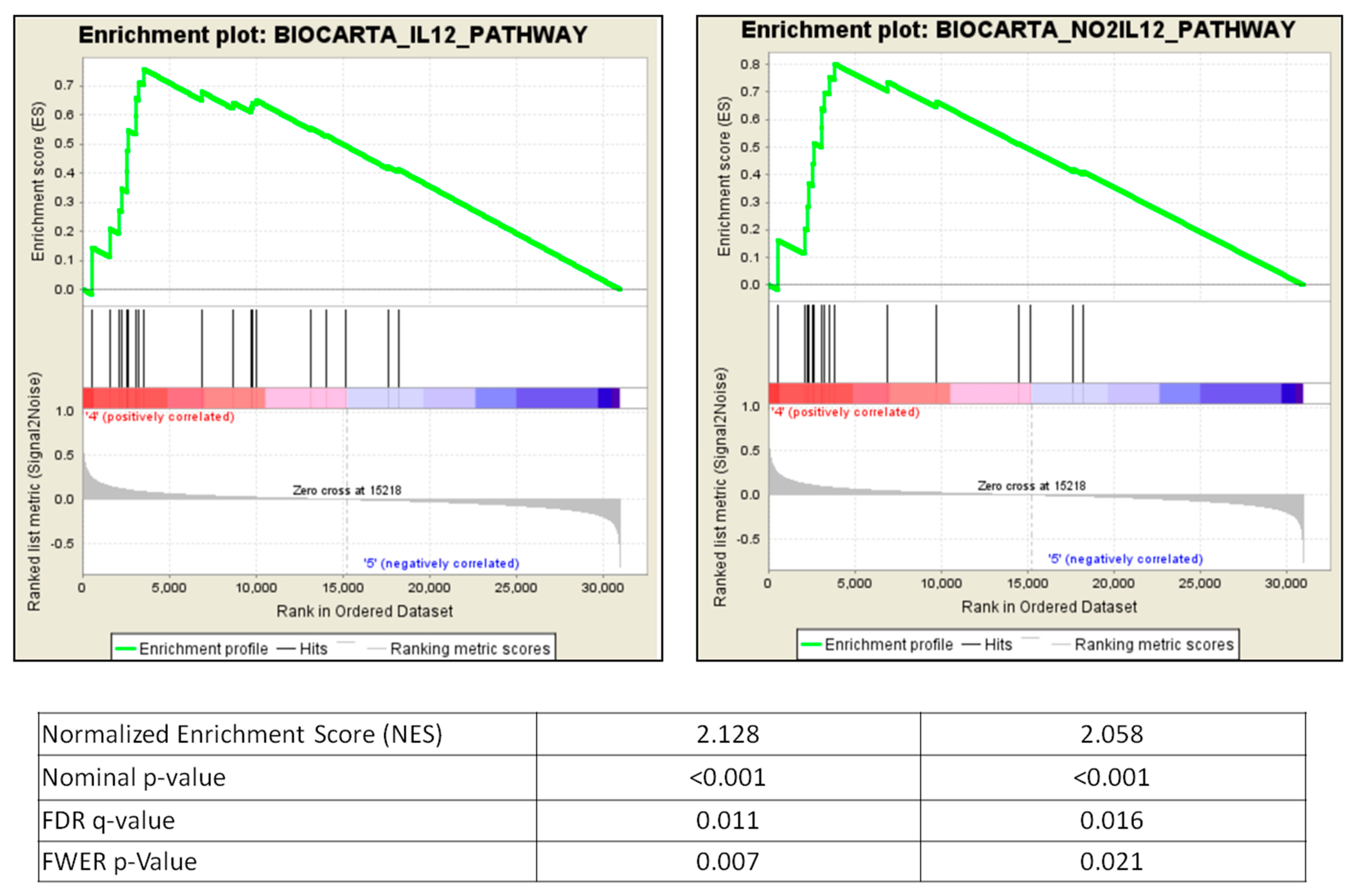
2.7. GSEA Pathways Involved in BAFF Inhibition
2.8. Changes of Immune Cell Fractions during Sensitization Using CIBERSORT
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Skin Allograft Transplant Procedure
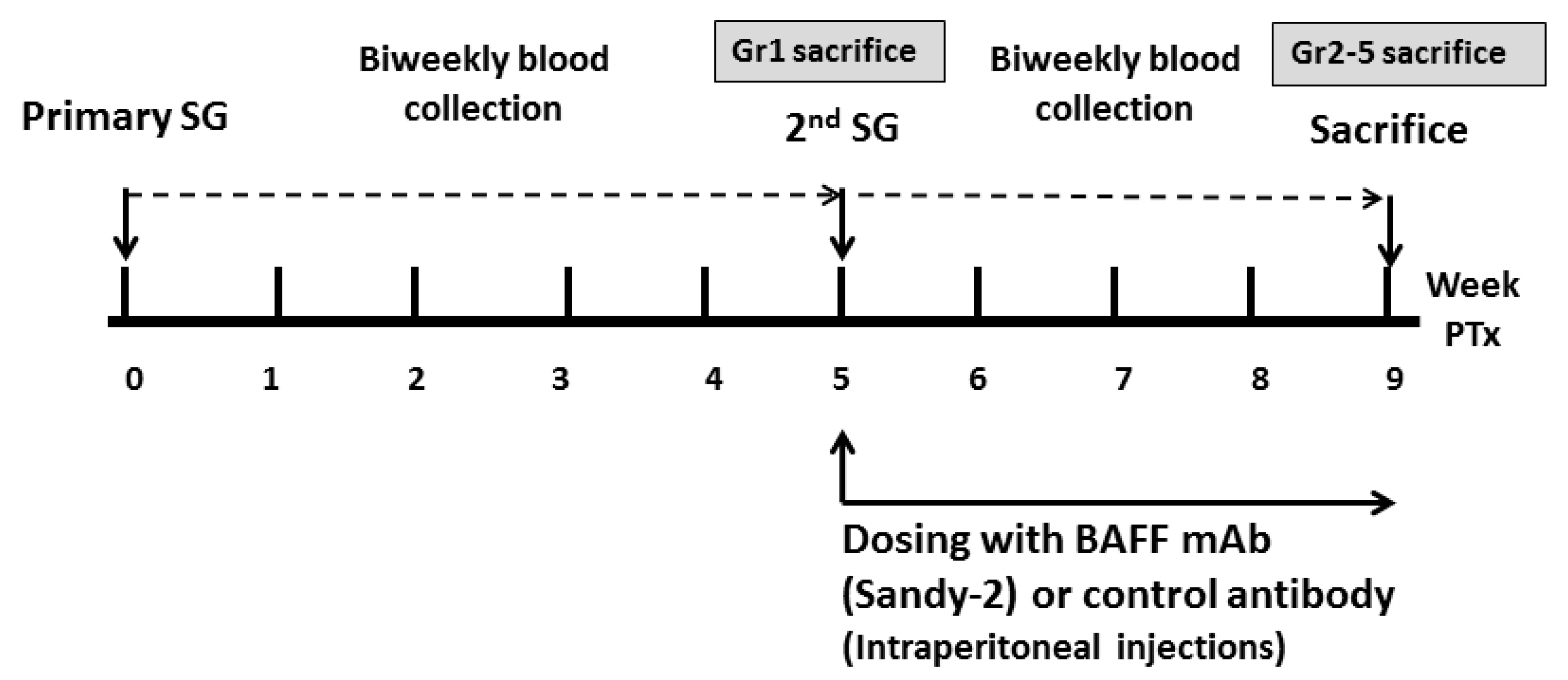
4.3. Experimental Design
4.4. Measurement of Serum Donor-Specific Anti-HLA.A2 Antibodies
4.5. Flow Cytometry Analysis
4.6. Microarray Analysis
4.6.1. mRNA Extraction and Quality Control
4.6.2. Affymetrix Whole Transcript Expression Arrays Methods
4.6.3. Raw Data Preparation and Analysis
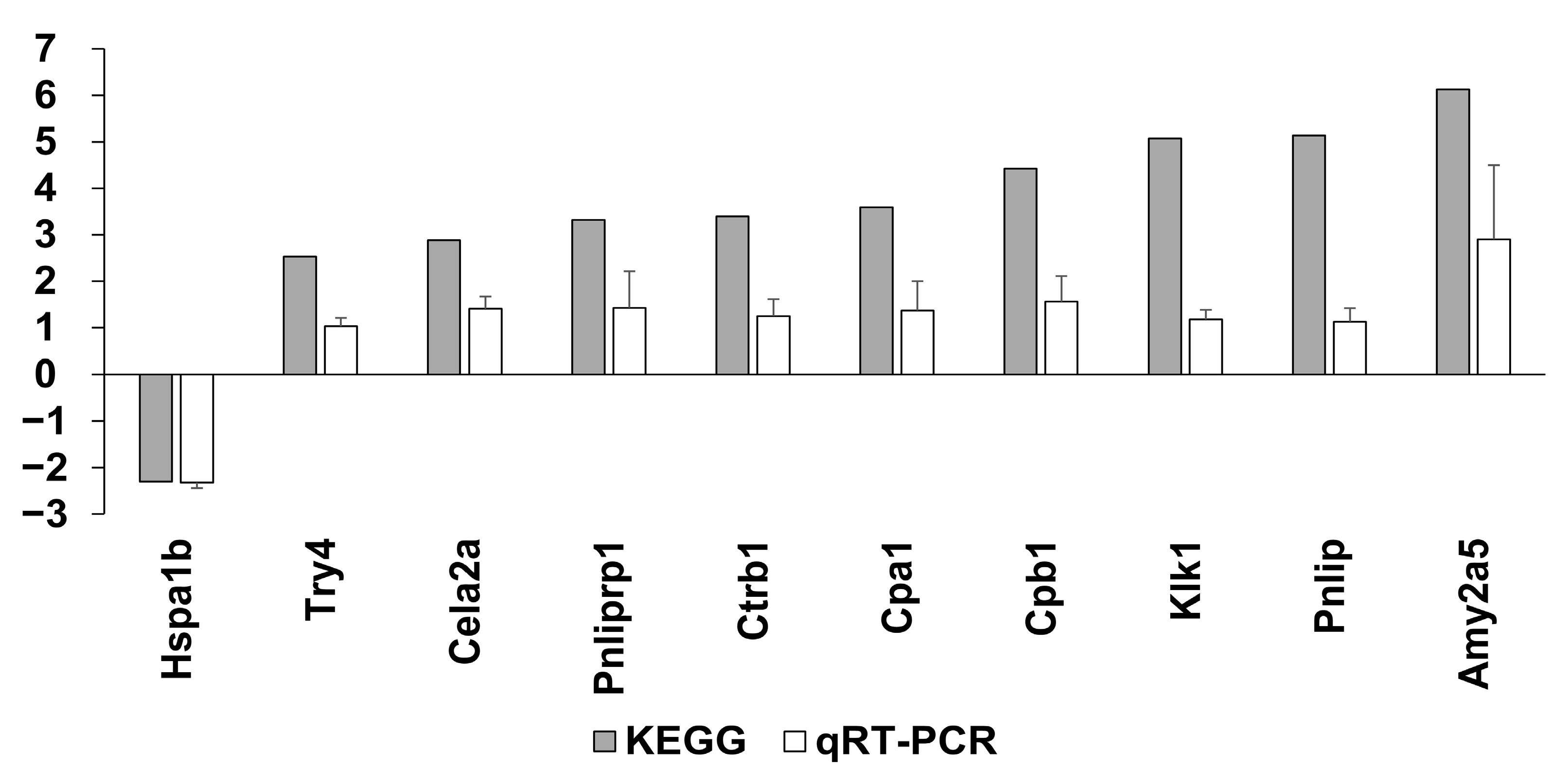
4.6.4. Validation Using Quantitative Real-Time PCR (qRT-PCR)
4.6.5. Subanalysis of Microarray Results Using CIBERSORT
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABMR | Antibody-mediated rejection |
| APRIL BAFF | A proliferation inducing ligand B cell activating factor |
| BAFFR | B cell activating factor receptor |
| BCMA | B cell maturation antigen |
| DEG | Differentially expressed gene |
| DSA | Donor-specific antibody |
| FACS | Fluorescence-activated cell sorting |
| FDR | False discovery rate |
| FC | Fold change |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GSEA | Gene Set Enrichment Analysis |
| Hsp | Heat shock protein |
| Anti-HLA.A2 Ab | HLA.A2-specific antibodies |
| HLA | Human leukocyte antibody |
| IQR | Interquartile range |
| IVIg | Intravenous immune globulin |
| KT | Kidney transplant |
| LPE | Local-pooled-error |
| LLPC | Long lived plasma cells |
| MFI | Mean fluorescence intensity |
| mAb | Monoclonal antibody |
| NES | Normalized enrichment score |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RMA | Robust multi-average |
| SLE | Systemic lupus erythematosus |
| SD | Standard deviation |
| TdT | Terminal deoxynucleotidyl transferase |
| TACI | Transmembrane activator and CAML interactor |
References
- Keith, D.S.; Vranic, G.M. Approach to the Highly Sensitized Kidney Transplant Candidate. Clin. J. Am. Soc. Nephrol. 2016, 11, 684–693. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, S.; Hwang, H.; Park, H.; Kwak, J.; Kwon, E.K.; Sung, H.Y.; Han, B. Positive Rates of Preliminary Crossmatches Among Transplantation Candidates Waitlisted for Different Organs in the Korean Network for Organ Sharing. Transplant. Proc. 2016, 48, 2464–2466. [Google Scholar] [CrossRef]
- Orandi, B.J.; Garonzik-Wang, J.M.; Massie, A.B.; Zachary, A.A.; Montgomery, J.R.; Van Arendonk, K.J.; Stegall, M.D.; Jordan, S.C.; Oberholzer, J.; Dunn, T.B.; et al. Quantifying the risk of incompatible kidney transplantation: A multicenter study. Am. J. Transplant. 2014, 14, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Akalin, E. A New Treatment Option for Highly Sensitized Patients Awaiting Kidney Transplantation. Am. J. Kidney Dis. 2018, 71, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Marfo, K.; Lu, A.; Ling, M.; Akalin, E. Desensitization protocols and their outcome. Clin. J. Am. Soc. Nephrol. 2011, 6, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Mackay, F.; Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 2009, 9, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Mackay, F.; Schneider, P.; Rennert, P.; Browning, J. BAFF AND APRIL: A tutorial on B cell survival. Annu. Rev. Immunol. 2003, 21, 231–264. [Google Scholar] [CrossRef]
- Sarantopoulos, S.; Stevenson, K.E.; Kim, H.T.; Bhuiya, N.S.; Cutler, C.S.; Soiffer, R.J.; Antin, J.H.; Ritz, J. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin. Cancer Res. 2007, 13, 6107–6114. [Google Scholar] [CrossRef]
- Pers, J.O.; Daridon, C.; Devauchelle, V.; Jousse, S.; Saraux, A.; Jamin, C.; Youinou, P. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann. N. Y. Acad. Sci. 2005, 1050, 34–39. [Google Scholar] [CrossRef]
- Stohl, W. Therapeutic targeting of the BAFF/APRIL axis in systemic lupus erythematosus. Expert Opin. Ther. Targets 2014, 18, 473–489. [Google Scholar] [CrossRef]
- Xu, H.; He, X.; Sun, J.; Shi, D.; Zhu, Y.; Zhang, X. The expression of B-cell activating factor belonging to tumor necrosis factor superfamily (BAFF) significantly correlated with C4D in kidney allograft rejection. Transplant. Proc. 2009, 41, 112–116. [Google Scholar] [CrossRef]
- Thaunat, O.; Patey, N.; Gautreau, C.; Lechaton, S.; Fremeaux-Bacchi, V.; Dieu-Nosjean, M.C.; Cassuto-Viguier, E.; Legendre, C.; Delahousse, M.; Lang, P.; et al. B cell survival in intragraft tertiary lymphoid organs after rituximab therapy. Transplantation 2008, 85, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Thibault-Espitia, A.; Foucher, Y.; Danger, R.; Migone, T.; Pallier, A.; Castagnet, S.; Gueguen, C.G.; Devys, A.; Gautier, A.C.; Giral, M.; et al. BAFF and BAFF-R levels are associated with risk of long-term kidney graft dysfunction and development of donor-specific antibodies. Am. J. Transplant. 2012, 12, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Banham, G.; Prezzi, D.; Harford, S.; Taylor, C.J.; Hamer, R.; Higgins, R.; Bradley, J.A.; Clatworthy, M.R. Elevated pretransplantation soluble BAFF is associated with an increased risk of acute antibody-mediated rejection. Transplantation 2013, 96, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Min, J.W.; Kim, K.W.; Kim, B.M.; Doh, K.C.; Choi, M.S.; Choi, B.S.; Park, C.W.; Yang, C.W.; Kim, Y.S.; Oh, E.J.; et al. Clinical Significance of Pre- and Post-Transplant BAFF Levels in Kidney Transplant Recipients. PLoS ONE 2016, 11, e0162964. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Komocsar, W.J.; Nantz, E.; Samaniego, M.D.; Henson, S.L.; Hague, J.A.; Lobashevsky, A.L.; Higgins, N.G.; Czader, M.; Book, B.K.; et al. Effect of Treatment Tabalumab, a B Cell-Activating Factor Inhibitor, on Highly Sensitized Patients with End-Stage Renal Disease Awaiting Transplantation. Am. J. Transplant. 2016, 16, 1266–1275. [Google Scholar] [CrossRef]
- Banham, G.D.; Flint, S.M.; Torpey, N.; Lyons, P.A.; Shanahan, D.N.; Gibson, A.; Watson, C.J.E.; O’Sullivan, A.M.; Chadwick, J.A.; Foster, K.E.; et al. Belimumab in kidney transplantation: An experimental medicine, randomised, placebo-controlled phase 2 trial. Lancet 2018, 391, 2619–2630. [Google Scholar] [CrossRef]
- Wu, G.; Chai, N.; Kim, I.; Klein, A.S.; Jordan, S.C. Monoclonal anti-interleukin-6 receptor antibody attenuates donor-specific antibody responses in a mouse model of allosensitization. Transplant. Immunol. 2013, 28, 138–143. [Google Scholar] [CrossRef]
- Kim, I.; Wu, G.; Chai, N.N.; Klein, A.S.; Jordan, S. Anti-interleukin 6 receptor antibodies attenuate antibody recall responses in a mouse model of allosensitization. Transplantation 2014, 98, 1262–1270. [Google Scholar] [CrossRef]
- Goodnow, C.C.; Vinuesa, C.G.; Randall, K.L.; Mackay, F.; Brink, R. Control systems and decision making for antibody production. Nat. Immunol. 2010, 11, 681–688. [Google Scholar] [CrossRef]
- Tangye, S.G.; Avery, D.T.; Deenick, E.K.; Hodgkin, P.D. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J. Immunol. 2003, 170, 686–694. [Google Scholar] [CrossRef]
- Sasaki, Y.; Casola, S.; Kutok, J.L.; Rajewsky, K.; Schmidt-Supprian, M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J. Immunol. 2004, 173, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.L.; Crowley, J.E.; Tomayko, M.M.; Steinel, N.; O’Neill, P.J.; Quinn, W.J., 3rd; Goenka, R.; Miller, J.P.; Cho, Y.H.; Long, V.; et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc. Natl. Acad. Sci. USA 2008, 105, 15517–15522. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.J.; Dillon, S.R.; Castigli, E.; Geha, R.S.; Xu, S.; Lam, K.P.; Noelle, R.J. Cutting edge: The dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 2008, 180, 3655–3659. [Google Scholar] [CrossRef] [PubMed]
- Boneparth, A.; Davidson, A. B-cell activating factor targeted therapy and lupus. Arthritis Res. Ther. 2012, 14 (Suppl. 4), S2. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Hiepe, F.; Dörner, T.; Hauser, A.E.; Hoyer, B.F.; Mei, H.; Radbruch, A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 2011, 7, 170–178. [Google Scholar] [CrossRef]
- Lai Kwan Lam, Q.; King Hung Ko, O.; Zheng, B.J.; Lu, L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc. Natl. Acad. Sci. USA 2008, 105, 14993–14998. [Google Scholar] [CrossRef]
- Mackay, F.; Leung, H. The role of the BAFF/APRIL system on T cell function. Semin. Immunol. 2006, 18, 284–289. [Google Scholar] [CrossRef]
- Stohl, W.; Yu, N. Promotion of T Regulatory Cells in Mice by B Cells and BAFF. J. Immunol. 2020, 204, 2416–2428. [Google Scholar] [CrossRef]
- Parsons, R.F.; Yu, M.; Vivek, K.; Zekavat, G.; Rostami, S.Y.; Ziaie, A.S.; Luo, Y.; Koeberlein, B.; Redfield, R.R.; Ward, C.D.; et al. Murine islet allograft tolerance upon blockade of the B-lymphocyte stimulator, BLyS/BAFF. Transplantation 2012, 93, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, H.; Su, X.; Luo, Y.; Li, X.; Yu, C.; Xie, Q.; Xia, X.; He, G.; Yang, L. Therapeutic effects of a novel BAFF blocker on arthritis. Signal. Transduct. Target. Ther. 2019, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.A.; Schmid, C.; Wilhelm, M.J.; Blasius, S.; Scheld, H.H.; Böcker, W.; Dockhorn-Dworniczak, B. Inducible heat shock protein 70 in rat cardiac allograft and its immunohistochemical localization in cardiac myocytes. Transplantation 1997, 64, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Land, W.G. Role of heat shock protein 70 in innate alloimmunity. Front. Immunol. 2011, 2, 89. [Google Scholar] [CrossRef] [PubMed]
- Honczarenko, M.; Le, Y.; Glodek, A.M.; Majka, M.; Campbell, J.J.; Ratajczak, M.Z.; Silberstein, L.E. CCR5-binding chemokines modulate CXCL12 (SDF-1)-induced responses of progenitor B cells in human bone marrow through heterologous desensitization of the CXCR4 chemokine receptor. Blood 2002, 100, 2321–2329. [Google Scholar] [CrossRef]
- Kim, S.J.; Caton, M.; Wang, C.; Khalil, M.; Zhou, Z.J.; Hardin, J.; Diamond, B. Increased IL-12 inhibits B cells’ differentiation to germinal center cells and promotes differentiation to short-lived plasmablasts. J. Exp. Med. 2008, 205, 2437–2448. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.D.; Read, K.A.; Sreekumar, B.K.; Jones, D.M.; Oestreich, K.J. IL-12 signaling drives the differentiation and function of a T(H)1-derived T(FH1)-like cell population. Sci. Rep. 2019, 9, 13991. [Google Scholar] [CrossRef]
- Ginzler, E.M.; Wax, S.; Rajeswaran, A.; Copt, S.; Hillson, J.; Ramos, E.; Singer, N.G. Atacicept in combination with MMF and corticosteroids in lupus nephritis: Results of a prematurely terminated trial. Arthritis Res. Ther. 2012, 14, R33. [Google Scholar] [CrossRef]
- Wu, G.D.; He, Y.; Chai, N.N.; Toyoda, M.; Dunn, R.; Kehry, M.R.; Klein, A.S.; Jordan, S.C. Anti-CD20 antibody suppresses anti-HLA antibody formation in a HLA-A2 transgenic mouse model of sensitization. Transpl. Immunol. 2008, 19, 178–186. [Google Scholar] [CrossRef]
- Kowalczyk-Quintas, C.; Schuepbach-Mallepell, S.; Vigolo, M.; Willen, L.; Tardivel, A.; Smulski, C.R.; Zheng, T.S.; Gommerman, J.; Hess, H.; Gottenberg, J.E.; et al. Antibodies That Block or Activate Mouse B Cell Activating Factor of the Tumor Necrosis Factor (TNF) Family (BAFF), Respectively, Induce B Cell Depletion or B Cell Hyperplasia. J. Biol. Chem. 2016, 291, 19826–19834. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, B.M.; Doh, K.C.; Kim, C.D.; Jeong, K.H.; Lee, S.H.; Yang, C.W.; Chung, B.H. Clinical significance of CD161+CD4+ T cells in the development of chronic antibody-mediated rejection in kidney transplant recipients. PLoS ONE 2018, 13, e0200631. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [PubMed]


| Symbol | Gene Annotation | Function | FC | p |
|---|---|---|---|---|
| Ighv1-78 | immunoglobulin heavy variable 1-78 | antigen binding and immunoglobulin production | −3.023 | 0.000 |
| Ighv1-31 | immunoglobulin heavy variable 1-31 | antigen binding and immunoglobulin production | −2.779 | 0.011 |
| Igkv3-4 | immunoglobulin kappa variable 3-4 | Immune response and immunoglobulin production | −2.531 | 0.001 |
| Gm24762 | predicted gene, 24762 | −2.116 | 0.011 | |
| Igkv9-123 | immunoglobulin kappa variable 9-123 | Immune response and immunoglobulin production | −1.974 | 0.042 |
| Igkv4-55 | immunoglobulin kappa variable 4-55 | antigen binding and immunoglobulin production | −1.614 | 0.005 |
| Ighv1-7 | immunoglobulin heavy variable V1-7 | antigen binding and immunoglobulin production | −1.610 | 0.011 |
| Apol11b | apolipoprotein L 11b | Lipid binding | 1.733 | 0.001 |
| Igkv11-125 | immunoglobulin kappa variable 11-125 | Immune response and immunoglobulin production | 2.027 | 0.006 |
| Ighv1-39 | immunoglobulin heavy variable 1-39 | antigen binding and immunoglobulin production | 2.074 | 0.025 |
| Ighv9-1 | immunoglobulin heavy variable 9-1 | antigen binding and immunoglobulin production | 2.242 | 0.000 |
| Igkv1-122 | immunoglobulin kappa chain variable 1-122 | Immune response and immunoglobulin production | 2.265 | 0.000 |
| Igkv14-126 | immunoglobulin kappa variable 14-126 | Immune response and immunoglobulin production | 2.436 | 0.000 |
| Mir669a-1 | microRNA 669a-1 | negative regulation of skeletal muscle cell differentiation and regulation of gene expression | 2.526 | 0.011 |
| Mir669p-1 | microRNA 669p-1 | Regulatory, pathogenic and control CD4+ T cells | 2.572 | 0.011 |
| Ear1 | eosinophil-associated, ribonuclease A family, member 1 | Endonuclease activity, hydrolase activity, nuclease activity, ribonuclease activity | 2.599 | 0.002 |
| Ighv12-3 | immunoglobulin heavy variable V12-3 | antigen binding and immunoglobulin production | 2.812 | 0.000 |
| Apol11a | apolipoprotein L 11a | Lipid binding | 3.039 | 0.000 |
| Ighv1-58 | immunoglobulin heavy variable 1-58 | antigen binding and immunoglobulin production | 3.739 | 0.011 |
| Pathways | Size | ES | NES | NOM p-Value | FDR q-Value | FWER p-Value |
|---|---|---|---|---|---|---|
| BIOCARTAIL12PATHWAY | 21 | 0.76 | 2.13 | 0 | 0.011 | 0.007 |
| BIOCARTANO2IL12PATHWAY | 17 | 0.8 | 2.06 | 0 | 0.017 | 0.021 |
| BIOCARTACSKPATHWAY | 19 | 0.75 | 2.03 | 0 | 0.014 | 0.027 |
| PIDIL12STAT4PATHWAY | 31 | 0.65 | 1.96 | 0.002 | 0.047 | 0.111 |
| PIDIL122PATHWAY | 57 | 0.56 | 1.94 | 0 | 0.047 | 0.138 |
| BIOCARTANKCELLSPATHWAY | 18 | 0.72 | 1.94 | 0 | 0.041 | 0.145 |
| REACTOMEGENERATIONOFSECONDMESSENGERMOLECULES | 20 | 0.72 | 1.93 | 0.002 | 0.042 | 0.171 |
| BIOCARTACTLA4PATHWAY | 16 | 0.73 | 1.92 | 0.006 | 0.039 | 0.18 |
| KEGGNATURALKILLERCELLMEDIATEDCYTOTOXICITY | 102 | 0.51 | 1.92 | 0 | 0.036 | 0.187 |
| REACTOMETCRSIGNALING | 44 | 0.57 | 1.86 | 0 | 0.078 | 0.401 |
| REACTOMEDEGRADATIONOFTHEEXTRACELLULARMATRIX | 25 | 0.63 | 1.85 | 0.007 | 0.082 | 0.449 |
| KEGGADHERENSJUNCTION | 73 | 0.52 | 1.84 | 0 | 0.078 | 0.459 |
| PIDTCRPATHWAY | 64 | 0.52 | 1.82 | 0.002 | 0.094 | 0.539 |
| KEGGCYTOKINECYTOKINERECEPTORINTERACTION | 224 | 0.43 | 1.82 | 0 | 0.089 | 0.546 |
| BIOCARTATH1TH2PATHWAY | 16 | 0.69 | 1.79 | 0.006 | 0.111 | 0.655 |
| STTCELLSIGNALTRANSDUCTION | 45 | 0.54 | 1.78 | 0.002 | 0.124 | 0.726 |
| PIDPTP1BPATHWAY | 49 | 0.54 | 1.75 | 0 | 0.168 | 0.84 |
| REACTOMECOSTIMULATIONBYTHECD28FAMILY | 54 | 0.5 | 1.75 | 0 | 0.16 | 0.842 |
| PIDTCPTPPATHWAY | 42 | 0.53 | 1.72 | 0 | 0.186 | 0.897 |
| REACTOMEIMMUNOREGULATORYINTERACTIONSBETWEENALYMPHOIDANDANON-LYMPHOIDCELL | 41 | 0.54 | 1.72 | 0.005 | 0.188 | 0.91 |
| PIDINTEGRINA9B1PATHWAY | 25 | 0.59 | 1.69 | 0 | 0.233 | 0.948 |
| KEGGLYSOSOME | 116 | 0.44 | 1.68 | 0.002 | 0.245 | 0.959 |
| IL12 Pathway | NO2-il12 Pathway | CSK Pathway | |||
|---|---|---|---|---|---|
| Probe | Rank Metric Score | Probe | Rank Metric Score | Probe | Rank Metric Score |
| CCR5 | 0.251 | CCR5 | 0.251 | CD4 | 0.119 |
| IL18R1 | 0.151 | IL12A | 0.127 | LCK | 0.118 |
| IL12A | 0.127 | IL12RB2 | 0.121 | ZAP70 | 0.117 |
| IL12RB2 | 0.121 | CD4 | 0.119 | CD3E | 0.109 |
| IL12RB1 | 0.111 | IL12RB1 | 0.111 | CD3D | 0.097 |
| ETV5 | 0.110 | CD3E | 0.109 | CD3G | 0.097 |
| CD3E | 0.109 | CD3D | 0.097 | CD247 | 0.093 |
| CD3D | 0.097 | CD3G | 0.097 | ||
| CD3G | 0.097 | CD247 | 0.093 | ||
| CD247 | 0.093 | CXCR3 | 0.087 | ||
| CXCR3 | 0.087 | NOS2 | 0.082 | ||
| Group | Name | Description |
|---|---|---|
| 1 | 1st Allogenic TP (1st TP CONT) | HLA-A2→B/6 |
| 2 | 2nd Syngenic TP (Syngenic CONT) | B/6→B/6 × 2 times |
| 3 | 2nd Allogenic TP (Allogenic CONT) | HLA-A2→B/6 × 2 times |
| 4 | 2nd Allogenic TP + BAFF inhibitor (BAFF group) | HLA-A2→B/6 × 2 times Anti-BAFF mAb administration |
| 5 | 2nd Allogenic TP + Control IgG1 (IgG1 CONT) | HLA-A2→B/6 × 2 times Control IgG1 Ab administration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, J.W.; Shin, Y.-J.; Lee, H.; Kim, B.-M.; Park, K.H.; Doh, K.C.; Kim, T.-M.; Lim, S.W.; Yang, C.W.; Oh, E.-J.; et al. BAFF Inhibition Effectively Suppresses the Development of Anti-HLA.A2 Antibody in the Highly Sensitized Mouse Model. Int. J. Mol. Sci. 2021, 22, 861. https://doi.org/10.3390/ijms22020861
Min JW, Shin Y-J, Lee H, Kim B-M, Park KH, Doh KC, Kim T-M, Lim SW, Yang CW, Oh E-J, et al. BAFF Inhibition Effectively Suppresses the Development of Anti-HLA.A2 Antibody in the Highly Sensitized Mouse Model. International Journal of Molecular Sciences. 2021; 22(2):861. https://doi.org/10.3390/ijms22020861
Chicago/Turabian StyleMin, Ji Won, Yoo-Jin Shin, Hyeyoung Lee, Bo-Mi Kim, Ki Hyun Park, Kyoung Chan Doh, Tae-Min Kim, Sun Woo Lim, Chul Woo Yang, Eun-Jee Oh, and et al. 2021. "BAFF Inhibition Effectively Suppresses the Development of Anti-HLA.A2 Antibody in the Highly Sensitized Mouse Model" International Journal of Molecular Sciences 22, no. 2: 861. https://doi.org/10.3390/ijms22020861
APA StyleMin, J. W., Shin, Y.-J., Lee, H., Kim, B.-M., Park, K. H., Doh, K. C., Kim, T.-M., Lim, S. W., Yang, C. W., Oh, E.-J., & Chung, B. H. (2021). BAFF Inhibition Effectively Suppresses the Development of Anti-HLA.A2 Antibody in the Highly Sensitized Mouse Model. International Journal of Molecular Sciences, 22(2), 861. https://doi.org/10.3390/ijms22020861






