The Complex Story of Plant Cyclic Nucleotide-Gated Channels
Abstract
1. Introduction
2. Heteromeric Channel Complexes Also Occur in Plants
3. Complex Formation Would Depend on the Co-Localisation and Relative Abundance of CNGC Isoforms
4. CNGCs Are Extensively Regulated—Formation of CNGC Complexes Generates Further Regulatory and Functional Complexity
4.1. CNGCs Are Regulated by cNMPs That May Be Generated by Soluble or Membrane Proteins
4.2. CNGCs Are Positively and Negatively Regulated by CaM, Potentially Affording Ca2+ Sensing and Feedback
4.3. CNGCs Are Regulated by Phosphorylation
4.4. CNGC Complexes Generate Further Functional and Regulatory Complexity
5. Could CNGCLs Modulate Complex Formation?
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Adenylyl cyclase |
| AGN | Alanine-glycine-asparagine |
| AHA | Arabidopsis H+-ATPase |
| AND | Alanine-asparagine-aspartic acid |
| Apo-CaM | Calmodulin without Ca2+ ligands |
| At | Arabidopsis thaliana |
| BAK1 | BRI-associated receptor kinase1 |
| BiFC | Bifluorescence complementation |
| Bo | Brassica oleracea |
| Bra | Brassica rapa |
| BRI1 | Brassinosteroid insensitive1 |
| Ca2+/CaM | Calmodulin with Ca2+ ligands |
| CaM | Calmodulin |
| CaMBD | Calmodulin-binding domain |
| cAMP | Cyclic adenosine monophosphate |
| cGMP | Cyclic guanosine monophosphate |
| CNDB | Cyclic nucleotide-binding domain |
| CNGC | Cyclic nucleotide-gated channel |
| CNGCL | A gene or protein which contains some, but not all the domains associated with cyclic nucleotide-gated channels |
| cNMP | Cyclic nucleotide monophosphate |
| CPK32 | Calcium-dependent protein kinase 32 |
| CT | Carboxy-terminal domain |
| db-cAMP | Dibutyryl-cyclic adenosine monophosphate |
| db-cGMP | Dibutyryl-cyclic guanosine monophosphate |
| DMI1 | Does not make infections 1 |
| flg22 | Flagellin 22 peptide |
| FLS2 | Flagellin Sensitive 2 |
| GC | Guanylyl cyclase |
| GNL | Glycine-asparagine-leucine |
| GQG | Glycine-glutamine-glycine |
| GQN | Glycine-glutamine-asparagine |
| GQS | Glycine-glutamine-serine |
| GUS | β-glucuronidase |
| HEK293 | Human embryonic kidney cell line 293 |
| Hv | Hordeum vulgare |
| IQ | Isoleucine-glutamine |
| KUP | K+ uptake permease |
| Lj | Lotus japonicus |
| LRRAC1 | Leucine-rich repeat adenylyl cyclase1 |
| Mt | Medicago truncatula |
| Ntab | Nicotiana tabacum |
| NOGC1 | Nitric oxide-dependent guanylate cyclase1 |
| NT | N-terminal domain |
| Os | Oryza sativa |
| OSCA1.3 | hyperOsmolality-induced [Ca2+]i increase 1.3 |
| PepR1 | Plant elicitor peptide recptor1 |
| Pp | Physcomitrella patens |
| PSKR1 | Phytosulfokine receptor1 |
| RLCK185 | Receptor-like cytoplasmic kinase 185 |
| SERK4 | Somatic embryogenesis receptor kinase 4 |
| Sl | Solanum lycopersicum |
| WAKL10 | Wall-associated kinase (WAK)-like 1 |
| Zj | Ziziphus jujube |
Appendix A

References
- Leng, Q.; Mercier, R.W.; Yao, W.; Berkowitz, G.A. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999, 121, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Mercier, R.W.; Hua, B.-G.; Fromm, H.; Berkowitz, G.A. Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiol. 2002, 128, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Verma, R.; Gehring, C.; Yamaguchi, Y.; Zhao, Y.; Ryan, C.A.; Berkowitz, G.A. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc. Natl. Acad. Sci. USA 2010, 107, 21193–21198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Munemasa, S.; Nishimura, N.; Ren, H.-M.; Robert, N.; Han, M.; Puzõrjova, I.; Kollist, H.; Lee, S.; Mori, I.; et al. Identification of cyclic GMP-activated nonselective Ca2+-permeable cation channels and associated CNGC5 and CNGC6 genes in Arabidopsis guard cells. Plant Physiol. 2013, 163, 578–590. [Google Scholar] [CrossRef]
- Gao, Q.-F.; Fei, C.-F.; Dong, J.-Y.; Gu, L.-L.; Wang, Y.-F. Arabidopsis CNGC18 is a Ca2+-permeable channel. Mol. Plant 2014, 7, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-F.; Gu, L.-L.; Wang, H.-Q.; Fei, C.-F.; Fang, X.; Hussain, J.; Sun, S.-J.; Dong, J.-Y.; Liu, H.; Wang, Y.-F. Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 3096–3101. [Google Scholar] [CrossRef]
- Mori, I.C.; Nobukiyo, Y.; Nakahara, Y.; Shibasaka, M.; Furuichi, T.; Katsuhara, M. A cyclic nucleotide-gated channel, HvCNGC2-3, is activated by the co-presence of Na+ and K+ and permeable to Na+ and K+ non-selectively. Plants 2018, 7. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, C.; Tian, W.; Li, L.; Zhu, H. Electrophysiological studies revealed CaM1-mediated regulation of the Arabidopsis calcium channel CNGC12. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Dietrich, P.; Moeder, W.; Yoshioka, K. Plant cyclic nucleotide-gated channels: New insights on their functions and regulation. Plant Physiol. 2020, 184, 27–38. [Google Scholar] [CrossRef]
- Blanco, E.; Fortunato, S.; Viggiano, L.; de Pinto, M.C. Cyclic AMP: A polyhedral signalling molecule in plants. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.; Moeder, W.; Yoshioka, K. Biological roles of cyclic-nucleotide-gated ion channels in plants: What we know and don’t know about this 20 member ion channel family. Botany 2009, 87, 668–677. [Google Scholar] [CrossRef]
- Duszyn, M.; Świeżawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Cyclic nucleotide gated channels (CNGCs) in plant signalling—Current knowledge and perspectives. Plant Physiol. 2019, 241. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Sharma, M.; Pandey, G.K. Role of cyclic nucleotide gated channels in stress management in plants. Curr. Genom. 2016, 17, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Moeder, W.; Urquhart, W.; Ung, H.; Yoshioka, K. The role of cyclic nucleotide-gated ion channels in plant immunity. Mol. Plant 2011, 4, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.; Sanders, D.; et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef]
- Zeng, H.; Zhao, B.; Wu, H.; Zhu, Y.; Chen, H. Comprehensive in silico characterization and expression profiling of nine gene families associated with calcium transport in soybean. Agronomy 2020, 10. [Google Scholar] [CrossRef]
- Nawaz, Z.; Kakar, K.U.; Ullah, R.; Yu, S.; Zhang, J.; Shu, Q.-Y.; Ren, X. Genome-wide identification, evolution and expression analysis of cyclic nucleotide-gated channels in tobacco (Nicotiana tabacum L.). Genomics 2019, 111, 142–158. [Google Scholar] [CrossRef]
- Nawaz, Z.; Kakar, K.U.; Saand, M.A.; Shu, Q.-Y. Cyclic nucleotide-gated ion channel gene family in rice, identification, characterization and experimental analysis of expression response to plant hormones, biotic and abiotic stresses. BMC Genom. 2014, 15, 1–18. [Google Scholar] [CrossRef]
- Guo, J.; Islam, M.A.; Lin, H.; Ji, C.; Duan, Y.; Liu, P.; Zeng, Q.; Day, B.; Kang, Z.; Guo, J. Genome-wide identification of cyclic nucleotide-gated ion channel gene family in wheat and functional analyses of TaCNGC14 and TaCNGC16. Front. Plant Sci. 2018, 9, 18. [Google Scholar] [CrossRef]
- Hao, L.; Qiao, X. Genome-wide identification and analysis of the CNGC gene family in maize. PeerJ 2018, 6, e5816. [Google Scholar] [CrossRef] [PubMed]
- Kakar, K.U.; Nawaz, Z.; Kakar, K.; Ali, E.; Almoneafy, A.A.; Ullah, R.; Ren, X.; Shu, Q.-Y. Comprehensive genomic analysis of the CNGC gene family in Brassica oleracea: Novel insights into synteny, structures, and transcript profiles. BMC Genom. 2017, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, S.; Ren, J.; Ye, X.; jiang, X.; Liu, Z. Genome-wide identification and functional analysis of the cyclic nucleotide-gated channel gene family in Chinese cabbage. 3 Biotech 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ali, R.; Berkowitz, G.A. Characterization of plant phenotypes associated with loss-of-function of AtCNGC1, a plant cyclic nucleotide gated cation channel. Plant Physiol. Biochem. 2006, 44, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Belloeil, C.; Ianna, M.L.; Shin, R. Arabidopsis CNGC family members contribute to heavy metal ion uptake in plants. Int. J. Mol. Sci. 2019, 20, 413. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Kaplan, B.; Bouché, N.; Arazi, T.; Dolev, D.; Talke, I.N.; Maathuis, F.J.M.; Sanders, D.; Bouchez, D.; Fromm, H. Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous Arabidopsis CNGC1 gene confer Pb2+ tolerance. Plant J. 2000, 24, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.; DeFalco, T.A.; Moeder, W.; Yoshioka, K. The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol. 2013, 163, 611–624. [Google Scholar] [CrossRef]
- Finka, A.; Cuendet, A.F.H.; Maathuis, F.J.M.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef]
- Tian, W.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Dahlbeck, D.; Hu, S.; Zhang, L.; Niu, Q.; Li, L.; et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef]
- Ali, R.; Ma, W.; Lemtiri-Chlieh, F.; Tsaltas, D.; Leng, Q.; von Bodman, S.; Berkowitz, G.A. Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 2007, 19, 1081–1095. [Google Scholar] [CrossRef]
- Clough, S.J.; Fengler, K.A.; Yu, I.; Lippok, B.; Smith, R.K.; Bent, A.F. The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 2000, 97, 9323–9328. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; DeFalco, T.A.; Karia, P.; Snedden, W.A.; Moeder, W.; Yoshioka, K.; Dietrich, P. Calmodulin as a Ca2+-sensing subunit of Arabidopsis cyclic nucleotide-gated channel complexes. Plant Cell Physiol. 2017, 58, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Genger, R.K.; Jurkowski, G.I.; McDowell, J.M.; Lu, H.; Jung, H.W.; Greenberg, J.T.; Bent, A.F. Signaling pathways that regulate the enhanced disease resistance of Arabidopsis “Defense, No Death” mutants. MPMI 2008, 21, 1285–1296. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Tang, S.; Pan, J.; Yu, Y.; Han, J.; Li, Y.; Du, X.; Nan, Z.; Sun, Q. AtCNGC2 is involved in jasmonic acid-induced calcium mobilization. J. Exp. Bot. 2016, 67, 809–819. [Google Scholar] [CrossRef]
- Ma, W.; Qi, Z.; Smigel, A.; Walker, R.K.; Verma, R.; Berkowitz, G.A. Ca2+, cAMP, and transduction of non-self perception during plant immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 20995–21000. [Google Scholar] [CrossRef]
- Ma, W.; Smigel, A.; Walker, R.K.; Moeder, W.; Yoshioka, K.; Berkowitz, G.A. Leaf senescence signaling: The Ca2+-conducting Arabidopsis cyclic nucleotide gated channel2 acts through nitric oxide to repress senescence programming. Plant Physiol. 2010, 154, 733–743. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, Y.; Ma, C.; Miao, R.; Wu, C.; Long, Y.; Ge, T.; Wu, Z.; Hou, X.; Zhang, J.; et al. CNGC2 is a Ca2+ influx channel that prevents accumulation of apoplastic Ca2+ in the leaf. Plant Physiol. 2017, 173, 1342–1354. [Google Scholar] [CrossRef]
- Yu, I.; Parker, J.; Bent, A.F. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc. Natl. Acad. Sci. USA 1998, 95, 7819–7824. [Google Scholar] [CrossRef]
- Gobert, A.; Park, G.; Amtmann, A.; Sanders, D.; Maathuis, F.J.M. Arabidopsis thaliana Cyclic Nucleotide Gated Channel 3 forms a non-selective ion transporter involved in germination and cation transport. J. Exp. Bot. 2006, 57, 791–800. [Google Scholar] [CrossRef]
- Balagué, C.; Lin, B.; Alcon, C.; Flottes, G.; Malmström, S.; Köhler, C.; Neuhaus, G.; Pelletier, G.; Gaymard, F.; Roby, D. HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide–gated channel ion channel family. Plant Cell 2003, 15, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Massange-Sánchez, J.A.; Palmeros-Suárez, P.A.; Espitia-Rangel, E.; Rodríguez-Arévalo, I.; Sánchez-Segura, L.; Martínez-Gallardo, N.A.; Alatorre-Cobos, F.; Tiessen, A.; Délano-Frier, J.P. Overexpression of Grain Amaranth (Amaranthus hypochondriacus) AhERF or AhDOF transcription factors in Arabidopsis thaliana increases water deficit- and salt-stress tolerance, respectively, via contrasting stress-amelioration mechanisms. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Q.; Yang, Y.; Zhang, A.; Fei, C.-F.; Gu, L.-L.; Sun, S.-J.; Xu, W.; Wang, L.; Liu, H.; Wang, Y.-F. Three CNGC family members, CNGC5, CNGC6, and CNGC9, are required for constitutive growth of Arabidopsis root hairs as Ca2+-permeable channels. Plant Commun. 2020, 1, 100001. [Google Scholar] [CrossRef] [PubMed]
- Brost, C.; Studtrucker, T.; Reimann, R.; Denninger, P.; Czekalla, J.; Krebs, M.; Fabry, B.; Schumacher, K.; Grossmann, G.; Dietrich, P. Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. Plant J. 2019, 99, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Han, X.; Wu, J.; Zheng, S.; Shang, Z.; Sun, D.; Zhou, R.; Li, B. A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—Is involved in heat shock responses. Plant J. 2012, 70, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chai, X.; Gao, Q.; Zhou, L.; Zhang, S.; Li, L.; Luan, S. Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev. Cell 2019, 48, 710–725. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Rato, C.; Brown, E.; Rogers, S.; Mooneyham, A.; Frietsch, S.; Myers, C.T.; Poulsen, L.R.; Malhó, R.; Harper, J.F. Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS ONE 2013, 8, e55277. [Google Scholar] [CrossRef]
- Borsics, T.; Webb, D.; Andeme-Ondzighi, C.; Staehelin, L.A.; Christopher, D.A. The cyclic nucleotide-gated calmodulin-binding channel AtCNGC10 localizes to the plasma membrane and influences numerous growth responses and starch accumulation in Arabidopsis thaliana. Planta 2007, 225, 563–573. [Google Scholar] [CrossRef]
- Jin, Y.; Jing, W.; Zhang, Q.; Zhang, W. Cyclic nucleotide gated channel 10 negatively regulates salt tolerance by mediating Na+ transport in Arabidopsis. J. Plant Res. 2015, 128, 211–220. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Marshall, C.B.; Munro, K.; Kang, H.-G.; Moeder, W.; Ikura, M.; Snedden, W.A.; Yoshioka, K. Multiple calmodulin-binding sites positively and negatively regulate Arabidopsis CYCLIC NUCLEOTIDE-GATED CHANNEL12. Plant Cell 2016, 28, 1738–1751. [Google Scholar] [CrossRef]
- Urquhart, W.; Gunawardena, A.H.L.A.N.; Moeder, W.; Ali, R.; Berkowitz, G.A.; Yoshioka, K. The chimeric cyclic nucleotide-gated ion channel ATCNGC11/12 constitutively induces programmed cell death in a Ca2+ dependent manner. Plant Mol. Biol. 2007, 65, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Kachroo, P.; Tsui, F.; Sharma, S.B.; Shah, J.; Klessig, D.F. Environmentally sensitive, SA-dependent defense responses in the cpr22 mutant of Arabidopsis. Plant J. 2001, 26, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Moeder, W.; Kang, H.-G.; Kachroo, P.; Masmoudi, K.; Berkowitz, G.; Klessig, D.F. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 2006, 18, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Dindas, J.; Scherzer, S.; Roelfsema, M.R.G.; von Meyer, K.; Müller, H.M.; Al-Rasheid, K.A.S.; Palme, K.; Dietrich, P.; Becker, D.; Bennett, M.J.; et al. AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nat. Commun. 2018, 9, 1174. [Google Scholar] [CrossRef]
- Shih, H.-W.; DePew, C.L.; Miller, N.D.; Monshausen, G.B. The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr. Biol. 2015, 25, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Zeb, Q.; Wang, X.; Hou, C.; Zhang, X.; Dong, M.; Zhang, S.; Zhang, Q.; Ren, Z.; Tian, W.; Zhu, H.; et al. The interaction of CaM7 and CNGC14 regulates root hair growth in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 887–896. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, Y.; Tian, W.; Dong, M.; Zhu, H.; Luan, S.; Li, L. Arabidopsis CNGC14 mediates calcium influx required for tip growth in root hairs. Mol. Plant 2017, 10, 1004–1006. [Google Scholar] [CrossRef]
- Leitão, N.; Dangeville, P.; Carter, R.; Charpentier, M. Nuclear calcium signatures are associated with root development. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Tang, C.; Ishka, M.R.; Brown, E.; Groves, N.R.; Myers, C.T.; Rato, C.; Poulsen, L.R.; McDowell, S.; Miller, G.; et al. A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 2013, 161, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Ladwig, F.; Dahlke, R.I.; Stührwohldt, N.; Hartmann, J.; Harter, K.; Sauter, M. Phytosulfokine regulates growth in Arabidopsis through a response module at the plasma membrane that includes CYCLIC NUCLEOTIDE-GATED CHANNEL17, H+-ATPase, and BAK1. Plant Cell 2015, 27, 1718–1729. [Google Scholar] [CrossRef]
- Chang, F.; Yan, A.; Zhao, L.-N.; Wu, W.-H.; Yang, Z. A putative calcium-permeable cyclic nucleotide-gated channel, CNGC18, regulates polarized pollen tube growth. J. Integr. Plant Biol. 2007, 49, 1261–1270. [Google Scholar] [CrossRef]
- Frietsch, S.; Wang, Y.-F.; Sladek, C.; Poulsen, L.R.; Romanowsky, S.M.; Schroeder, J.I.; Harper, J.F. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. USA 2007, 104, 14531–14536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lan, W.; Jiang, Y.; Fang, W.; Luan, S. A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol. Plant 2014, 7, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Jogawat, A.; Meena, M.K.; Kundu, A.; Varma, M.; Vadassery, J. Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J. Exp. Bot. 2020, 71, 2752–2768. [Google Scholar] [CrossRef] [PubMed]
- Kugler, A.; Köhler, B.; Palme, K.; Wolff, P.; Dietrich, P. Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biol. 2009, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.K.; Prajapati, R.; Krishna, D.; Divakaran, K.; Pandey, Y.; Reichelt, M.; Mathew, M.K.; Boland, W.; Mithöfer, A.; Vadassery, J. The Ca2+ channel CNGC19 regulates Arabidopsis defense against spodoptera herbivory. Plant Cell 2019, 31, 1539–1562. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, G.; Li, B.; de Souza Vespoli, L.; Liu, H.; Moeder, W.; Chen, S.; de Oliveira, M.V.V.; Ariádina de Souza, S.; Shao, W.; et al. The receptor kinases BAK1/SERK4 regulate Ca2+ channel-mediated cellular homeostasis for cell death containment. Curr. Biol. 2019, 29, 3778–3790. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Seifert, R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002, 82, 769–824. [Google Scholar] [CrossRef]
- Liu, D.T.; Tibbs, G.R.; Siegelbaum, S.A. Subunit stoichiometry of cyclic nucleotide-gated channels and effects of subunit order on channel function. Neuron 1996, 16, 983–990. [Google Scholar] [CrossRef]
- Michalakis, S.; Becirovic, E.; Biel, M. Retinal cyclic nucleotide-gated channels: From pathophysiology to therapy. Int. J. Mol. Sci. 2018, 19, 749. [Google Scholar] [CrossRef]
- Shuart, N.G.; Haitin, Y.; Camp, S.S.; Black, K.D.; Zagotta, W.N. Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat. Commun. 2011, 2, 457. [Google Scholar] [CrossRef]
- Dreyer, I.; Porée, F.; Schneider, A.; Mittelstädt, J.; Bertl, A.; Sentenac, H.; Thibaud, J.-B.; Mueller-Roeber, B. Assembly of plant Shaker-like Kout channels requires two distinct sites of the channel α-subunit. Biophys. J. 2004, 87, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Lebaudy, A.; Pascaud, F.; Véry, A.-A.; Alcon, C.; Dreyer, I.; Thibaud, J.-B.; Lacombe, B. Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J. Biol. Chem. 2010, 285, 6265–6274. [Google Scholar] [CrossRef] [PubMed]
- Naso, A.; Dreyer, I.; Pedemonte, L.; Testa, I.; Gomez-Porras, J.L.; Usai, C.; Mueller-Rueber, B.; Diaspro, A.; Gambale, F.; Picco, C. The role of the C-terminus for functional heteromerization of the plant channel KDC1. Biophys. J. 2009, 96, 4063–4074. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nieves-Cordones, M.; Chavanieu, A.; Jeanguenin, L.; Alcon, C.; Szponarski, W.; Estaran, S.; Chérel, I.; Zimmermann, S.; Sentenac, H.; Gaillard, I. Distinct amino acids in the C-linker domain of the Arabidopsis K+ channel KAT2 determine its subcellular localization and activity at the plasma membrane. Plant Physiol. 2014, 164, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Chen, L.-M.; Liu, W.-X.; Shen, L.-K.; Wang, F.-L.; Zhou, Y.; Zhang, Z.; Wu, W.-H.; Wang, Y. AtKC1 and CIPK23 synergistically modulate AKT1-mediated low-potassium stress responses in Arabidopsis. Plant Physiol. 2016, 170, 2264–2277. [Google Scholar] [CrossRef]
- Charpentier, M.; Sun, J.; Martins, T.V.; Radhakrishnan, G.V.; Findlay, K.; Soumpourou, E.; Thouin, J.; Véry, A.-A.; Sanders, D.; Morris, R.J.; et al. Nuclear-localized cyclic nucleotide–gated channels mediate symbiotic calcium oscillations. Science 2016, 352, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Zelman, A.K.; Dawe, A.; Gehring, C.; Berkowitz, G.A. Evolutionary and structural perspectives of plant cyclic nucleotide-gated cation channels. Front. Plant Sci. 2012, 3, 95. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Liu, Z.; Dai, L.; Zhang, M.; Wang, L.; Zhao, J.; Liu, M. Genome-wide identification of CNGC genes in Chinese jujube (Ziziphus jujuba Mill.) and ZjCNGC2 mediated signalling cascades in response to cold stress. BMC Genomics 2020, 21, 191–216. [Google Scholar] [CrossRef]
- Christopher, D.A.; Borsics, T.; Yuen, C.Y.; Ullmer, W.; Andème-Ondzighi, C.; Andres, M.A.; Kang, B.-H.; Staehelin, L.A. The cyclic nucleotide gated cation channel AtCNGC10 traffics from the ER via Golgi vesicles to the plasma membrane of Arabidopsis root and leaf cells. BMC Plant Biol. 2007, 7, 48. [Google Scholar] [CrossRef]
- Baxter, J.; Moeder, W.; Urquhart, W.; Shahinas, D.; Chin, K.; Christendat, D.; Kang, H.-G.; Angelova, M.; Kato, N.; Yoshioka, K. Identification of a functionally essential amino acid for Arabidopsis cyclic nucleotide gated ion channels using the chimeric AtCNGC11/12 gene. Plant J. 2008, 56, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Kugler, A.; Hoth, S.; Dietrich, P. An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide-gated channel. Plant Cell Physiol. 2013, 54, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.C.Y.; Christopher, D.A. The group IV-A cyclic nucleotide-gated channels, CNGC19 and CNGC20, localize to the vacuole membrane in Arabidopsis thaliana. AoB PLANTS 2013, 5. [Google Scholar] [CrossRef]
- Lemtiri-Chlieh, F.; Berkowitz, G.A. Cyclic adenosine monophosphate regulates calcium channels in the plasma membrane of Arabidopsis leaf guard and mesophyll cells. J. Biol. Chem. 2004, 279, 35306–35312. [Google Scholar] [CrossRef]
- Hua, B.-G.; Mercier, R.W.; Leng, Q.; Berkowitz, G.A. Plants do it differently. A new basis for potassium/sodium selectivity in the pore of an ion channel. Plant Physiol. 2003, 132, 1353–1361. [Google Scholar] [CrossRef]
- Mercier, R.W.; Rabinowitz, N.M.; Ali, R.; Gaxiola, R.A.; Berkowitz, G.A. Yeast hygromycin sensitivity as a functional assay of cyclic nucleotide gated cation channels. Plant Physiol. Biochem. 2004, 42, 529–536. [Google Scholar] [CrossRef]
- Ali, R.; Zielinski, R.E.; Berkowitz, G.A. Expression of plant cyclic nucleotide-gated cation channels in yeast. J. Exp. Bot. 2006, 57, 125–138. [Google Scholar] [CrossRef]
- Li, X.; Borsics, T.; Harrington, H.M.; Christopher, D.A. Arabidopsis AtCNGC10 rescues potassium channel mutants of E. coli, yeast and Arabidopsis and is regulated by calcium/calmodulin and cyclic GMP in E. coli. Funct. Plant Biol. 2005, 32, 643–653. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, A.; Ren, Y.; Wu, F.; Wang, G.; Xu, Y.; Lei, C.; Zhu, S.; Pan, T.; et al. A cyclic nucleotide-gated channel mediates cytoplasmic calcium elevation and disease resistance in rice. Cell Res. 2019, 29, 820–831. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.; Wang, Y.; Wang, J.; Yu, Y.; Long, Y.; Wang, Y.; Zhang, H.; Ren, Y.; Chen, J.; et al. OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PLoS Genet. 2017, 13, e1006906. [Google Scholar] [CrossRef]
- Schuurink, R.C.; Shartzer, S.F.; Fath, A.; Jones, R.L. Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc. Natl. Acad. Sci. USA 1998, 95, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.; Merkle, T.; Neuhaus, G. Characterisation of a novel gene family of putative cyclic nucleotide- and calmodulin-regulated ion channels in Arabidopsis thaliana. Plant J. 1999, 18, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Arazi, T.; Sunkar, R.; Kaplan, B.; Fromm, H. A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J. 1999, 20, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Fu, Z.; Su, D.; Zhang, Y.; Li, M.; Pan, Y.; Li, H.; Li, S.; Grassucci, R.A.; Ren, Z.; et al. Mechanism of ligand activation of a eukaryotic cyclic nucleotide-gated channel. Nat. Struct. Biol. 2020, 27, 625–634. [Google Scholar] [CrossRef]
- Jammes, F.; Hu, H.-C.; Villiers, F.; Bouten, R.; Kwak, J.M. Calcium-permeable channels in plant cells. FEBS J. 2011, 278, 4262–4276. [Google Scholar] [CrossRef]
- Lemtiri-Chlieh, F.; Arold, S.T.; Gehring, C. Mg2+ is a missing link in plant cell Ca2+ signalling and homeostasis—a study on Vicia faba guard cells. Int. J. Mol. Sci. 2020, 21, 3771. [Google Scholar] [CrossRef]
- Hua, B.-G.; Mercier, R.W.; Zielinski, R.E.; Berkowitz, G.A. Functional interaction of calmodulin with a plant cyclic nucleotide gated cation channel. Plant Physiol. Biochem. 2003, 41, 945–954. [Google Scholar] [CrossRef]
- Zagotta, W.N.; Siegelbaum, S.A. Structure and function of cyclic nucleotide-gated channels. Annu. Rev. Neurosci. 1996, 19, 235–263. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.N.; Coutts, K.B.; Tester, M.A.; Davies, J.M. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J. Cell Sci. 2003, 116, 81–88. [Google Scholar] [CrossRef]
- Kaplan, B.; Sherman, T.; Fromm, H. Cyclic nucleotide-gated channels in plants. FEBS Lett. 2007, 581, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Isner, J.-C.; Maathuis, F.J.M. cGMP signalling in plants: From enigma to main stream. Funct. Plant Biol. 2018, 45, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Świeżawska, B.; Duszyn, M.; Jaworski, K.; Szmidt-Jaworska, A. Downstream targets of cyclic nucleotides in plants. Front. Plant Sci. 2018, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Gross, I.; Durner, J. In search of enzymes with a role in 3′, 5′-cyclic guanosine monophosphate metabolism in plants. Front. Plant Sci. 2016, 7, 576. [Google Scholar] [CrossRef] [PubMed]
- Al-Younis, I.; Wong, A.; Gehring, C. The Arabidopsis thaliana K+-uptake permease 7 (AtKUP7) contains a functional cytosolic adenylate cyclase catalytic centre. FEBS Lett. 2015, 589, 3848–3852. [Google Scholar] [CrossRef]
- Al-Younis, I.; Wong, A.; Lemtiri-Chlieh, F.; Schmöckel, S.; Tester, M.; Gehring, C.; Donaldson, L. The Arabidopsis thaliana K+-Uptake Permease 5 (AtKUP5) contains a functional cytosolic adenylate cyclase essential for K+ transport. Front. Plant Sci. 2018, 9, 1645. [Google Scholar] [CrossRef]
- Bianchet, C.; Wong, A.; Quaglia, M.; Alqurashi, M.; Gehring, C.; Ntoukakis, V.; Pasqualini, S. An Arabidopsis thaliana leucine-rich repeat protein harbors an adenylyl cyclase catalytic center and affects responses to pathogens. J. Plant Physiol. 2019, 232, 12–22. [Google Scholar] [CrossRef]
- Ludidi, N.; Gehring, C. Identification of a novel protein with guanylyl cyclase activity in Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 6490–6494. [Google Scholar] [CrossRef]
- Mulaudzi, T.; Ludidi, N.; Ruzvidzo, O.; Morse, M.; Hendricks, N.; Iwuoha, E.; Gehring, C. Identification of a novel Arabidopsis thaliana nitric oxide-binding molecule with guanylate cyclase activity in vitro. FEBS Lett. 2011, 585, 2693–2697. [Google Scholar] [CrossRef]
- Kwezi, L.; Ruzvidzo, O.; Wheeler, J.I.; Govender, K.; Iacuone, S.; Thompson, P.E.; Gehring, C.; Irving, H.R. The phytosulfokine (PSK) receptor is capable of guanylate cyclase activity and enabling cyclic GMP-dependent signaling in plants. J. Biol. Chem. 2011, 286, 22580–22588. [Google Scholar] [CrossRef]
- Kwezi, L.; Meier, S.; Mungur, L.; Ruzvidzo, O.; Irving, H.; Gehring, C. The Arabidopsis thaliana Brassinosteroid Receptor (AtBRI1) contains a domain that functions as a guanylyl cyclase in vitro. PLoS ONE 2007, 2, e449. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Ruzvidzo, O.; Morse, M.; Donaldson, L.; Kwezi, L.; Gehring, C. The Arabidopsis Wall Associated Kinase-Like 10 gene encodes a functional guanylyl cyclase and is co-expressed with pathogen defense related genes. PLoS ONE 2010, 5, e8904. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Wang, X.-Y.; Xu, Y.-P.; He, Y.-H.; Cai, X.-Z. Characterization of tomato protein kinases embedding guanylate cyclase catalytic center motif. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Muleya, V.; Wheeler, J.I.; Ruzvidzo, O.; Freihat, L.; Manallack, D.T.; Gehring, C.; Irving, H.R. Calcium is the switch in the moonlighting dual function of the ligand-activated receptor kinase phytosulfokine receptor 1. Cell Commun. Signal. 2014, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qiu, Y.; Peng, Y.; Ning, J.; Song, G.; Yang, Y.; Deng, M.; Men, Y.; Zhao, X.; Wang, Y.; et al. Close temporal relationship between oscillating cytosolic K+ and growth in root hairs of Arabidopsis. Int. J. Mol. Sci. 2020, 21, 6184. [Google Scholar] [CrossRef]
- Ahn, S.J.; Shin, R.; Schachtman, D.P. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 2004, 134, 1135–1145. [Google Scholar] [CrossRef]
- Han, M.; Wu, W.; Wu, W.-H.; Wang, Y. Potassium transporter KUP7 is involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol. Plant 2016, 9, 437–446. [Google Scholar] [CrossRef]
- Donaldson, L.; Ludidi, N.; Knight, M.R.; Gehring, C.; Denby, K. Salt and osmotic stress cause rapid increases in Arabidopsis thaliana cGMP levels. FEBS Lett. 2004, 569, 317–320. [Google Scholar] [CrossRef]
- Shabala, S.; Wu, H.; Bose, J. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Sci. 2015, 241, 109–119. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Sanders, D. Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol. 2001, 127, 1617–1625. [Google Scholar] [CrossRef]
- Demidchik, V.; Tester, M. Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol. 2002, 128, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Bowen, H.C.; Maathuis, F.J.M.; Shabala, S.N.; Tester, M.A.; White, P.J.; Davies, J.M. Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. Plant J. 2002, 32, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Kiegle, E.; Moore, C.A.; Haseloff, J.; Tester, M.A.; Knight, M.R. Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J. 2000, 23, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.-T.; Han, X.-W.; Wei, S.-S.; Shang, Z.-L.; Wang, J.; Yang, D.-W.; Fan, X.; Gao, F.; Zheng, S.-Z.; Bai, J.-T.; et al. Arabidopsis cyclic nucleotide-gated channel 6 is negatively modulated by multiple calmodulin isoforms during heat shock. J. Exp. Bot. 2020, 71, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wu, S.; Gao, X.; Zhang, Y.; Shan, L.; He, P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, L.; Boller, T. FLS2: An LRR receptor–like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 2000, 5, 1003–1011. [Google Scholar] [CrossRef]
- Ma, Y.; Walker, R.K.; Zhao, Y.; Berkowitz, G.A. Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 19852–19857. [Google Scholar] [CrossRef]
- Jeworutzki, E.; Roelfsema, M.R.G.; Anschütz, U.; Krol, E.; Elzenga, J.T.M.; Felix, G.; Boller, T.; Hedrich, R.; Becker, D. Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca2+-associated opening of plasma membrane anion channels. Plant J. 2010, 62, 367–378. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Zhang, N.; Guo, Y.-J.; Gong, B.-Q.; Li, J.-F. Split nano luciferase complementation for probing protein-protein interactions in plant cells. J. Integr. Plant Biol. 2020, 62, 1065–1079. [Google Scholar] [CrossRef]
- Thor, K.; Peiter, E. Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol. 2014, 204, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Thor, K.; Jiang, S.; Michard, E.; George, J.; Scherzer, S.; Huang, S.; Dindas, J.; Derbyshire, P.; Leitão, N.; DeFalco, T.A.; et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 2020, 585, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-R.; Xu, Y.-P.; Cai, X.-Z. SlCNGC1 and SlCNGC14 suppress Xanthomonas oryzae pv. oryzicola-induced hypersensitive response and non-host resistance in tomato. Front. Plant Sci. 2018, 9, 9. [Google Scholar] [CrossRef]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The evolution of calcium-based signalling in plants. Curr. Biol. 2017, 27, 667–679. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, D.M.; Altshuler-Keylin, S.; Zhang, X.-D.; He, C.; Morales-Phan, C.; Yu, Y.; Kaye, J.A.; Brueggemann, C.; Chen, T.-Y.; L’Etoile, N.D. Contribution of the cyclic nucleotide gated channel subunit, CNG-3, to olfactory plasticity in Caenorhabditis elegans. Sci. Rep. 2017, 7, 169. [Google Scholar] [CrossRef]
- Wilson, C.M.; Stecyk, J.A.W.; Couturier, C.S.; Nilsson, G.E.; Farrell, A.P. Phylogeny and effects of anoxia on hyperpolarization-activated cyclic nucleotide-gated channel gene expression in the heart of a primitive chordate, the Pacific hagfish (Eptatretus stoutii). J. Exp. Biol. 2013, 216, 4462–4472. [Google Scholar] [CrossRef]
- Chiasson, D.M.; Haage, K.; Sollweck, K.; Brachmann, A.; Dietrich, P.; Parniske, M. A quantitative hypermorphic CNGC allele confers ectopic calcium flux and impairs cellular development. eLife 2017, 6. [Google Scholar] [CrossRef]
- Lelle, M.; Otte, M.; Bonus, M.; Gohlke, H.; Benndorf, K. Fluorophore-labeled cyclic nucleotides as potent agonists of cyclic nucleotide-regulated ion channels. ChemBioChem 2020, 21, 2311–2320. [Google Scholar] [CrossRef]
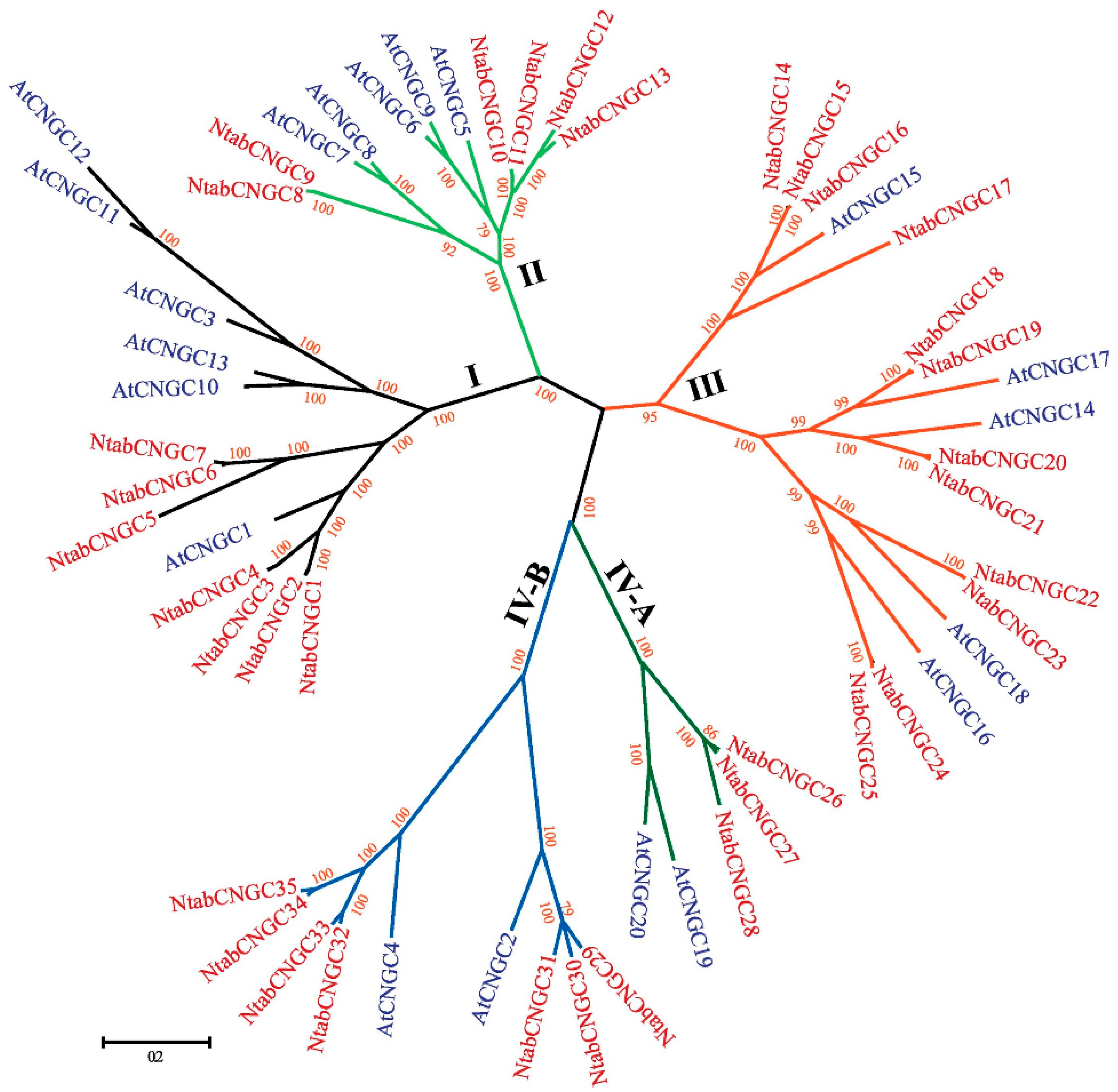

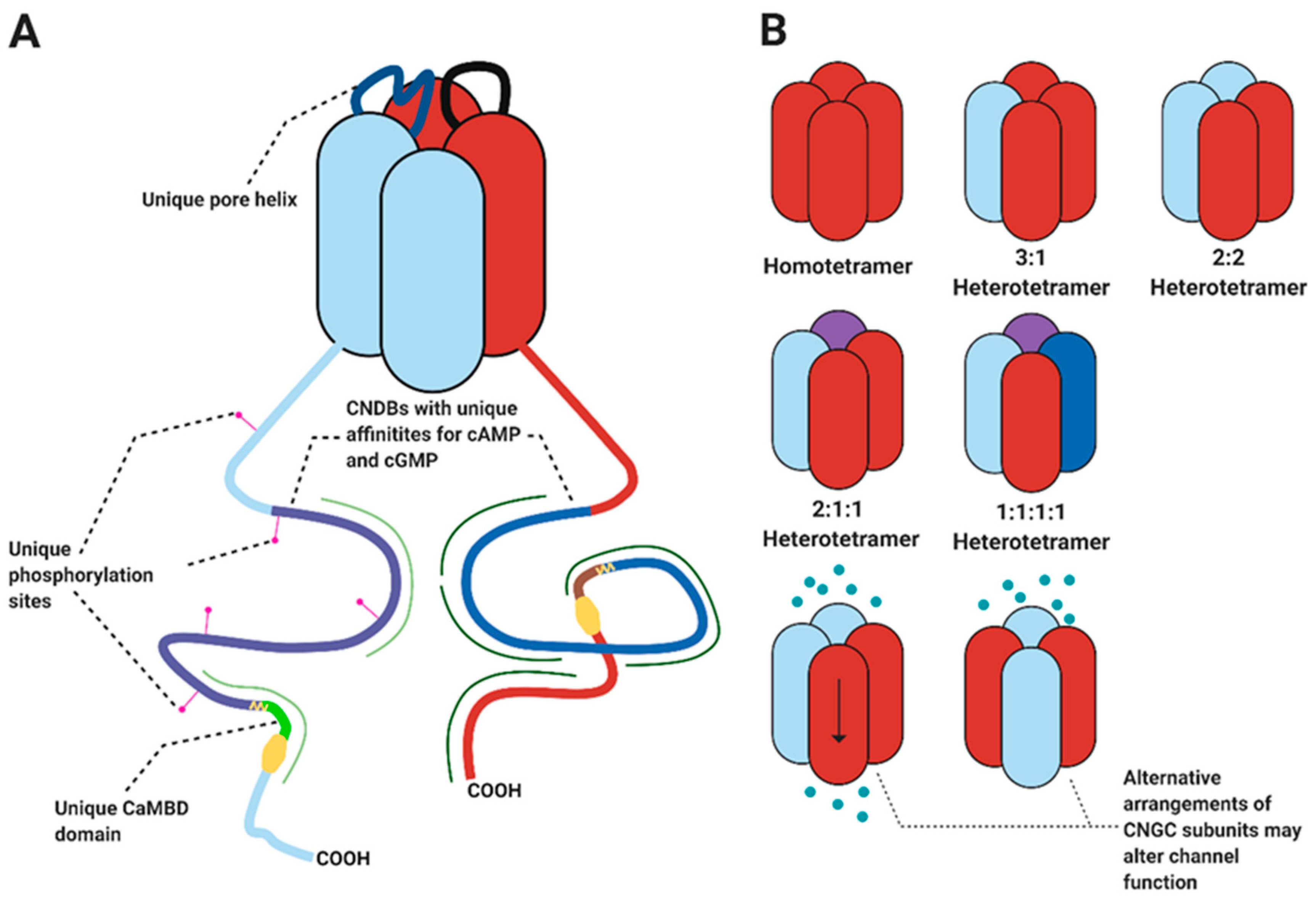
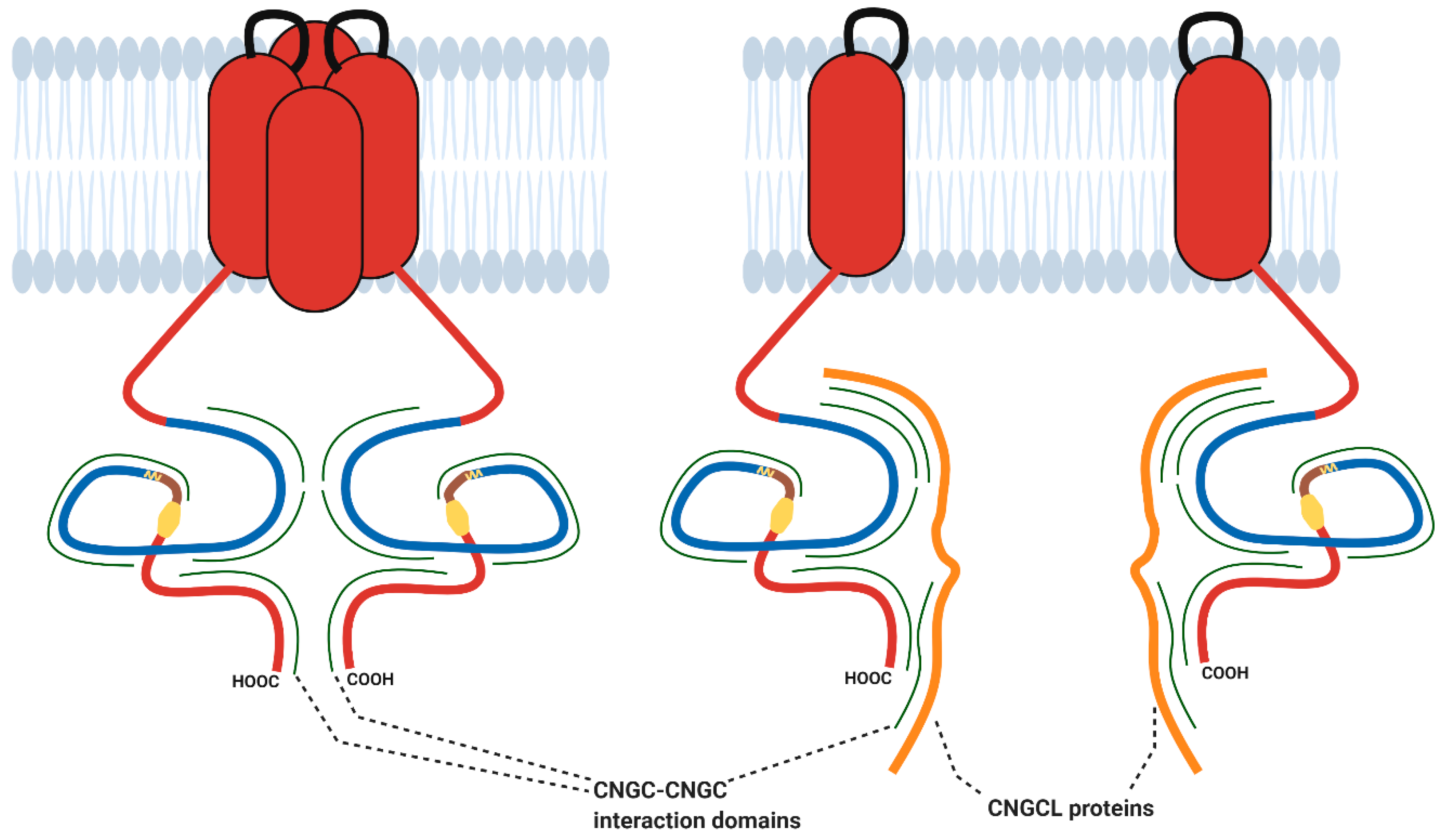
| Gene | Proposed Physiological or Developmental Process | References |
|---|---|---|
| AtCNGC1 | Negative regulation of Pb2+ tolerance; primary root growth; gravitropism | [24,25,26] |
| AtCNGC2 | Pathogen defence; programmed cell death; nitric oxide generation; suppression of leaf senescence; flowering time; thermotolerance (heat and chill); Ca2+ transport in leaves and Ca2+ sensitivity; jasmonic acid-induced Ca2+ entry | [27,28,29,30,31,32,33,34,35,36,37,38,39] |
| AtCNGC3 | Germination; salt tolerance; Na+ and K+ uptake | [40] |
| AtCNGC4 | Pathogen defence; programmed cell death; flowering time; thermotolerance (heat and chill); Ca2+ tolerance | [27,28,29,32,38,41] |
| AtCNGC5 | cGMP-activated Ca2+ entry in guard cells; salt tolerance; root hair growth; auxin signalling | [4,42,43] |
| AtCNGC6 | cGMP-activated Ca2+ entry in guard cells; thermotolerance (heat); root hair growth; auxin signalling | [4,43,44,45] |
| AtCNGC7 | Pollen tube growth | [46,47] |
| AtCNGC8 | Pollen tube growth | [46,47] |
| AtCNGC9 | Root hair growth; auxin signalling | [43,44] |
| AtCNGC10 | Negative regulation of salt tolerance; K+, Na+ and Pb2+ uptake; K+ homeostasis; negative regulation of Pb2+ tolerance; regulation of starch granule size; gravitropism; flowering time; hypocotyl elongation | [25,48,49] |
| AtCNGC11 | Pathogen defence; programmed cell death; Pb2+ and Cd2+ uptake; Pb2+ tolerance; negative regulation of Cd2+ tolerance | [15,25,50,51,52,53] |
| AtCNGC12 | Pathogen defence; programmed cell death | [15,50,51,52,53] |
| AtCNGC13 | Pb2+ uptake; negative regulation of Pb2+ tolerance | [25] |
| AtCNGC14 | Root hair growth; gravitropism; auxin signalling | [43,44,54,55,56,57] |
| AtCNGC15 | Pb2+ and Cd2+ uptake; Pb2+ tolerance; root development | [25,58] |
| AtCNGC16 | Heat and drought tolerance in pollen; negative regulation of Cd2+ tolerance | [25,59] |
| AtCNGC17 | Growth regulation; salt tolerance | [42,60] |
| AtCNGC18 | Pollen tube growth and guidance | [6,46,61,62,63] |
| AtCNGC19 | Response to salt; Pb2+ and Cd2+ uptake; negative regulation of Pb2+ tolerance; herbivory response; pathogen defence; endophyte response; regulating cell death | [25,64,65,66,67] |
| AtCNGC20 | Response to salt; pathogen defence; regulating cell death | [65,67] |
| CNGCs | System | Tested Cations | Tested with cNMPs? | Results | References |
|---|---|---|---|---|---|
| AtCNGC1 | HEK293— whole cell | Tested K+ and Na+ conductance | Yes | Application of 100 μM db-cAMP stimulated AtCNGC1 K+ and Na+ conductance. No K+ or Na+ conductance was observed in the absence of db-cAMP. | [85] |
| AtCNGC1 | Yeast | Tested Ca2+ uptake | No | In the presence of yeast pheromone α factor, AtCNGC1 in a Ca2+ uptake-deficient yeast mutant increased colony growth, indirectly demonstrating Ca2+ conduction. | [24] |
| AtCNGC1, AtCNGC2, AtCNGC4 | Yeast | Tested K+ uptake | Yes | Addition of 100 μM db-cAMP stimulated growth of a K+ uptake-deficient yeast mutant expressing AtCNGC1, AtCNGC2, and AtCNGC4. | [86] |
| AtCNGC1, AtCNGC2, AtCNGC4 | Yeast | Tested K+ and Ca2+ uptake | Yes | AtCNGC1M2 (deletion in C-terminal domain) expression in a Ca2+ uptake yeast mutant resulted in growth, indicating Ca2+ permeability of AtCNGC1. Expression of AtCNGC2 and AtCNGC4 enhanced growth of a K+ uptake-deficient yeast mutant. Application of 100 μM db-cAMP increased growth of yeast mutant transformed with AtCNGC1M2. | [87] |
| AtCNGC2 | Yeast | Tested K+ uptake | Yes | In the presence of 10 μM db-cAMP or db-cGMP, transfection with AtCNGC2 enhanced growth of a K+ uptake-deficient yeast mutant. | [1] |
| AtCNGC2 | Xenopus oocytes— whole cell | Tested K+ conductance | Yes | Application of 10 μM db-cAMP stimulated AtCNGC2 K+ conductance. No K+ conductance was observed in the absence of db-cNMPs. | [1] |
| AtCNGC2 | Xenopus oocytes— whole cell | Tested K+, Na+, Li+, Cs+ and Rb+ conductance | Yes | In the presence of 100 μM db-cAMP, AtCNGC2 conducted K+, Li+, Cs+ and Rb+. Na+ conductance was significantly less. No data were reported concerning conductance in the absence of db-cAMP. | [2] |
| AtCNGC2 | Xenopus oocytes— inside-out patch | Tested K+ conductance | Yes | Application of 100 μM cAMP stimulated AtCNGC2 K+ conductance. No K+ conductance was observed in the absence of db-cAMP. | [2] |
| AtCNGC2 | Xenopus oocytes— inside-out patch | Tested K+ and Na+ conductance | Yes | Application of 100 μM cAMP stimulated AtCNGC2 K+ conductance, but not Na+ conductance. No K+ or Na+ conductance was observed in the absence of cAMP. Mutation of N416 and D417 in the pore resulted in Na+ conductance similar to K+ conductance. | [85] |
| AtCNGC2 | HEK293— whole cell and inside-out patch | Tested K+ and Na+ conductance | Yes | Application of 100 μM db-cAMP stimulated AtCNGC2 K+ conductance, but not Na+ conductance. No K+ or Na+ conductance was observed in the absence of db-cAMP. Mutation of N416 and D417 in the pore region resulted in Na+ conductance similar to K+ conductance. | [2,85] |
| AtCNGC2 | Guard cell protoplasts— whole cell | Tested Ba2+ conductance (as a proxy for Ca2+) | Yes | Application of 1 mM db-cAMP stimulated AtCNGC2-dependent Ca2+ conductance. | [30] |
| AtCNGC2 | HEK293T— whole cell | Tested Ca2+ conductance | Yes | Application of 200 μM 8Br-cAMP stimulated AtCNGC2 Ca2+ conductance. No data were reported concerning Ca2+ conductance in the absence of db-cAMP. | [38] |
| AtCNGC2, AtCNGC4 | Mesophyll cell protoplasts— whole cell | Tested Ba2+ conductance (as a proxy for Ca2+) | No | Wild-type mesophyll cell protoplasts conducted Ca2+ in response to H2O2 or flg22. Ca2+ conductance was lost in Atcngc2 or Atcngc4 loss-of function mutants, as well as the Atcngc2 Atcngc4 double mutant. | [29] |
| AtCNGC2, AtCNGC4 | Xenopus oocytes— whole cell | Tested Ca2+, Mg2+, Ba2+, Sr2+, K+ and Na+ conductance | No | Independently, AtCNGC2 or AtCNGC4 did not conduct Ca2+ in the absence of cNMPs. Co-expression of AtCNGC2 and AtCNGC4 produced Ca2+, Sr2+, Ba2+ and K+-permeable (Na+ and Mg2+-impermeable) channels in the absence of cNMPs. | [29] |
| AtCNGC3 | Yeast | Tested Na+ and K+ uptake | No | Yeast expressing CNGC3 accumulated more Na+ and K+, suggesting a pathway for Na+ and K+ transport. | [40] |
| AtCNCG4 | Xenopus oocytes— inside-out patch | Tested K+, Na+ and Cs+ conductance | Yes | Application of 500 μM cAMP or cGMP stimulated AtCNGC4 K+, Na+ and Cs+ conductance. Compared to K+, outward conductance of Cs+ was significantly lower. No conduction of K+, Na+ or Cs+ was observed in the absence of cNMPs. | [41] |
| AtCNGC5, AtCNGC6 | Guard cell protoplasts— whole cell | Tested Mg2+, Ba2+ and Ca2+ conductance | Yes | Application of 500 μM 8Br-cGMP stimulated Mg2+, Ca2+ and Ba2+ conductance. Mg2+ conductance was lost in Atcngc5 Atcngc6 double mutants. AtCNGC1, AtCNGC2 and AtCNGC20 did not appear to contribute to these guard cell 8Br-cGMP-activated currents. | [4] |
| AtCNGC5, AtCNGC6 | HEK293T— whole cell | Tested Ca2+ and Na+ conductance | No | HEK293 cells expressing CNGC5 or CNGC6 displayed inward currents carried by Ca2+, not Na+. | [43] |
| AtCNGC6 | Root protoplasts— whole cell | Tested Ca2+ conductance | Yes | Application of 50 μM db-cAMP stimulated AtCNGC6-dependent Ca2+ conductance, application of a phosphodiesterase inhibitor also stimulated Ca2+ conductance. | [45] |
| AtCNGC7, AtCNGC8 | Xenopus oocytes— whole cell | Tested Ca2+ conductance | No | AtCNGC7 or AtCNGC8 Ca2+ conductivity was not observed in the absence of cNMPs. | [46] |
| AtCNGC7, AtCNGC8, AtCNGC9, AtCNGC10, AtCNGC16 and AtCNGC18 | HEK293T— whole cell | Tested Ca2+ and K+ conductance | Yes | Application of 100 μM 8Br-cAMP or 100 μM 8Br-cGMP stimulated AtCNGC7, AtCNGC8, AtCNGC9, AtCNGC10, AtCNGC16 and AtCNGC18 Ca2+ conductance. Ca2+ conductance did not require 8Br-cNMP application. No significant K+ conductance reported. | [6] |
| AtCNGC10 | E. coli and yeast | Tested K+ uptake | Yes | AtCNGC10 complemented E. coli and yeast K+ uptake mutants. In E. coli, co-expression of AtCNGC10 and CaM inhibited cell growth, but cGMP overcame this. | [88] |
| AtCNGC10 | HEK293— whole cell | Tested K+ conductance | Yes | In the presence of 100 μM db-cGMP, AtCNGC10 conducted K+. No data were reported concerning conductance in the absence of db-cAMP. | [80] |
| AtCNGC10 | Yeast | Tested K+ and Na+ uptake | No | AtCNGC10-transformed yeast accumulated more Na+ in the presence of 20 mM NaCl. Expression rescued growth of K+ uptake-deficient yeast. | [49] |
| AtCNGC11, AtCNGC12 | Yeast | Tested K+ uptake | Yes | Growth of K+ uptake-deficient yeast was complemented by AtCNGC11, AtCNGC12 or the chimeric AtCNGC11/12. Growth was enhanced by 100 μM db-cAMP but not db-cGMP. | [53] |
| AtCNGC11, AtCNGC12 | Yeast | Tested Ca2+ uptake | No | Expression of AtCNGC11, AtCNGC12 or AtCNGC11/12 complemented growth of Ca2+ uptake-deficient yeast. | [51] |
| AtCNGC11, AtCNGC12 | Yeast | Tested K+ uptake | No | AtCNGC11/12 or AtCNGC12 restored growth of K+ uptake-deficient yeast. | [81] |
| AtCNGC11, AtCNGC12 | Xenopus oocytes— whole cell | Tested Ca2+ conductance | Yes | Expression of AtCNGC12 caused a Ca2+ conductance that was not enhanced by cNMPs. AtCNGC11 expression did not cause a Ca2+ conductance, even with cNMPs. Co-expression did not affect the AtCNGC12-dependent conductance. | [8] |
| AtCNGC14 | Xenopus oocytes— whole cell | Tested Ca2+ conductance | No | AtCNGC14 Ca2+ conductivity was observed in the absence of cNMPs. It was not tested whether application of cNMPs would increase Ca2+ conductance. | [57] |
| AtCNGC14 | Xenopus oocytes— whole cell | Tested Ca2+ conductance | No | AtCNGC14 inward Ca2+ currents were observed in the absence of cNMPs. | [56] |
| AtCNGC18 | E. coli | Tested Ca2+ uptake | No | Expression of AtCNGC18 in E. coli caused Ca2+ accumulation. | [62] |
| AtCNGC18 | HEK293T— whole cell | Tested Ca2+ conductance | Yes | Application of 100 μM 8Br-cAMP or 100 μM 8Br-cGMP stimulated greater AtCNGC18 Ca2+ conductance, but not 20 μM 8Br-cNMP. Ca2+ conductance did not require 8Br-cNMP application. | [5] |
| AtCNGC18 | Xenopus oocytes— whole cell | Tested Ca2+ conductance | Yes | In the presence of 100 μM db-cAMP, AtCNGC18 conducted Ca2+. No data were reported concerning conductance in the absence of db-cAMP. | [63] |
| AtCNGC18 | Pollen tube protoplasts— whole cell | Tested Ca2+ conductance | Yes | Application of 100 μM 8Br-cGMP stimulated AtCNGC18-dependent Ca2+ conductance. Ca2+ conductance was not apparent in the absence of 8Br-cGMP. | [6] |
| AtCNGC18 | Xenopus oocytes— whole cell | Tested Ca2+ conductance | No | AtCNGC18 Ca2+ conductivity was observed in the absence of cNMPs. Co-expression of AtCNGC18 with AtCNGC7 or AtCNGC8 eliminated Ca2+ conductivity. | [46] |
| AtCNGC19 | Xenopus oocytes— whole cell | Tested Ca2+, Na+ and K+ conductance | Yes | In the presence of 300 μM db-cAMP, AtCNGC19 elicited Ca2+ inward currents but not K+ and Na+ currents. | [66] |
| AtCNGC19, AtCNGC20 | Xenopus oocytes— whole cell | Tested Ca2+ conductance | No | AtCNGC19 and AtCNGC20 conductivity was observed in the absence of cNMPs. It was not tested whether application of cNMPs would increase Ca2+ conductance. Co-expression of AtCNGC19 and AtCNGC20 increased conductance compared to independently expressed AtCNGC19 and AtCNGC20. | [67] |
| PpCNGCb | Moss protoplasts cell attached | Tested Ba2+ conductance (as a proxy for Ca2+) | No | Ba2+ conductivity did not require application of cNMPs, but it is possible that there were endogenous cNMPs. Ba2+ conductivity was altered in Ppcngcb mutants. | [28] |
| HvCNGC2-3 | Xenopus oocytes— whole cell | Tested K+ and Na+ conductance | Yes | Application of 10 μM 8Br-cGMP stimulated HvCNGC2-3 Na+ and K+ conductivity only in the co-presence of both ions. No Na+ or K+ conductance was observed in the absence of cGMP, or 10 μM 8Br-cAMP. Ca2+ conductivity was not observed. | [7] |
| OsCNGC9 | HEK293— whole cell | Tested Ca2+ and K+ conductance | No | OsCNGC9 Ca2+ conductivity was observed in the absence of cNMPs. Comparatively little K+ conductivity was observed. It was not tested whether application of cNMPs would increase Ca2+ or K+ conductance. | [89] |
| OsCNGC13 | HEK293— whole cell | Tested Ca2+ and K+ conductance | No | OsCNGC13 mediated Ca2+ inward currents but not K+ currents. It was not tested whether application of cNMPs would increase Ca2+ conductance. | [90] |
| MtCNGC15 | Xenopus oocytes— whole cell | Tested Ba2+ and Ca2+ conductance | No | MtCNGC15 Ca2+ conductivity did not require application of cNMPs. It was not tested whether application of cNMPs would increase Ca2+ conductance. | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarratt-Barnham, E.; Wang, L.; Ning, Y.; Davies, J.M. The Complex Story of Plant Cyclic Nucleotide-Gated Channels. Int. J. Mol. Sci. 2021, 22, 874. https://doi.org/10.3390/ijms22020874
Jarratt-Barnham E, Wang L, Ning Y, Davies JM. The Complex Story of Plant Cyclic Nucleotide-Gated Channels. International Journal of Molecular Sciences. 2021; 22(2):874. https://doi.org/10.3390/ijms22020874
Chicago/Turabian StyleJarratt-Barnham, Edwin, Limin Wang, Youzheng Ning, and Julia M. Davies. 2021. "The Complex Story of Plant Cyclic Nucleotide-Gated Channels" International Journal of Molecular Sciences 22, no. 2: 874. https://doi.org/10.3390/ijms22020874
APA StyleJarratt-Barnham, E., Wang, L., Ning, Y., & Davies, J. M. (2021). The Complex Story of Plant Cyclic Nucleotide-Gated Channels. International Journal of Molecular Sciences, 22(2), 874. https://doi.org/10.3390/ijms22020874





