Apolipoprotein CIII Is an Important Piece in the Type-1 Diabetes Jigsaw Puzzle
Abstract
1. Introduction
2. Apolipoprotein CIII, Structure and Function
3. ApoCIII Gene Regulation
3.1. Insulin
3.2. Glucose
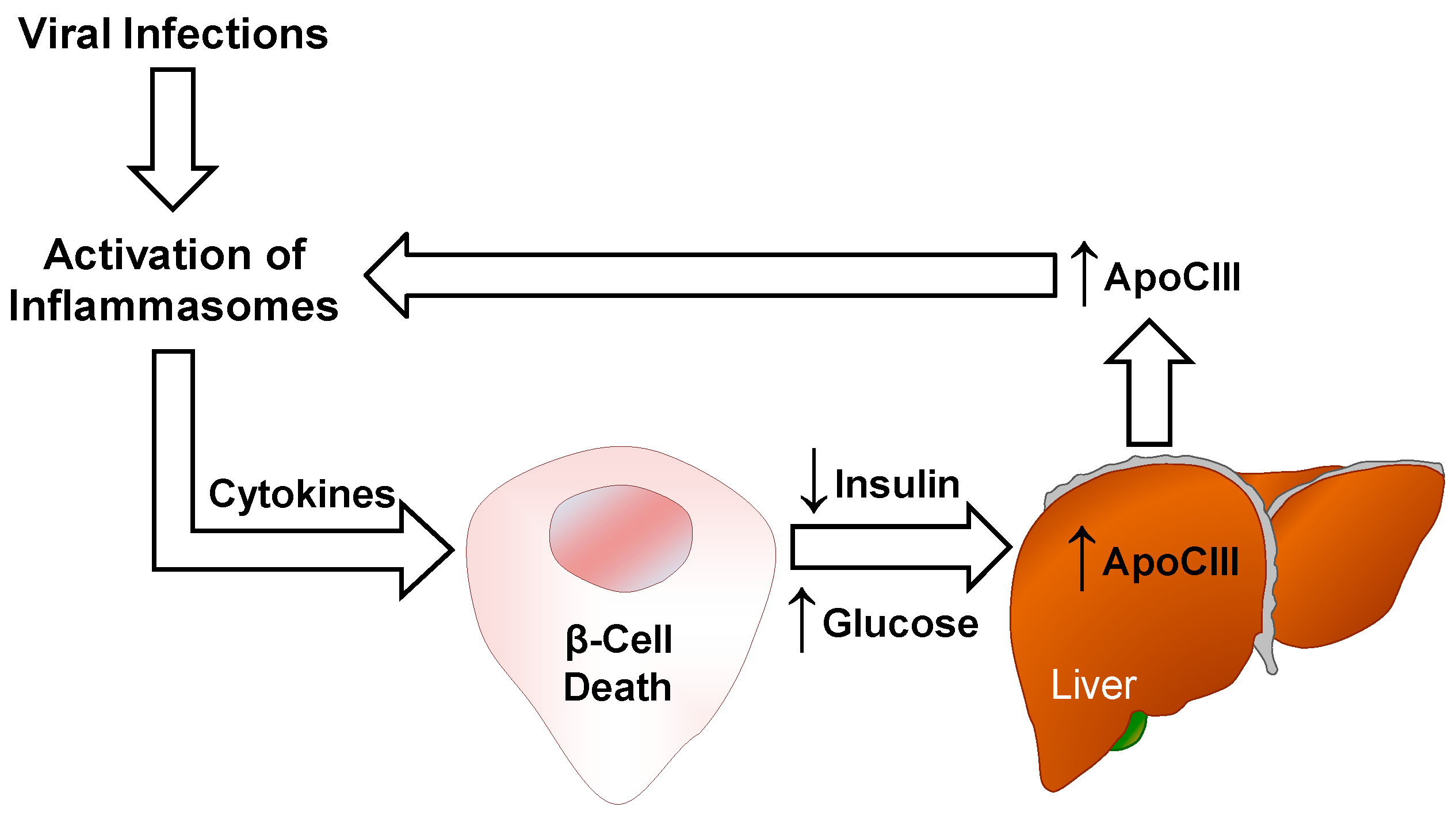
3.3. Cytokines
4. ApoCIII and Inflammation
4.1. Vascular Effects
4.2. Inflammasomes
5. ApoCIII and Autoimmunity
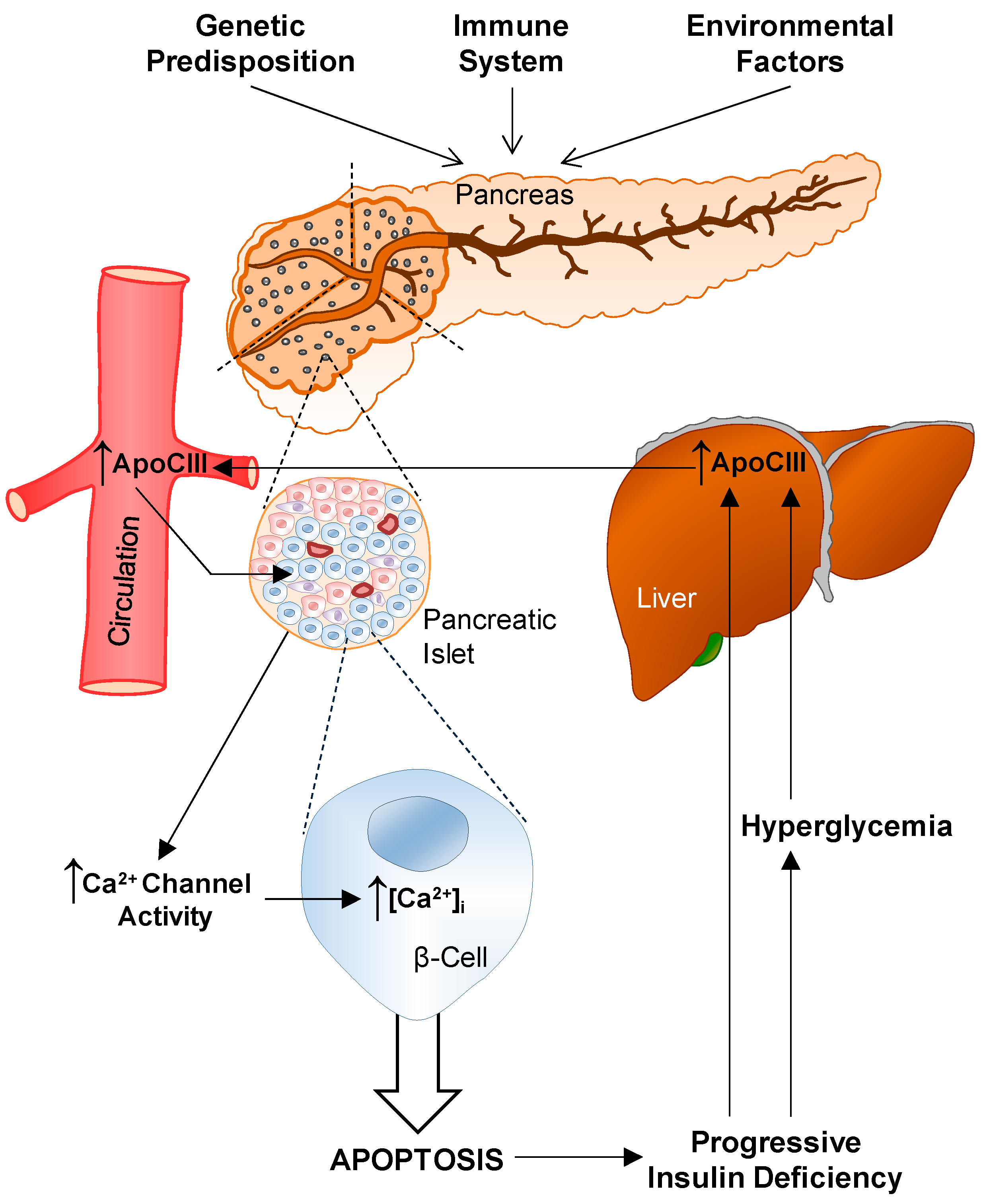
6. T1D and ApoCIII
6.1. Serum
6.2. Voltage-Gated L-Type Ca2+ Channels
6.3. Cytokines
6.4. In Vivo Effects
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| apoCIII | Apolipoprotein CIII |
| BB | Biobreeding rat |
| C/EBPδ/NF-IL6-β | CAAT enhancer-binding protein δ/nuclear factor/IL6- β |
| CVD | Cardiovascular disease |
| CaV | Voltage-gated Ca2+-channels |
| ChREBP | Carbohydrate response element–binding protein |
| DAMPs | Danger-associated molecular patterns |
| DPBB | Diabetes-prone BB rat |
| EC | Endothelial cells |
| ERK1/2 | Extracellular signal-regulated kinases 1/2 |
| Foxo1 | Forkhead box O1 |
| G-banding | Giemsa-banding |
| HDL | High-density lipoprotein |
| HNF-4α | Hepatocyte nuclear receptor-4α |
| HLA | Human leukocyte antigens |
| ICAM-1 | Intracellular adhesion molecule |
| IL | Interleukin |
| IDL | Intermediate-density lipoproteins |
| IRE | Insulin/phorbol ester responsive element |
| IκΒ | Inhibitor of κΒ |
| LDL | Low-density lipoprotein |
| LDLR | LDL receptor |
| LPL | Lipoprotein lipase |
| LRP1 | LDL-related protein 1 |
| LXR | Liver X receptors |
| MAPK | Mitogen activated protein kinase |
| NF-κB | Nuclear factor kappa-B |
| NLRP3 | Nod-like pyrin domain-containing 3 |
| PAMPs | Pathogen-associated molecular patterns |
| PK | Pyruvate kinase |
| PKA | Protein kinase A |
| PKCα | Protein kinase C α |
| SCIMP | SLP adaptor and CSK-interacting membrane protein |
| SLE | Systemic lupus erythematosus |
| SR-BI | Scavenger-receptor class BI |
| Src | Proto-oncogene tyrosine-protein kinase Src |
| T1D | Type-1 diabetes |
| T2D | Type-2 diabetes |
| TEDDY | The Environmental Determinants of Diabetes in the Young |
| Tgs | Triglycerides |
| TNF-α | Tumor necrosis factor α |
| TRL | Triglyceride-rich lipoprotein |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VLDL | Very low-density lipoproteins |
| [Ca2+]i | Cytoplasmic-free Ca2+ concentration |
References
- Barrett, J.C.; Clayton, D.G.; Concannon, P.; Akolkar, B.; Cooper, J.D.; Erlich, H.A.; Julier, C.; Morahan, G.; Nerup, J.; Nierras, C.; et al. Type 1 Diabetes Genetics Consortium. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009, 41, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Herold, K.; Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010, 464, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.C.; Porto, L.C.; Oliveira, R.V.; Secco, D.; Hanhoerderster, L.; Pizarro, M.H.; Barros, B.S.V.; Mello, L.G.N.; Muniz, L.H.; Silva, D.A.; et al. HLA class II genotyping of admixed Brazilian patients with type 1 diabetes according to self-reported color/race in a nationwide study. Sci. Rep. 2020, 10, 6628. [Google Scholar] [CrossRef] [PubMed]
- Dayan, C.M.; Korah, M.; Tatovic, D.; Bundy, B.N.; Herold, K.C. Changing the landscape for type 1 diabetes: The first step to prevention. Lancet 2019, 394, 1286–1296. [Google Scholar] [CrossRef]
- Steck, A.K.; Vehik, K.; Bonifacio, E.; Lernmark, A.; Ziegler, A.G.; Hagopian, W.A.; She, J.; Simell, O.; Akolkar, B.; Krischer, J.; et al. TEDDY Study Group. Predictors of Progression from the Appearance of Islet Autoantibodies to Early Childhood Diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015, 38, 808–813. [Google Scholar] [CrossRef]
- Rewers, M.; Ludvigsson, J. Environmental risk factors for type 1 diabetes. Lancet 2016, 387, 2340–2348. [Google Scholar] [CrossRef]
- Brown, W.V.; Levy, R.I.; Fredrickson, D.S. Studies of the proteins in human plasma very low density lipoproteins. J. Biol. Chem. 1969, 244, 5687–5694. [Google Scholar] [CrossRef]
- Brewer, H.B., Jr.; Shulman, R.; Herbert, P.; Ronan, R.; Wehrly, K. The complete amino acid sequence of alanine apolipoprotein (apoC-3), and apolipoprotein from human plasma very low density lipoproteins. J. Biol. Chem. 1974, 249, 4975–4984. [Google Scholar] [CrossRef]
- Zannis, V.I.; Cole, F.S.; Jackson, C.L.; Kurnit, D.M.; Karathanasis, S.K. Distribution of apolipoprotein A-I, C-II, C-III, and E mRNA in fetal human tissues. Time-dependent induction of apolipoprotein E mRNA by cultures of human monocyte-macrophages. Biochemistry 1985, 24, 4450–4455. [Google Scholar] [CrossRef]
- Reue, K.; Leff, T.; Breslow, J.L. Human apolipoprotein CIII gene expression is regulated by positive and negative cis-acting elements and tissue-specific protein factors. J. Biol. Chem. 1988, 263, 6857–6864. [Google Scholar] [CrossRef]
- Ogami, K.; Hadzopoulou-Cladaras, M.; Cladaras, C.; Zannis, V.I. Promoter elements and factors required for hepatic and intestinal transcription of the human ApoCIII gene. J. Biol. Chem. 1990, 265, 9808–9815. [Google Scholar] [CrossRef]
- West, G.; Rodia, C.; Li, D.; Johnson, Z.; Dong, H.; Kohan, A.B. Key differences between apoC-III regulation and expression in intestine and liver. Biochem. Biophys. Res. Commun. 2017, 491, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Vaith, P.; Assmann, G.; Uhlenbruck, G. Characterization of the oligosaccharide side chain of apolipoprotein C-III from human plasma very low density lipoproteins. Biochim. Biophys. Acta 1978, 541, 234–240. [Google Scholar] [CrossRef]
- Ito, Y.; Breslow, J.L.; Chait, B.T. Apolipoprotein C-III0 lacks carbohydrate residues: Use of mass spectrometry to study apolipoprotein structure. J. Lipid. Res. 1989, 30, 1781–1787. [Google Scholar] [CrossRef]
- Kashyap, M.L.; Srivastava, L.S.; Hynd, B.A.; Gartside, P.S.; Perisutti, G. Quantitation of human apolipoprotein C-III and its subspecie by radioimmunoassay and analytical isoelectric focusing: Abnormal plasma triglyceride-rich lipoprotein apolipoprotein C-III subspecie concentrations in hypertriglyceridemia. J. Lipid. Res. 1981, 22, 800–810. [Google Scholar] [CrossRef]
- Roghani, A.; Zannis, V.I. Mutagenesis of the glycosylation site of human ApoCIII. O-linked glycosylation is not required for ApoCIII secretion and lipid binding. J. Biol. Chem. 1988, 263, 17925–17932. [Google Scholar] [CrossRef]
- Mauger, J.F.; Couture, P.; Bergeron, N.; Lamarche, B. Apolipoprotein C-III isoforms: Kinetics and relative implication in lipid metabolism. J. Lipid. Res. 2006, 47, 1212–1218. [Google Scholar] [CrossRef]
- Kegulian, N.C.; Ramms, B.; Horton, S.; Trenchevska, O.; Nedelkov, D.; Graham, M.J.; Lee, R.G.; Esko, J.D.; Yassine, H.N.; Gordts, P.L.S.M. ApoC-III Glycoforms Are Differentially Cleared by Hepatic TRL (Triglyceride-Rich Lipoprotein) Receptors. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2145–2156. [Google Scholar] [CrossRef]
- Olivieri, O.; Chiariello, C.; Martinelli, N.; Castagna, A.; Speziali, G.; Girelli, D.; Pizzolo, F.; Bassi, A.; Cecconi, D.; Robotti, E.; et al. Sialylated isoforms of apolipoprotein C-III and plasma lipids in subjects with coronary artery disease. Clin. Chem. Lab. Med. 2018, 56, 1542–1550. [Google Scholar] [CrossRef]
- Juntti-Berggren, L.; Refai, E.; Appelskog, I.; Andersson, M.; Imreh, G.; Dekki, N.; Uhles, S.; Yu, L.; Griffiths, W.J.; Zaitsev, S.; et al. Apolipoprotein CIII promotes Ca2+-dependent-cell death in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2004, 101, 10090–10094. [Google Scholar] [CrossRef]
- Alaupovic, P. Significance of apolipoproteins for structure, function, and classification of plasma lipoproteins. Methods Enzymol. 1996, 263, 32–60. [Google Scholar] [PubMed]
- Khoo, C.; Campos, H.; Judge, H.; Sacks, F.M. Effects of estrogenic oral contraceptives on the lipoprotein B particle system defined by apolipoproteins E and C-III content. J. Lipid. Res. 1999, 40, 202–212. [Google Scholar] [PubMed]
- Jong, M.C.; Hofker, M.H.; Havekes, L.M. Role of ApoCs in lipoprotein metabolism: Functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Campos, H.; Perlov, D.; Khoo, C.; Sacks, F.M. Distinct patterns of lipoproteins with apoB defined by presence of apoE or apoC-III in hypercholesterolemia and hypertriglyceridemia. J. Lipid. Res. 2001, 42, 1239–1249. [Google Scholar] [PubMed]
- Taskinen, M.R.; Borén, J. Why Is Apolipoprotein CIII Emerging as a Novel Therapeutic Target to Reduce the Burden of Cardiovascular Disease? Curr. Atheroscler. Rep. 2016, 18, 59. [Google Scholar] [CrossRef]
- Zvintzou, E.; Lhomme, M.; Chasapi, S.; Filou, S.; Theodoropoulos, V.; Xapapadaki, E.; Kontush, A.; Spyroulias, G.; Tellis, C.C.; Tselepis, A.D.; et al. Pleiotropic effects of apolipoprotein C3 on HDL functionality and adipose tissue metabolic activity. J. Lipid. Res. 2017, 58, 1869–1883. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Le, N.A.; Goldberg, I.J.; Gibson, J.C.; Rubinstein, A.; Wang-Iverson, P.; Norum, R.; Brown, W.V. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J. Clin. Investig. 1986, 78, 1287–1295. [Google Scholar] [CrossRef]
- Ebara, T.; Ramakrishnan, R.; Steiner, G.; Shachter, N.S. Chylomicronemia due to apolipoprotein CIII overexpression in apolipoprotein E-null mice. Apolipoprotein CIII-induced hypertriglyceridemia is not mediated by effects on apolipoprotein E. J. Clin. Investig. 1997, 99, 2672–2681. [Google Scholar] [CrossRef]
- Lambert, D.A.; Smith, L.C.; Pownall, H.; Sparrow, J.T.; Nicolas, J.P.; Gotto, A.M., Jr. Hydrolysis of phospholipids by purified milk lipoprotein lipase. Effect of apoprotein CII, CIII, A and E, and synthetic fragments. Clin. Chim. Acta 2000, 291, 19–33. [Google Scholar] [CrossRef]
- Larsson, M.; Vorrsjö, E.; Talmud, P.; Lookene, A.; Olivecrona, G. Apolipoproteins C-I and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J. Biol. Chem. 2013, 288, 33997–34008. [Google Scholar] [CrossRef]
- Sacks, F.M. The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia. Curr. Opin. Lipidol. 2015, 26, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Gordts, P.L.; Nock, R.; Son, N.H.; Ramms, B.; Lew, I.; Gonzales, J.C.; Thacker, B.E.; Basu, D.; Lee, R.G.; Mullick, A.E.; et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J. Clin. Investig. 2016, 126, 2855–2866. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, S.K.; McPherson, J.; Zannis, V.I.; Breslow, J.L. Linkage of human apolipoproteins A-I and C-III genes. Nature 1983, 304, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Bruns, G.A.; Karathanasis, S.K.; Breslow, J.L. Human apolipoprotein A-I--C-III gene complex is located on chromosome 11. Arteriosclerosis 1984, 4, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, S.K. Apolipoprotein multigene family: Tandem organization of human apolipoprotein AI, CIII, and AIV genes. Proc. Natl. Acad. Sci. USA 1985, 82, 6374–6378. [Google Scholar] [CrossRef]
- Kan, H.Y.; Georgopoulos, S.; Zannis, V. A hormone response element in the human apolipoprotein CIII (ApoCIII) enhancer is essential for intestinal expression of the ApoA-I and ApoCIII genes and contributes to the hepatic expression of the two linked genes in transgenic mice. J. Biol. Chem. 2000, 275, 30423–30431. [Google Scholar] [CrossRef]
- Guardiola, M.; Oliva, I.; Guillaumet, A.; Martín-Trujillo, Á.; Rosales, R.; Vallvé, J.C.; Sabench, F.; Del Castillo, D.; Zaina, S.; Monk, D.; et al. Tissue-specific DNA methylation profiles regulate liver-specific expression of the APOA1/C3/A4/A5 cluster and can be manipulated with demethylating agents on intestinal cells. Atherosclerosis 2014, 237, 528–535. [Google Scholar] [CrossRef]
- Altomonte, J.; Cong, L.; Harbaran, S.; Richter, A.; Xu, J.; Meseck, M.; Dong, H.H. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J. Clin. Investig. 2004, 114, 1493–1503. [Google Scholar] [CrossRef]
- Caron, S.; Verrijken, A.; Mertens, I.; Samanez, C.H.; Mautino, G.; Haas, J.T.; Duran-Sandoval, D.; Prawitt, J.; Francque, S.; Vallez, E.; et al. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 513–519. [Google Scholar] [CrossRef]
- Lacorte, J.M.; Beigneux, A.; Parant, M.; Chambaz, J. Repression of apoC-III gene expression by TNFalpha involves C/EBPdelta/NF-IL6beta via an IL-1 independent pathway. FEBS Lett. 1997, 415, 217–220. [Google Scholar] [CrossRef]
- Lacorte, J.M.; Ktistaki, E.; Beigneux, A.; Zannis, V.I.; Chambaz, J.; Talianidis, I. Activation of CAAT enhancer-binding protein delta (C/EBPdelta) by interleukin-1 negatively influences apolipoprotein C-III expression. J. Biol. Chem. 1997, 272, 23578–23584. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Breslow, J.L.; Li, W.; Leff, T. Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: Correlation with changes in plasma triglyceride levels. J. Lipid. Res. 1994, 35, 1918–1924. [Google Scholar] [CrossRef]
- Li, W.W.; Dammerman, M.M.; Smith, J.D.; Metzger, S.; Breslow, J.L.; Leff, T. Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J. Clin. Investig. 1995, 96, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Packard, C.J.; Taskinen, M.R. The Roles of ApoC-III on the Metabolism of Triglyceride-Rich Lipoproteins in Humans. Front. Endocrinol. 2020, 11, 474. [Google Scholar] [CrossRef]
- Nakae, J.; Kitamura, T.; Silver, D.L.; Accili, D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Investig. 2001, 108, 1359–1367. [Google Scholar] [CrossRef]
- Nakae, J.; Biggs, W.H., 3rd; Kitamura, T.; Cavenee, W.K.; Wright, C.V.; Arden, K.C.; Accili, D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 2002, 32, 245–253. [Google Scholar] [CrossRef]
- Shih, H.M.; Liu, Z.; Towle, H.C. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J. Biol. Chem. 1995, 270, 21991–21997. [Google Scholar] [CrossRef]
- Yamashita, H.; Takenoshita, M.; Sakurai, M.; Bruick, R.K.; Henzel, W.J.; Shillinglaw, W.; Arnot, D.; Uyeda, K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. USA 2001, 98, 9116–9121. [Google Scholar] [CrossRef]
- Iizuka, K.; Bruick, R.K.; Liang, G.; Horton, J.D.; Uyeda, K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA 2004, 101, 7281–7286. [Google Scholar] [CrossRef]
- Towle, H.C. Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol. Metab. 2005, 16, 489–494. [Google Scholar] [CrossRef]
- Adamson, A.W.; Suchankova, G.; Rufo, C.; Nakamura, M.T.; Teran-Garcia, M.; Clarke, S.D.; Gettys, T.W. Hepatocyte nuclear factor-4alpha contributes to carbohydrate-induced transcriptional activation of hepatic fatty acid synthase. Biochem. J. 2006, 399, 285–295. [Google Scholar] [CrossRef]
- Cha, J.Y.; Repa, J.J. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J. Biol. Chem. 2007, 282, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Kardassis, D.; Tzameli, I.; Hadzopoulou-Cladaras, M.; Talianidis, I.; Zannis, V. Distal apolipoprotein C-III regulatory elements F to J act as a general modular enhancer for proximal promoters that contain hormone response elements. Synergism between hepatic nuclear factor-4 molecules bound to the proximal promoter and distal enhancer sites. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 222–232. [Google Scholar] [PubMed]
- Gruber, P.J.; Torres-Rosado, A.; Wolak, M.L.; Leff, T. Apo CIII gene transcription is regulated by a cytokine inducible NF-kappa B element. Nucleic. Acids. Res. 1994, 22, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Aikawa, M.; Alcaide, P.; Luscinskas, F.W.; Libby, P.; Sacks, F.M. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation 2006, 114, 681–687. [Google Scholar] [CrossRef]
- Kawakami, A.; Aikawa, M.; Libby, P.; Alcaide, P.; Luscinskas, F.W.; Sacks, F.M. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation 2006, 113, 691–700. [Google Scholar] [CrossRef]
- Lee, S.J.; Campos, H.; Moye, L.A.; Sacks, F.M. LDL containing apolipoprotein CIII is an independent risk factor for coronary events in diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 853–858. [Google Scholar] [CrossRef]
- Katzmann, J.L.; Werner, C.M.; Stojakovic, T.; März, W.; Scharnagl, H.; Laufs, U. Apolipoprotein CIII predicts cardiovascular events in patients with coronary artery disease: A prospective observational study. Lipids Health Dis. 2020, 19, 116. [Google Scholar] [CrossRef]
- Rocha, N.A.; East, C.; Zhang, J.; McCullough, P.A. ApoCIII as a Cardiovascular Risk Factor and Modulation by the Novel Lipid-Lowering Agent Volanesorsen. Curr. Atheroscler. Rep. 2017, 19, 62. [Google Scholar] [CrossRef]
- Kawakami, A.; Aikawa, M.; Nitta, N.; Yoshida, M.; Libby, P.; Sacks, F.M. Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase C alpha-mediated nuclear factor-kappaB activation. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 219–225. [Google Scholar] [CrossRef]
- Hiukka, A.; Ståhlman, M.; Pettersson, C.; Levin, M.; Adiels, M.; Teneberg, S.; Leinonen, E.S.; Hultén, L.M.; Wiklund, O.; Oresic, M.; et al. ApoCIII-enriched LDL in type 2 diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes 2009, 58, 2018–2126. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Reiser, J.; Jankowski, V.; Alansary, D.; Hahm, E.; Triem, S.; Klug, M.; Schunk, S.J.; Schmit, D.; Kramann, R.; et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat. Immunol. 2020, 21, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Papadopoulou-Marketou, N.; Chrousos, G.P.; Kanaka-Gantenbein, C. On type 1 diabetes mellitus pathogenesis. Endocr. Connect. 2018, 7, R38–R46. [Google Scholar] [CrossRef]
- Morgan, P.E.; Sturgess, A.D.; Hennessy, A.; Davies, M.J. Serum protein oxidation and apolipoprotein CIII levels in people with systemic lupus erythematosus with and without nephritis. Free Radic. Res. 2007, 41, 1301–1312. [Google Scholar] [CrossRef]
- Araújo, D.M.; Rodrigues, C.E.M.; Gonçalves, N.G.G.; Rabelo-Júnior, C.N.; Lobo, M.D.P.; Moreira, R.A.; Monteiro-Moreira, A.C.O. Proteins Involved in the Induction of Procoagulant Activity and Autoimmune Response in Patients with Primary Antiphospholipid Syndrome. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620905338. [Google Scholar] [CrossRef]
- Størling, J.; Juntti-Berggren, L.; Olivecrona, G.; Prause, M.C.; Berggren, P.O.; Mandrup-Poulsen, T. Apolipoprotein CIII reduces proinflammatory cytokine-induced apoptosis in rat pancreatic islets via the Akt prosurvival pathway. Endocrinology 2011, 152, 3040–3048. [Google Scholar] [CrossRef]
- Frostegård, J. SLE, atherosclerosis and cardiovascular disease. J. Intern. Med. 2005, 257, 485–495. [Google Scholar] [CrossRef]
- Esdaile, J.M.; Abrahamowicz, M.; Grodzicky, T.; Li, Y.; Panaritis, C.; du Berger, R.; Côte, R.; Grover, S.A.; Fortin, P.R.; Clarke, A.E.; et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001, 44, 2331–2337. [Google Scholar] [CrossRef]
- Briones, E.R.; Mao, S.J.; Palumbo, P.J.; O’Fallon, W.M.; Chenoweth, W.; Kottke, B.A. Analysis of plasma lipids and apolipoproteins in insulin-dependent and noninsulin-dependent diabetics. Metabolism 1984, 33, 42–49. [Google Scholar] [CrossRef]
- Joven, J.; Vilella, E.; Costa, B.; Turner, P.R.; Richart, C.; Masana, L. Concentrations of lipids and apolipoproteins in patients with clinically well-controlled insulin-dependent and non-insulin-dependent diabetes. Clin. Chem. 1989, 35, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.W.; Laker, M.F.; Alberti, K.G. The contribution of lipids to coronary heart disease in diabetes mellitus. J. Intern. Med. Suppl. 1994, 736, 41–46. [Google Scholar]
- Bren, N.D.; Rastogi, A.; Kottke, B.A. Quantification of human plasma apolipoproteins C-I, C-II, and C-III by radioimmunoassays. Mayo Clin. Proc. 1993, 68, 657–664. [Google Scholar] [CrossRef]
- Nestel, P.J.; Fidge, N.H. Apoprotein C metabolism in man. Adv. Lipid Res. 1982, 19, 55–83. [Google Scholar] [PubMed]
- Blackett, P.; Sarale, D.C.; Fesmire, J.; Harmon, J.; Weech, P.; Alaupovic, P. Plasma apolipoprotein C-III levels in children with type I diabetes. South Med. J. 1988, 81, 469–473. [Google Scholar] [CrossRef]
- Al Muhtaseb, N.; al Yousuf, A.; Bajaj, J.S. Apolipoprotein A-I, A-II, B, C-II, and C-III in children with insulin-dependent diabetes mellitus. Pediatrics 1992, 89, 936–941. [Google Scholar]
- Manzato, E.; Zambon, A.; Lapolla, A.; Zambon, S.; Braghetto, L.; Crepaldi, G.; Fedele, D. Lipoprotein abnormalities in well-treated type II diabetic patients. Diabetes Care 1993, 16, 469–475. [Google Scholar] [CrossRef]
- Reverter, J.L.; Sentí, M.; Rubiés-Prat, J.; Lucas, A.; Salinas, I.; Pizarro, E.; Pedro-Botet, J.; Sanmartí, A. Lipoprotein composition in the insulin-deficient non-acidotic phase of type I diabetic patients and early evolution after the start of insulin therapy. Clin. Chim. Acta 1993, 223, 113–120. [Google Scholar] [CrossRef]
- Kanter, J.E.; Shao, B.; Kramer, F.; Barnhart, S.; Shimizu-Albergine, M.; Vaisar, T.; Graham, M.J.; Crooke, R.M.; Manuel, C.R.; Haeusler, R.A.; et al. Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes. J. Clin. Investig. 2019, 129, 4165–4179. [Google Scholar] [CrossRef]
- Dekki, N.; Nilsson, R.; Norgren, S.; Rössner, S.M.; Appelskog, I.; Marcus, C.; Simell, O.; Pugliese, A.; Alejandro, R.; Ricordi, C.; et al. Type 1 diabetic serum interferes with pancreatic beta-cell Ca2+-handling. Biosci. Rep. 2007, 27, 321–326. [Google Scholar] [CrossRef]
- Juntti-Berggren, L.; Larsson, O.; Rorsman, P.; Ammälä, C.; Bokvist, K.; Wåhlander, K.; Nicotera, P.; Dypbukt, J.; Orrenius, S.; Hallberg, A.; et al. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science 1993, 261, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.; Shi, Y.; Yang, G.; Li, Y.; Yu, J.; Berggren, P.-O. Ionic mechanisms in pancreatic β cell signaling. Cell Mol. Life Sci. 2014, 71, 4149–4177. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shi, Y.; Yu, J.; Li, Y.; Yu, L.; Welling, A.; Hofmann, F.; Striessnig, J.; Juntti-Berggren, L.; Berggren, P.O.; et al. CaV1.2 and CaV1.3 channel hyperactivation in mouse islet β cells exposed to type 1 diabetic serum. Cell Mol. Life Sci. 2015, 72, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, G.; Yu, J.; Yu, L.; Westenbroek, R.; Catterall, W.A.; Juntti-Berggren, L.; Berggren, P.O.; Yang, S.N. Apolipoprotein CIII hyperactivates β cell CaV1 channels through SR-BI/β1 integrin-dependent coactivation of PKA and Src. Cell Mol. Life Sci. 2014, 71, 1289–1303. [Google Scholar] [CrossRef]
- Refai, E.; Dekki, N.; Yang, S.N.; Imreh, G.; Cabrera, O.; Yu, L.; Yang, G.; Norgren, S.; Rössner, S.M.; Inverardi, L.; et al. Transthyretin constitutes a functional component in pancreatic β-cell stimulus-secretion coupling. Proc. Natl. Acad. Sci. USA 2005, 102, 17020–17025. [Google Scholar] [CrossRef]
- Sol, E.M.; Sundsten, T.; Bergsten, P. Role of MAPK in apolipoprotein CIII-induced apoptosis in INS-1E cells. Lipids Health Dis. 2009, 8, 3. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Jing, G.; Shalev, A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes 2012, 61, 848–856. [Google Scholar] [CrossRef]
- Åvall, K.; Berggren, P.O.; Juntti-Berggren, L. The yin and yang of apolipoprotein CIII. Diabetes Metab. 2018, 44, 303–304. [Google Scholar] [CrossRef]
- Norum, R.A.; Lakier, J.B.; Goldstein, S.; Angel, A.; Goldberg, R.B.; Block, W.D.; Noffze, D.K.; Dolphin, P.J.; Edelglass, J.; Bogorad, D.D.; et al. Familial deficiency of apolipoproteins A-I and C-III and precocious coronary-artery disease. N. Engl. J. Med. 1982, 306, 1513–1519. [Google Scholar] [CrossRef]
- Duivenvoorden, I.; Teusink, B.; Rensen, P.C.; Romijn, J.A.; Havekes, L.M.; Voshol, P.J. Apolipoprotein C3 deficiency results in diet-induced obesity and aggravated insulin resistance in mice. Diabetes 2005, 54, 664–671. [Google Scholar] [CrossRef]
- Nakhooda, A.F.; Like, A.A.; Chappel, C.I.; Murray, F.T.; Marliss, E.B. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes 1977, 26, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Nakhooda, A.F.; Like, A.A.; Chappel, C.I.; Wei, C.N.; Marliss, E.B. The spontaneously diabetic Wistar rat (the "BB" rat). Studies prior to and during development of the overt syndrome. Diabetologia 1978, 14, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, R.; Refai, E.; Höög, A.; Crooke, R.M.; Graham, M.; Olivecrona, G.; Berggren, P.O.; Juntti-Berggren, L. Lowering apolipoprotein CIII delays onset of type 1 diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 10685–10689. [Google Scholar] [CrossRef] [PubMed]
- Hokanson, J.E.; Kinney, G.L.; Cheng, S.; Erlich, H.A.; Kretowski, A.; Rewers, M. Susceptibility to type 1 diabetes is associated with ApoCIII gene haplotypes. Diabetes 2006, 55, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Blankenhorn, D.H.; Alaupovic, P.; Wickham, E.; Chin, H.P.; Azen, S.P. Prediction of angiographic change in native human coronary arteries and aortocoronary bypass grafts. Lipid and nonlipid factors. Circulation 1990, 81, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Gervaise, N.; Garrigue, M.A.; Lasfargues, G.; Lecomte, P. Triglycerides, apo C3 and Lp B:C3 and cardiovascular risk in type II diabetes. Diabetologia 2000, 43, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J.; Azen, S.P.; Alaupovic, P.; Pogoda, J.M.; LaBree, L.; Hemphill, L.C.; Kramsch, D.M.; Blankenhorn, D.H. Triglyceride- and cholesterol-rich lipoproteins have a differential effect on mild/moderate and severe lesion progression as assessed by quantitative coronary angiography in a controlled trial of lovastatin. Circulation 1994, 90, 42–49. [Google Scholar] [CrossRef]
- Koren, E.; Corder, C.; Mueller, G.; Centurion, H.; Hallum, G.; Fesmire, J.; McConathy, W.D.; Alaupovic, P. Triglyceride enriched lipoprotein particles correlate with the severity of coronary artery disease. Atherosclerosis 1996, 122, 105–115. [Google Scholar] [CrossRef]
- Krauss, R.M.; Kesäniemi, Y.A. Cardiovascular disease and hyperlipidaemia. Curr. Opin. Lipidol. 1994, 5, 249–251. [Google Scholar] [CrossRef]
- Luc, G.; Fievet, C.; Arveiler, D.; Evans, A.E.; Bard, J.M.; Cambien, F.; Fruchart, J.C.; Ducimetiere, P. Apolipoproteins C-III and E in apoB- and non-apoB-containing lipoproteins in two populations at contrasting risk for myocardial infarction: The ECTIM study. Etude Cas Témoins sur ’Infarctus du Myocarde. J. Lipid. Res. 1996, 37, 508–517. [Google Scholar] [CrossRef]
- Sacks, F.M.; Alaupovic, P.; Moye, L.A.; Cole, T.G.; Sussex, B.; Stampfer, M.J.; Pfeffer, M.A.; Braunwald, E. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation 2000, 102, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.L.; McHenry, M.B.; Lok, K.H.; Hunter, S.J.; Le, N.A.; Jenkins, A.J.; Zheng, D.; Semler, A.J.; Brown, W.V.; DCCT/EDIC Research Group; et al. Apolipoprotein C-III protein concentrations and gene polymorphisms in type 1 diabetes: Associations with lipoprotein subclasses. Metabolism 2004, 53, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.L.; McHenry, M.B.; Lok, K.H.; Hunter, S.J.; Le, N.A.; Jenkins, A.J.; Zheng, D.; Semler, A.; Page, G.; Brown, W.V.; et al. DCCT/EDIC Research Group. Apolipoprotein C-III protein concentrations and gene polymorphisms in Type 1 diabetes: Associations with microvascular disease complications in the DCCT/EDIC cohort. J. Diabetes Complicat. 2005, 19, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Jenkins, A.J.; Stoner, J.A.; Zhang, Y.; Klein, R.L.; Lopes-Virella, M.F.; Garvey, W.T.; Schade, D.S.; Wood, J.; Alaupovic, P.; et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Apolipoprotein-defined lipoprotein subclasses, serum apolipoproteins, and carotid intima-media thickness in T1D. J. Lipid Res. 2018, 595, 872–883. [Google Scholar] [CrossRef]
- Aroner, S.A.; Koch, M.; Mukamal, K.J.; Furtado, J.D.; Stein, J.H.; Tattersall, M.C.; McClelland, R.L.; Jensen, M.K. High-Density Lipoprotein Subspecies Defined by Apolipoprotein C-III and Subclinical Atherosclerosis Measures: MESA (The Multi-Ethnic Study of Atherosclerosis). J. Am. Heart Assoc. 2018, 7, e007824. [Google Scholar] [CrossRef]
- Aroner, S.A.; Furtado, J.D.; Sacks, F.M.; Tsai, M.Y.; Mukamal, K.J.; McClelland, R.L.; Jensen, M.K. Apolipoprotein C-III and its defined lipoprotein subspecies in relation to incident diabetes: The Multi-Ethnic Study of Atherosclerosis. Diabetologia 2019, 62, 981–992. [Google Scholar] [CrossRef]
- Atzmon, G.; Rincon, M.; Schechter, C.B.; Shuldiner, A.R.; Lipton, R.B.; Bergman, A.; Barzilai, N. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006, 4, e113. [Google Scholar] [CrossRef]
- Pollin, T.I.; Damcott, C.M.; Shen, H.; Ott, S.H.; Shelton, J.; Horenstein, R.B.; Post, W.; McLenithan, J.C.; Bielak, L.F.; Peyser, P.A.; et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 2008, 322, 1702–1705. [Google Scholar] [CrossRef]
- TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute; Crosby, J.; Peloso, G.M.; Auer, P.L.; Crosslin, D.R.; Stitziel, N.O.; Lange, L.A.; Lu, Y.; Tang, Z.Z.; Zhang, H.; et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 2014, 371, 22–31. [Google Scholar]
- Jørgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 2014, 371, 32–41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valladolid-Acebes, I.; Berggren, P.-O.; Juntti-Berggren, L. Apolipoprotein CIII Is an Important Piece in the Type-1 Diabetes Jigsaw Puzzle. Int. J. Mol. Sci. 2021, 22, 932. https://doi.org/10.3390/ijms22020932
Valladolid-Acebes I, Berggren P-O, Juntti-Berggren L. Apolipoprotein CIII Is an Important Piece in the Type-1 Diabetes Jigsaw Puzzle. International Journal of Molecular Sciences. 2021; 22(2):932. https://doi.org/10.3390/ijms22020932
Chicago/Turabian StyleValladolid-Acebes, Ismael, Per-Olof Berggren, and Lisa Juntti-Berggren. 2021. "Apolipoprotein CIII Is an Important Piece in the Type-1 Diabetes Jigsaw Puzzle" International Journal of Molecular Sciences 22, no. 2: 932. https://doi.org/10.3390/ijms22020932
APA StyleValladolid-Acebes, I., Berggren, P.-O., & Juntti-Berggren, L. (2021). Apolipoprotein CIII Is an Important Piece in the Type-1 Diabetes Jigsaw Puzzle. International Journal of Molecular Sciences, 22(2), 932. https://doi.org/10.3390/ijms22020932



