Real-Time Fluorescence Image-Guided Oncolytic Virotherapy for Precise Cancer Treatment
Abstract
:1. Introduction: Intravital Imaging
2. Fluorescence Ubiquitination Cell Cycle Indicator (FUCCI) Imaging Guides Precise Timing of Cancer Chemotherapy after Adenoviral S-Phase Cell-Cycle Decoy
2.1. FUCCI Imaging Visualizes Quiescent Cancer Stem Cells (CSCs) Resistant to Conventional Therapy
2.2. FUCCI Imaging Demonstrates that an Oncolytic Adenovirus Decoys Quiescent Cancer Stem Cells to Cycle to S-Phase. Cytotoxic Chemotherapy Can then Be Presicely Administrated to Eradicate the Decoyed Cancer Cells
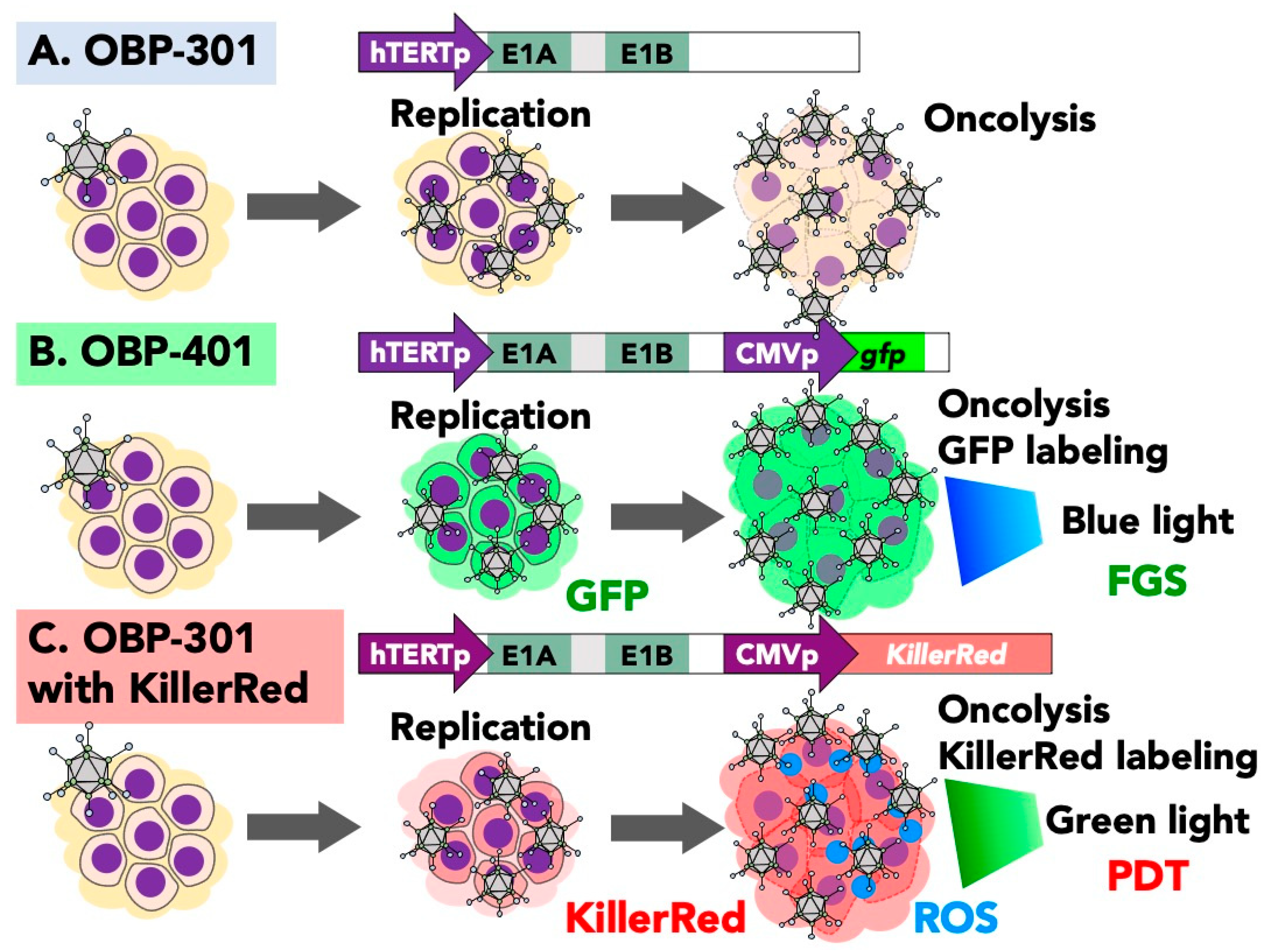
3. Oncolytic GFP Reporter Virus for Fluorescence-Guided Surgery (FGS)
3.1. Fluorescence-Guided Surgery (FGS) with Adenoviral GFP: Efficacy and Limitations
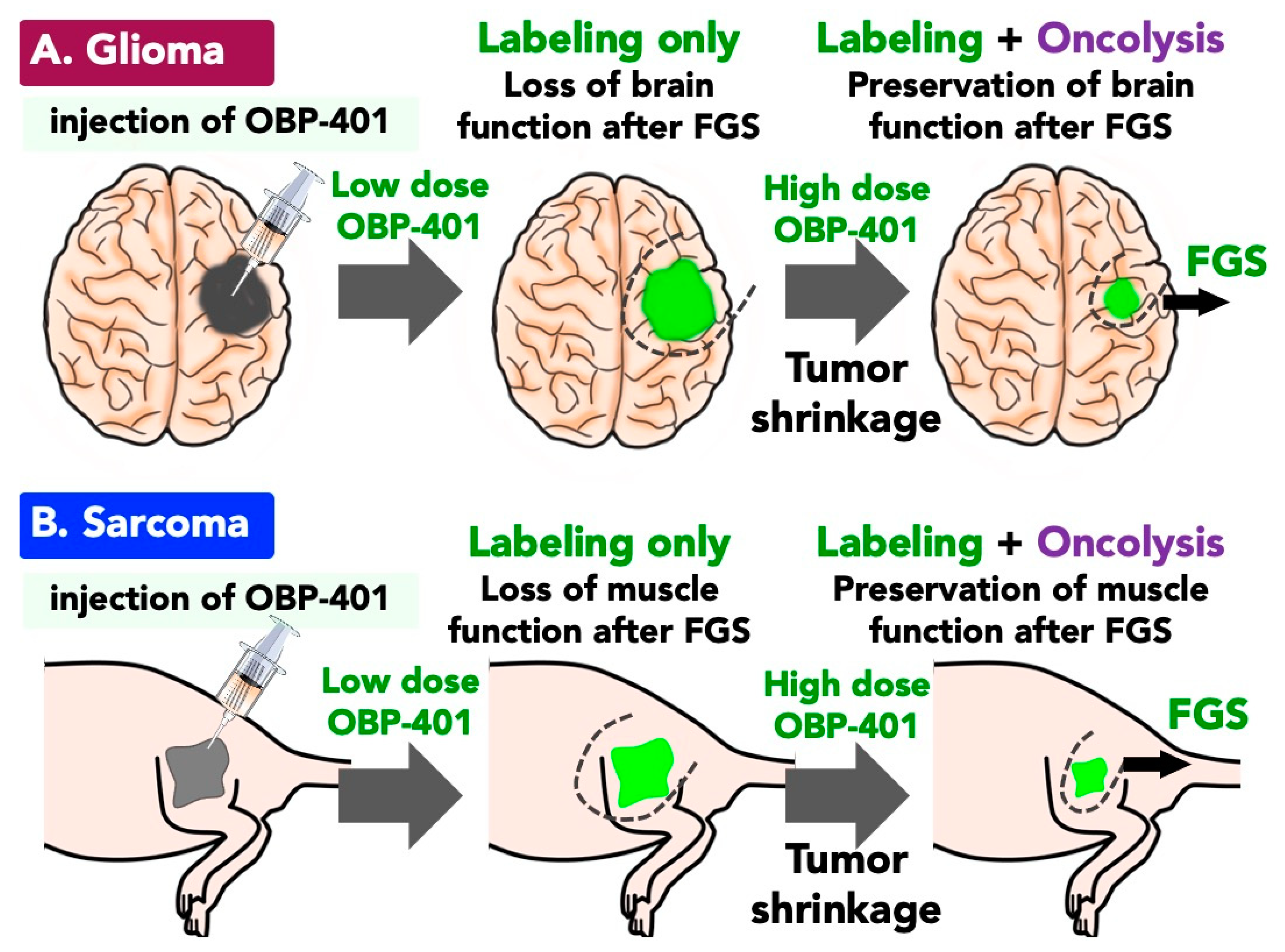
3.2. OBP-401-Based-FGS for GBM with Oncolysis of Invading GBM Cells
3.3. Function-Preserving FGS with OBP-401 for Soft Tissue Sarcoma
3.4. FGS with OBP-401 for Osteosarcoma without Specific Markers
3.5. OBP-401-Based FGS for Liver and Lung Metastasis
4. Preclinical FGS with Patient-Derived Orthotopic Xenograft (PDOX) Mouse Models
5. Photodynamic Virotherapy with OBP-301 Expressing KillerRed
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Shimomura, O. Structure of the chromophore of Aequorea green fluorescent protein. FEBS Lett. 1979, 104, 220–222. [Google Scholar] [CrossRef] [Green Version]
- Tsein, R. The green fluorescence protein. Anuu. Rev. Biochem. 1998, 67, 509–554. [Google Scholar] [CrossRef]
- Zhang, J.; Campbell, R.E.; Ting, A.Y.; Tsien, R.Y. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 2002, 3, 906–918. [Google Scholar] [CrossRef]
- Newman, R.H.; Fosbrink, M.D.; Zhang, J. Genetically encodable fluorescent biosensors for tracking signaling dynamics in living cells. Chem. Rev. 2011, 111, 3614–3666. [Google Scholar] [CrossRef] [Green Version]
- Ni, Q.; Mehta, S.; Zhang, J. Live-cell imaging of cell signaling using genetically encoded fluorescent reporters. FEBS J. 2018, 285, 203–219. [Google Scholar] [CrossRef]
- Hoffman, R.M. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat. Rev. Cancer 2005, 5, 796–806. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Yang, M. Color-coded fluorescence imaging of tumor-host interactions. Nat. Protoc. 2006, 1, 928–935. [Google Scholar] [CrossRef]
- Condeelis, J.S.; Segall, J.E. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 2003, 3, 921–930. [Google Scholar] [CrossRef]
- Chishima, T.; Miyagi, Y.; Wang, X.; Yamaoka, H.; Shimada, H.; Moossa, A.R.; Hoffman, R.M. Cancer invasion and mi-crometastasis visualized in live tissue by green fluorescent protein expression. Cancer Res. 1997, 57, 2042–2047. [Google Scholar]
- Yang, M.; Baranov, E.; Moossa, A.R.; Penman, S.; Hoffman, R.M. Visualizing gene expression by whole-body fluorescence imaging. Proc. Natl. Acad. Sci. USA 2000, 97, 12278–12282. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Baranov, E.; Jiang, P.; Sun, F.X.; Li, X.M.; Li, L.; Hasegawa, S.; Chishima, T.; Shimada, H.; Moosa, A.R.; et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastasis. Proc. Natl. Acad. Sci. USA 2000, 97, 1206–1211. [Google Scholar] [CrossRef] [Green Version]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Ntziachristos, V.; Ripoll, J.; Wang, L.V.; Weissleder, R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320. [Google Scholar] [CrossRef]
- Hawkins, L.K.; Lemoine, N.R.; Kirn, D. Oncolytic biotherapy: A novel therapeutic plafform. Lancet Oncol. 2002, 3, 17–26. [Google Scholar] [CrossRef]
- Chiocca, E.A. Oncolytic viruses. Nat. Rev. Cancer 2002, 2, 938–950. [Google Scholar] [CrossRef]
- Eager, R.M.; Nemunaitis, J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011, 18, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Russell, S.J.; Peng, K.-W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef] [Green Version]
- Curiel, D.T. The development of conditionally replicative adenoviruses for cancer therapy. Clin. Cancer Res. 2000, 6, 3395–3399. [Google Scholar]
- Rein, D.T.; Breidenbach, M.; Curiel, D.T. Current developments in adenovirus-based cancer gene therapy. Futur. Oncol. 2006, 2, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Curiel, D.T. Current issues and future directions of oncolytic adenoviruses. Mol. Ther. 2010, 18, 243–250. [Google Scholar] [CrossRef]
- Kawashima, T.; Kagawa, S.; Kobayashi, N.; Shirakiya, Y.; Umeoka, T.; Teraishi, F.; Taki, M.; Kyo, S.; Tanaka, N.; Fujiwara, T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004, 10, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Nemunaitis, J.; Tong, A.W.; Pappen, B.; Burke, J.; Ichimaru, D.; Urata, Y.; Fujiwara, T.; Nemunaitis, M.; Senzer, N.; Phadke, A.P.; et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (Telomelysin) for various solid tumors. Mol. Ther. 2010, 18, 429–434. [Google Scholar] [CrossRef]
- Kuroda, S.; Fujiwara, T.; Urata, Y.; Kagawa, S.; Fujiwara, T.; Shirakawa, Y.; Yamasaki, Y.; Yano, S.; Uno, F.; Tazawa, H.; et al. Telomerase-dependent oncolytic adenovirus sensitizes human cancer cells to ionizing radiation via inhibition of DNA repair machinery. Cancer Res. 2010, 70, 9339–9348. [Google Scholar] [CrossRef] [Green Version]
- Tazawa, H.; Kuroda, S.; Hasei, J.; Kagawa, S.; Fujiwara, T. Impact of autophagy in oncolytic adenoviral therapy for cancer. Int. J. Mol. Sci. 2017, 18, 1479. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T. Multidisciplinary oncolytic virotherapy for gastrointestinal cancer. Ann. Gastroenterol. Surg. 2019, 3, 396–404. [Google Scholar] [CrossRef]
- Kishimoto, H.; Kojima, T.; Watanabe, Y.; Kagawa, S.; Fujiwara, T.; Uno, F.; Teraishi, F.; Kyo, S.; Mizuguchi, H.; Hashimoto, Y.; et al. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat. Med. 2006, 12, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Takehara, K.; Tazawa, H.; Urata, Y.; Kagawa, S.; Fujiwara, T.; Okada, N.; Hashimoto, Y.; Kikuchi, S.; Kuroda, S.; Kishimoto, H.; et al. Targeted photodynamic virotherapy armed with a genetically encoded photosensitizer. Mol. Cancer Ther. 2016, 15, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.C.; Kolb, E.; Sampson, V.B. Development of chemotherapy with cell-cycle inhibitors for adult and pediatric cancer therapy. Cancer Res. 2018, 78, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Lytle, N.K.; Barber, A.G.; Reya, T. Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer 2018, 18, 669–680. [Google Scholar] [CrossRef]
- Aguirre-Ghiso, J.A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 2007, 7, 834–846. [Google Scholar] [CrossRef] [Green Version]
- Goss, P.E.; Chambers, A.F. Does tumour dormancy offer a therapeutic target? Nat. Rev. Cancer 2010, 10, 871–877. [Google Scholar] [CrossRef]
- Aguirre-Ghiso, J.A.; Bragado, P.; Sosa, M.S. Targeting dormant cancer. Nat. Med. 2013, 19, 276–277. [Google Scholar] [CrossRef]
- Isherwood, B.; Timpson, P.; McGhee, E.J.; Anderson, K.I.; Canel, M.; Serrels, A.; Brunton, V.G.; Carragher, N.O. Live cell in vitro and in vivo imaging applications: Accelerating drug discovery. Pharmaceutics 2011, 3, 141–170. [Google Scholar] [CrossRef] [Green Version]
- Sakaue-Sawano, A.; Kurokawa, H.; Morimura, T.; Tanyu, A.; Osawa, H.; Kashiwagi, S.; Fukami, K.; Miyata, T.; Miyoshi, H.; Imamura, T.; et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle pro-gression. Cell 2008, 132, 487–489. [Google Scholar] [CrossRef] [Green Version]
- Sakaue-Sawano, A.; Kobayashi, T.; Ohtawa, K.; Miyawaki, A. Drug-induced cell cycle modulation leading to cell-cycle arrest, nuclear mis-segregation, or endoreplication. BMC Cell Biol. 2011, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Bajar, B.T.; Lam, A.J.; Badiee, R.K.; Oh, Y.-H.; Chu, J.; Zhou, X.X.; Kim, N.; Kim, B.B.; Chung, M.; Yablonovitch, A.L.; et al. Fluorescent indicators for simultaneous reporting of all four cell cycle phases. Nat. Methods 2016, 13, 993–996. [Google Scholar] [CrossRef] [Green Version]
- Sakaue-Sawano, A.; Yo, M.; Komatsu, N.; Hiratsuka, T.; Kogure, T.; Hoshida, T.; Goshima, N.; Matsuda, M.; Miyoshi, H.; Miyawaki, A. Genetically encoded tools for optical dissection of the mammalian cell cycle. Mol. Cell. 2017, 68, 626. [Google Scholar] [CrossRef]
- Yano, S.; Tazawa, H.; Kagawa, S.; Hoffman, R.M.; Fujiwara, T.; Hashimoto, Y.; Shirakawa, Y.; Kuroda, S.; Nishizaki, M.; Kishimoto, H.; et al. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem–like cells in s/G2/M phases. Clin. Cancer Res. 2013, 19, 6495–6505. [Google Scholar] [CrossRef] [Green Version]
- Cobrinik, D. Pocket proteins and cell cycle control. Oncogene 2005, 24, 2796–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyson, N.; Harlow, E. Adenovirus E1A targets key regulators of cell proliferation. Cancer Surv. 1992, 12, 161–195. [Google Scholar] [PubMed]
- Zheng, X.; Rao, X.-M.; Gomez-Gutierrez, J.G.; Hao, H.; McMasters, K.M.; Zhou, H.S. Adenovirus E1B55K region is required to enhance cyclin e expression for efficient viral DNA replication. J. Virol. 2008, 82, 3415–3427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, S.; Zhang, Y.; Miwa, S.; Tome, Y.; Hiroshima, Y.; Uehara, F.; Yamamoto, M.; Suetsugu, A.; Kishimoto, H.; Tazawa, H.; et al. Spatial–temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle 2014, 13, 2110–2119. [Google Scholar] [CrossRef] [Green Version]
- Chittajallu, D.R.; Florian, S.; Kohler, R.H.; Iwamoto, Y.; Orth, J.D.; Weissleder, R.; Danuser, G.; Mitchison, T.J. In vivo cell-cycle profiling in xenograft tumors by quantitative intravital microscopy. Nat. Methods 2015, 12, 577–585. [Google Scholar] [CrossRef]
- Yano, S.; Tazawa, H.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. FUCCI real-time cell-cycle imaging as a guide for designing improved cancer therapy: A review of innovative strategies to target quiescent chemo-resistant cancer cells. Cancers 2020, 12, 2655. [Google Scholar] [CrossRef]
- Yano, S.; Hoffman, R.M. Real-time determination of the cell-cycle position of individual cells within live tumors using FUCCI cell-cycle imaging. Cells 2018, 7, 168. [Google Scholar] [CrossRef] [Green Version]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Büchler, M.W. Most pancreatic cancer resections are R1 resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef]
- Vos, E.; Gaal, J.; Verhoef, C.; Brouwer, K.; Van Deurzen, C.; Koppert, L.B. Focally positive margins in breast conserving surgery: Predictors, residual disease, and local recurrence. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 1846–1854. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation-a new cutting edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef]
- Hernot, S.; Van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Delong, J.C.; Hoffman, R.M.; Bouvet, M. Current status and future perspectives of fluorescence-guided surgery for cancer. Expert Rev. Anticancer. Ther. 2016, 16, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaya, T.; Nakamura, Y.A.; Choyke, P.L.; Kobayashi, H. Fluorescence-Guided Surgery. Front. Oncol. 2017, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Stammes, M.A.; Bugby, S.L.; Porta, T.; Pierzchalski, K.; Devling, T.; Otto, C.; Dijkstra, J.; Vahrmeijer, A.L.; De Geus-Oei, L.-F.; Mieog, J.S.D. Modalities for image- and molecular-guided cancer surgery. BJS 2018, 105, e69–e83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioux, S.; Choi, H.S.; Frangioni, J.V. Image-guided surgery using invisible near-infrared light: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 237–255. [Google Scholar] [CrossRef] [Green Version]
- Vahrmeijer, A.L.; Hutteman, M.; Van Der Vorst, J.R.; Van De Velde, C.J.H.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keereweer, S.; Van Driel, P.; Snoeks, T.J.; Kerrebijn, J.; De Jong, R.; Robert, J.B.; Vahrmeijer, A.; Sterenborg, H.J.; Löwik, C. Optical image-guided cancer surgery: Challenges and limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; De Jong, J.S.; Arts, H.J.G.; Van Der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Shum, C.F.; Bahler, C.D.; Low, P.S.; Ratliff, T.L.; Kheyfets, S.V.; Natarajan, J.P.; Sandusky, G.E.; Sundaram, C.P. Novel use of folate-targeted intraoperative fluorescence, otl38, in robot-assisted laparoscopic partial nephrectomy: Report of the first three cases. J. Endourol. Case Rep. 2016, 2, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Van Manen, L.; Handgraaf, H.J.M.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018, 118, 283–300. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Cho, S.S.; Stummer, W.; Tanyi, J.L.; Vahrmeijer, A.L.; Rosenthal, E.; Smith, B.L.; Henderson, E.R.; Roberts, D.W.; Lee, A.; et al. Review of clinical trials in intraoperative molecular imaging during cancer surgery. J. Biomed. Opt. 2019, 24, 120901. [Google Scholar] [CrossRef] [PubMed]
- Valente, S.A.; Al-Hilli, Z.; Radford, D.M.; Yanda, C.; Tu, C.; Grobmyer, S.R. Near infrared fluorescent lymph node mapping with indocyanine green in breast cancer patients: A prospective trial. J. Am. Coll. Surg. 2019, 228, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Gazyakan, E.; Yang, W.; Engel, H.; Hünerbein, M.; Kneser, U.; Hirche, C. Indocyanine green fluorescence-guided sentinel node biopsy: A meta-analysis on detection rate and diagnostic performance. Eur. J. Surg. Oncol. (EJSO) 2014, 40, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Niebling, M.G.; Pleijhuis, R.G.; Bastiaannet, E.; Brouwers, A.H.; Van Dam, G.M.; Hoekstra, H.J. A systematic review and meta-analyses of sentinel lymph node identification in breast cancer and melanoma, a plea for tracer mapping. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 466–473. [Google Scholar] [CrossRef]
- Ogawa, M.; Regino, C.A.; Choyke, P.L.; Kobayashi, H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol. Cancer Ther. 2009, 8, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Harlaar, N.J.; Koller, M.; De Jongh, S.J.; Van Leeuwen, B.L.; Hemmer, P.H.; Kruijff, S.; Van Ginkel, R.J.; Been, L.B.; De Jong, J.S.; Kats-Ugurlu, G.; et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: A single-centre feasibility study. Lancet Gastroenterol. Hepatol. 2016, 1, 283–290. [Google Scholar] [CrossRef] [Green Version]
- LaFreniere, A.-S.; Shine, J.J.; Nicholas, C.R.; Temple-Oberle, C.F. The use of indocyanine green and near-infrared fluorescence imaging to assist sentinel lymph node biopsy in cutaneous melanoma: A systematic review. Eur. J. Surg. Oncol. (EJSO) 2020. [Google Scholar] [CrossRef]
- Weele, E.J.T.; van Scheltinga, A.G.T.T.; Linssen, M.D.; Nagengast, W.B.; Lindner, I.; Jorritsma-Smit, A.; de Vries, E.G.E.; Kosterink, J.G.W.; Lub-de Hooge, M.N. Development, preclinical safety, formulation, and stability of clinical grade bevaci-zumab-800CW, a new near infrared fluorescent imaging against for the first I human use. Eur. J. Pharm. Biopharm. 2016, 104, 226–234. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Aldave, G.; Tejada, S.; Pay, E.; Marigil, M.; Bejarano, B.; Idoate, M.A.; Díez-Valle, R. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic acid-guided surgery. Neurosurgery 2013, 72, 915–921. [Google Scholar] [CrossRef]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomized, controlled trial. Lancet Oncol. 2012, 12, 997–1003. [Google Scholar] [CrossRef]
- Eyüpoglu, I.Y.; Hore, N.; Savaskan, N.E.; Grummich, P.; Roessler, K.; Buchfelder, M.; Ganslandt, O. Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS ONE 2012, 7, e44885. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, H.; Zhao, M.; Hayashi, K.; Urata, Y.; Tanaka, N.; Fujiwara, T.; Penman, S.; Hoffman, R.M. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 14514–14517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, S.; Miwa, S.; Fujiwara, T.; Hoffman, R.M.; Kishimoto, H.; Toneri, M.; Hiroshima, Y.; Yamamoto, M.; Bouvet, M.; Urata, Y.; et al. Experimental curative fluorescence-guided surgery of highly invasive glioblastoma multiforme selectively labeled with a killer-reporter adenovirus. Mol. Ther. 2015, 23, 1182–1188. [Google Scholar] [CrossRef] [Green Version]
- Pisters, P.W.; Leung, D.H.; Woodruff, J.; Shi, W.; Brennan, M.F. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J. Clin. Oncol. 1996, 14, 1679–1689. [Google Scholar] [CrossRef]
- Coindre, J.M.; Terrier, P.; Bui, N.B.; Bonichon, F.; Collin, F.; Le Doussal, V.; Mandard, A.M.; Vilain, M.; Jacquemier, J.; Duplay, H.; et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J. Clin. Oncol. 1996, 14, 869–877. [Google Scholar] [CrossRef]
- Eilber, F.C.; Rosen, G.; Nelson, S.D. High-grade extremity soft tissue sarcomas: Factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003, 237, 218–226. [Google Scholar] [CrossRef]
- Weitz, J.; Antonescu, C.R.; Brennan, M.F. Localized extremity soft tissue sarcoma: Improved knowledge with unchanged survival over time. J. Clin. Oncol. 2003, 21, 2719–2725. [Google Scholar] [CrossRef]
- Clark, M.A.; Fisher, C.; Judson, I.; Thomas, J.M. Soft-tissue sarcoma in adults. N. Engl. J. Med. 2005, 353, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Trovik, C.S.; Bauer, H.C.; Alvegard, T.A. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000, 36, 710–716. [Google Scholar] [CrossRef]
- Lewis, J.J.; Leung, D.; Espat, J.; Woodruff, J.M.; Brennan, M.F. Effect of reresection in extremity soft tissue sarcoma. Ann. Surg. 2000, 231, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Miwa, S.; Kishimoto, H.; Uehara, F.; Tazawa, H.; Toneri, M.; Hiroshima, Y.; Yamamoto, M.; Urata, Y.; Kagawa, S.; et al. Targeting tumors with a killer-reporter adenovirus for curative fluorescence-guided surgery of soft-tissue sarcoma. Oncotarget 2015, 6, 13133–13148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002, 20, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Longhi, A.; Versari, M.; Mercuri, M.; Briccoli, A.; Picci, P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Cancer 2006, 106, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Therapeutic targets for bone and soft-tissue sarcomas. Int. J. Mol. Sci. 2019, 20, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, S.; Miwa, S.; Kishimoto, H.; Urata, Y.; Tazawa, H.; Kagawa, S.; Bouvet, M.; Fujiwara, T.; Hoffman, R.M. Eradication of osteosarcoma by fluorescence-guided surgery with tumor labeling by a killer-reporter adenovirus. J. Orthop. Res. 2015, 34, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Miwa, S.; Hiroshima, Y.; Yano, S.; Zhang, Y.; Matsumoto, Y.; Uehara, F.; Yamamoto, M.; Kimura, H.; Hayashi, K.; Bouvet, M.; et al. Fluorescence-guided surgery improves outcome in an orthotopic osteosarcoma nude-mouse model. J. Orthop. Res. 2014, 32, 1596–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasawa, M.; Ishizawa, T.; Mise, Y.; Inoue, Y.; Ito, H.; Takahashi, Y.; Saiura, A. Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg. Endosc. 2017, 31, 5111–5118. [Google Scholar] [CrossRef]
- Syn, N.L.; Kabir, T.; Koh, Y.X.; Tan, H.L.; Wang, L.Z.; Chin, B.Z.; Wee, I.; Teo, J.Y.; Tai, B.C.; Goh, B.K. Resection for colorectal liver metastasis: A meta-analysis of individual patient data from randomized trials and propensity-score matched studies. Ann. Surg. 2020, 272, 253–265. [Google Scholar] [CrossRef]
- Liberale, G.; Bourgeois, P.; Larsimont, D.; Moreau, M.; Donckier, V.; Ishizawa, T. Indocyanine green fluorescence-guided surgery after IV injection in metastatic colorectal cancer: A systemic review. Eur. J. Surg. Oncol. 2017, 43, 1656–1667. [Google Scholar] [CrossRef]
- Giorgio, R.; Antonio, T.; Gianluca, B.; Gian, L.D.; Federica, G.; Francesco, D.M.; Fausto, C.; Raffaele, D.V. Fluorescence guided surgery in liver tumors: Applications and advantages. Acta Biomed. 2018, 89, 135–140. [Google Scholar]
- Yano, S.; Takehara, K.; Hoffman, R.M.; Miwa, S.; Kishimoto, H.; Hiroshima, Y.; Murakami, T.; Urata, Y.; Kagawa, S.; Bouvet, M.; et al. Improved resection and outcome of colon-cancer liver metastasis with fluorescence-guided surgery using in situ gfp labeling with a telomerase-dependent adenovirus in an orthotopic mouse model. PLoS ONE 2016, 11, e0148760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, S.; Zhang, Y.; Miwa, S.; Kishimoto, H.; Urata, Y.; Bouvet, M.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. Precise navigation surgery of tumours in the lung in mouse models enabled by in situ fluorescence labelling with a killer-reporter adenovirus. BMJ Open Respir. Res. 2015, 2, e000096. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, H.; Urata, Y.; Tanaka, N.; Fujiwara, T.; Hoffman, R.M. Selective metastatic tumor labeling with green fluorescent protein and killing by systemic administration of telomerase-dependent adenoviruses. Mol. Cancer Ther. 2009, 8, 3001–3008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstraw, P.; Ball, D.; Jett, J.R.; Le chevalier, T.; Lim, E.; Nicholson, A.G.; Shepherd, F.A. Non-small cell lung cancer. Lancet 2011, 378, 1727–1740. [Google Scholar] [CrossRef]
- Farjah, F.; Backhus, L.M.; Varghese, T.K.; Mulligan, M.S.; Cheng, A.M.; Alfonso-Cristancho, R.; Flum, D.R.; Wood, D.E. Ninety-Day Costs of Video-Assisted Thoracic Surgery Versus Open Lobectomy for Lung Cancer. Ann. Thorac. Surg. 2014, 98, 191–196. [Google Scholar] [CrossRef]
- Hirschburger, M.; Sauer, S.; Schwandner, T.; Schief, W.; Kuchenbuch, T.; Zoerb, C.; Jansen, H.; Grau, V.; Stertmann, W.; Rau, W.S.; et al. Extratumoral Spiral Fixed Wire Marking of Small Pulmonary Nodules for Thoracoscopic Resection. Thorac. Cardiovasc. Surg. 2008, 56, 106–109. [Google Scholar] [CrossRef]
- Eichfeld, U.; Dietrich, A.; Ott, R.; Kloeppel, R. Video-assisted thoracoscopic surgery for pulmonary nodules after comput-ed-tomography guided marker with a spiral wire. Ann Thorac Surg. 2005, 79, 316–317. [Google Scholar] [CrossRef]
- Lizza, N.; Eucher, P.; Haxhe, J.-J.; De Wispelaere, J.-F.; Johnson, P.M.; Delaunois, L. Thoracoscopic resection of pulmonary nodules after computed tomographic–guided coil labeling. Ann. Thorac. Surg. 2001, 71, 986–988. [Google Scholar] [CrossRef]
- Gobardhan, P.D.; Djamin, R.S.; Romme, P.J.H.J.; de Wit, P.E.J.; de Groot, H.G.W.; Adriaensen, T.; Turkenburg, J.L.; Veen, E.J. The use of iodine seed (I-125) as a marker for the localization of lung nodules in minimal invasive pulmonary surgery. Eur J Surg Oncol. 2013, 39, 945–950. [Google Scholar] [CrossRef]
- Okusanya, O.T.; DeJesus, E.M.; Jiang, J.X.; Judy, R.P.; Venegas, O.G.; Deshpande, C.G.; Heitjan, D.F.; Nie, S.; Low, P.S.; Singhal, S. Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J. Thorac. Cardiovasc. Surg. 2015, 150, 28–35.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Predina, J.D.; Newton, A.D.; Keating, J.; Barbosa, E.M., Jr.; Okusanya, O.; Xia, L.; Dunbar, A.; Connolly, C.; Baldassari, M.P.; Mizelle, J.; et al. Intraoperative molecular imaging combined with positron emission tomography improved surgical management of peripheral malignant pulmonary nodules. Ann. Surg. 2017, 266, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Predina, J.D.; Newton, A.D.; Xia, L.; Corbett, C.; Connolly, C.; Shin, M.; Sulyok, L.F.; Litzky, L.; Deshpande, C.; Nie, S.; et al. An open label trial of folate receptor-targeted intraoperative molecular imaging to localize pulmonary squamous cell carcinomas. Oncotarget 2018, 9, 13517–13529. [Google Scholar] [CrossRef]
- Predina, J.D.; Newton, A.D.; Low, P.S.; Singhal, S.; Keating, J.; Dunbar, A.; Connolly, C.; Baldassari, M.; Mizelle, J.; Xia, L.; et al. A phase I clinical trial of targeted intraoperative molecular imaging for pulmonary adenocarcinomas. Ann. Thorac. Surg. 2018, 105, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, S.; Takehara, K.; Kishimoto, H.; Tazawa, H.; Urata, Y.; Kagawa, S.; Bouvet, M.; Fujiwara, T.; Hoffman, R.M. Tumor-targeting adenovirus OBP-401 inhibits primary and metastatic tumor growth of triple-negative breast cancer in orthotopic nude-mouse models. Oncotarget 2016, 7, 85273–85282. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Takehara, K.; Kishimoto, H.; Tazawa, H.; Urata, Y.; Kagawa, S.; Bouvet, M.; Fujiwara, T.; Hoffman, R.M. OBP-401-GFP telomerase-dependent adenovirus illuminates and kills high-metastatic more effectively than low-metastatic triple-negative breast cancer in vitro. Cancer Gene Ther. 2017, 24, 45–47. [Google Scholar] [CrossRef]
- Yano, S.; Takehara, K.; Kishimoto, H.; Urata, Y.; Kagawa, S.; Bouvet, M.; Fujiwara, T.; Hoffman, R.M. Adenoviral targeting of malignant melanoma for fluorescence-guided surgery prevents recurrence in orthotopic nude-mouse models. Oncotarget 2015, 7, 18558–18572. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinska, E.; Byrne, A.T.; Caldas, C.; Clarle, R.B.; de Jong, S.; Jonkers, J.; Maelandsmo, G.M.; et al. For the EurOPDX Consortium Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, R.M. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer 2015, 15, 451–452. [Google Scholar] [CrossRef]
- Fu, X.Y.; Besterman, J.M.; Monosov, A.; Hoffman, R.M. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc. Natl. Acad. Sci. USA 1991, 88, 9345–9349. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Guadagni, F.; Hoffman, R.M. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc. Natl. Acad. Sci. USA 1992, 89, 5645–5649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsohima, Y.; Zhang, Y.; Zhang, N.; Maawy, A.; Sumiyuki, M.; Yamamoto, M.; Uehara, F.; Miwa, S.; Yano, S.; Murakami, T.; et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS ONE 2015, 10, e0117417. [Google Scholar]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.M.; Hong, S.; Kothapalli, S.R.; Hasan, A.; Ertsey, R.; et al. Inraoperative pancreatic cancer detection using tumor-specific multimodality molecular imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Hiroshima, Y.; Maawy, A.; Kishimoto, H.; Suetsugu, A.; Miwa, S.; Toneri, M.; Yamamoto, M.; Katz, M.H.; Fleming, J.B.; et al. Color-coding cancer and stromal cells with genetic reporters in a patient-derived orthotopic xenograft (PDOX) model of pancreatic cancer enhances fluorescence-guided surgery. Cancer Gene Ther. 2015, 22, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Bulina, M.E.; Chudakov, D.M.; Britanova, O.V.; Yanushevich, Y.G.; Staroverov, D.B.; Chepurnykh, T.V.; Merzlyak, E.M.; Shkrob, M.A.; Lukyanov, S.; Lukyanov, K.A. A genetically encoded photosensitizer. Nat. Biotechnol. 2006, 1, 95–99. [Google Scholar] [CrossRef]
- Nordgren, M.; Wang, B.; Apanasets, O.; Brees, C.; Van Veldhoven, P.; Fransen, M. Potential limitations in the use of KillerRed for fluorescence microscopy. J. Microsc. 2011, 245, 229–235. [Google Scholar] [CrossRef]
- Serebrovskaya, E.O.; Edelweiss, E.F.; Stremovskiy, O.A.; Lukyanov, K.A.; Chudakov, D.M.; Deyev, S.M. Targeting cancer cells by using an antireceptor antibody-photosensitizer fusion protein. Proc. Natl. Acad. Sci. USA 2009, 106, 9221–9225. [Google Scholar] [CrossRef] [Green Version]
- Takehara, K.; Yano, S.; Tazawa, H.; Kishimoto, H.; Narii, N.; Mizuguchi, H.; Urata, Y.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. Eradication of melanoma in vitro and in vivo via targeting with a Killer-Red-containing telomerase-dependent adenovirus. Cell Cycle 2017, 16, 1502–1508. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yano, S.; Tazawa, H.; Kishimoto, H.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. Real-Time Fluorescence Image-Guided Oncolytic Virotherapy for Precise Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 879. https://doi.org/10.3390/ijms22020879
Yano S, Tazawa H, Kishimoto H, Kagawa S, Fujiwara T, Hoffman RM. Real-Time Fluorescence Image-Guided Oncolytic Virotherapy for Precise Cancer Treatment. International Journal of Molecular Sciences. 2021; 22(2):879. https://doi.org/10.3390/ijms22020879
Chicago/Turabian StyleYano, Shuya, Hiroshi Tazawa, Hiroyuki Kishimoto, Shunsuke Kagawa, Toshiyoshi Fujiwara, and Robert M. Hoffman. 2021. "Real-Time Fluorescence Image-Guided Oncolytic Virotherapy for Precise Cancer Treatment" International Journal of Molecular Sciences 22, no. 2: 879. https://doi.org/10.3390/ijms22020879




