Metabolomic Profiles in Adipocytes Differentiated from Adipose-Derived Stem Cells Following Exercise Training or High-Fat Diet
Abstract
:1. Introduction
2. Results
2.1. Physical Characteristics of Animals
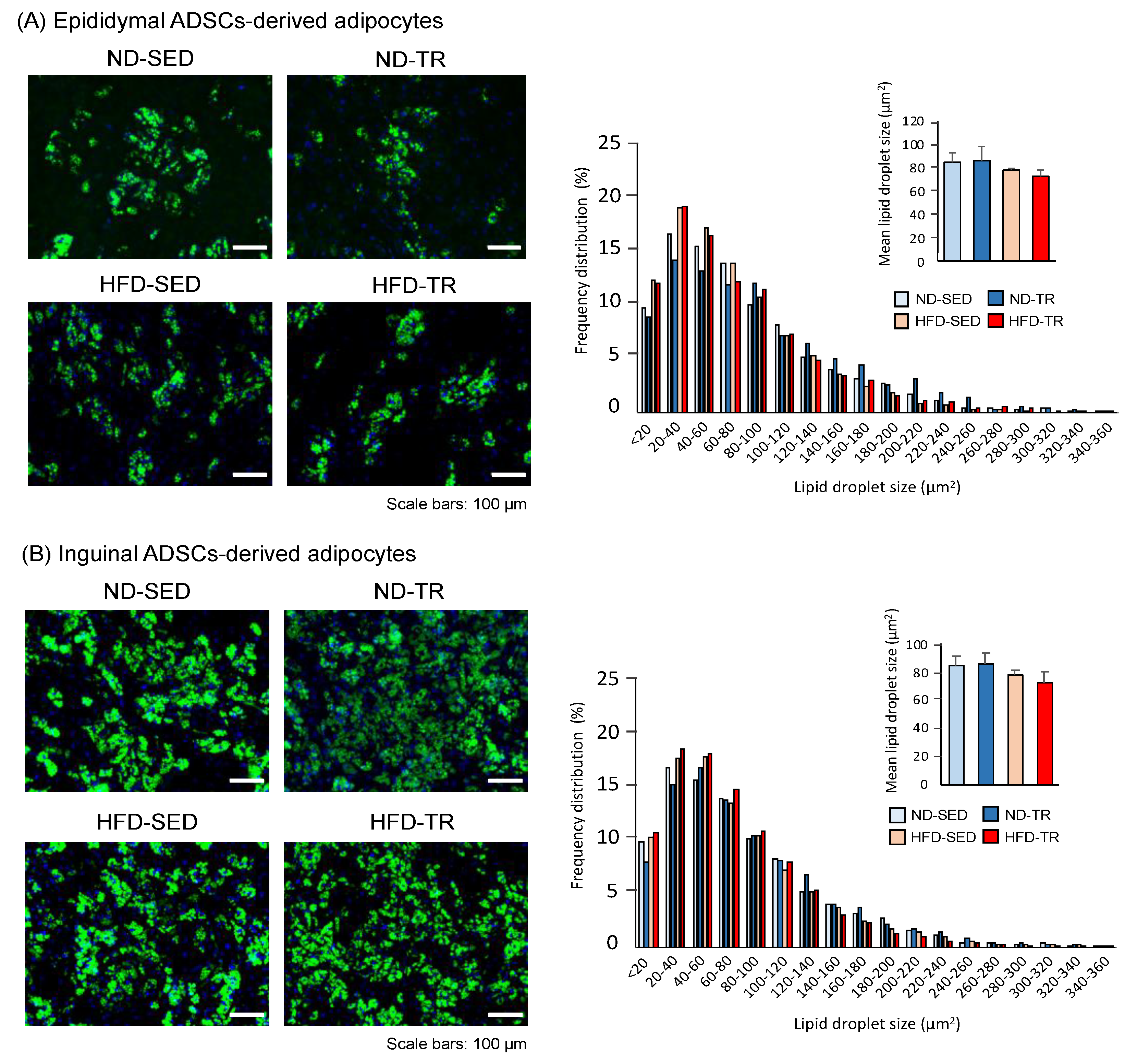
2.2. Cellular Phenotypes of Adipocytes Differentiated from ADSCs
2.3. Metabolomic Analysis
2.4. Effect of TR on the Accumulation of Metabolites in Adipocytes Differentiated from ADSC in ND Rats
2.5. Effect of HFD on Cellular Accumulation Levels of Metabolites in Adipocytes Differentiated from ADSCs
2.6. Effect of TR on Cellular Accumulation Levels of Metabolites in Adipocytes Differentiated from ADSC in HFD Rats
2.7. Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
2.8. Metabolism of the Tricarboxylic Acid (TCA) Cycle-Related Amino Acids
3. Discussion
4. Materials and Methods
4.1. Animal Care and TR Programme
4.2. Isolation and Preparation of SVF Cells from Adipose Tissues
4.3. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
4.4. Differentiation of ADSC into Mature Adipocytes
4.5. Fluorescent Staining and Size of Lipid Droplets
4.6. Metabolomic Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSCs | adipose-derived stem/progenitor cells |
| BCAA | branched-chain amino acid |
| BODIPY | boron dipyrrin |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ES | embryonic stem |
| HFD | high-fat diet |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ND | normal diet |
| PCA | principal component analysis |
| PGC1-α | peroxisome proliferator-activated receptor γ coactivator 1-α |
| PPAR | peroxisome proliferator-activated receptor |
| PRPP | 5-Phosphoribosyl 1-diphosphate |
| SAT | subcutaneous white adipose tissue |
| SED | sedentary control |
| SVF | stromal vascular fraction |
| TR | exercise training |
| mTORC1 | mammalian target of rapamycin complex 1 |
| VAT | visceral adipose tissue |
References
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose tissue-derived stem cells in regenerative medicine. Transfus. Med. Hemother. 2016, 43, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nat. Cell Biol. 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Ritter, A.; Friemel, A.; Roth, S.; Kreis, N.N.; Hoock, C.S.; Safdar, K.B.; Fischer, K.; Möllmann, C.; Solbach, C.; Louwen, F.; et al. Subcutaneous and visceral adipose-derived mesenchymal stem cells: Commonality and diversity. Cells 2019, 8, 1288. [Google Scholar] [CrossRef] [Green Version]
- Ejarque, M.; Ceperuelo-Mallafré, V.; Serena, C.; Maymo-Masip, E.; Duran, X.; Díaz-Ramos, A.; Millan-Scheiding, M.; Núñez-Álvarez, Y.; Núñez-Roa, C.; Gama, P.; et al. Adipose tissue mitochondrial dysfunction in human obesity is linked to a specific DNA methylation signature in adipose-derived stem cells. Int. J. Obes. 2018, 43, 1256–1268. [Google Scholar] [CrossRef] [Green Version]
- Lefevre, C.; Panthu, B.; Naville, D.; Guibert, S.; Pinteur, C.; Elena-Herrmann, B.; Vidal, H.; Rautureau, G.J.P.; Mey, A. Metabolic phenotyping of adipose-derived stem cells reveals a unique signature and intrinsic differences between fat pads. Stem Cells Int. 2019, 2019, 9323864. [Google Scholar] [CrossRef] [PubMed]
- Bódis, K.; Roden, M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur. J. Clin. Investig. 2018, 48, e13017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Kim, J.B. Two faces of white adipose tissue with heterogeneous adipogenic progenitors. Diabetes Metab. J. 2019, 43, 752–762. [Google Scholar] [CrossRef]
- Tran, T.T.; Yamamoto, Y.; Gesta, S.; Kahn, C.R. Beneficial effects of subcutaneous fat transplantation on metab-olism. Cell Metab. 2008, 7, 410–420. [Google Scholar] [CrossRef] [Green Version]
- Hocking, S.L.; Stewart, R.L.; Brandon, A.E.; Suryana, E.; Stuart, E.; Baldwin, E.M.; Kolumam, G.A.; Modrusan, Z.; Junutula, J.R.; Gun-ton, J.E.; et al. Subcutaneous fat transplantation alleviates diet-induced glucose intolerance and inflammation in mice. Diabetologia 2015, 58, 1587–1600. [Google Scholar] [CrossRef]
- Tchkonia, T.; Tchoukalova, Y.D.; Giorgadze, N.; Pirtskhalava, T.; Karagiannides, I.; Forse, R.A.; Koo, A.; Stevenson, M.; Chinnappan, D.; Cartwright, A.; et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am. J. Physiol. Endocrinol. Metabol. 2005, 288, E267–E277. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Giorgadze, N.; Pirtskhalava, T.; Thomou, T.; DePonte, M.; Koo, A.; Forse, R.A.; Chinnappan, D.; Martin-Ruiz, C.; von Zglinicki, T.; et al. Fat depot–specific characteristics are retained in strains de-rived from single human preadipocytes. Diabetes 2006, 55, 2571–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niesler, C.U.; Siddle, K.; Prins, J.B. Human preadipocytes display a depot-specific susceptibility to apoptosis. Diabetes 1998, 47, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Djian, P.; Roncari, A.K.; Hollenberg, C.H. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J. Clin. Investig. 1983, 72, 1200–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.; Montague, C.T.; Prins, J.B.; Holder, J.C.; Smith, S.A.; Sanders, L.; Digby, J.E.; Sewter, C.P.; Lazar, M.A.; Chatterjee, V.K.; et al. Activators of peroxisome proliferator-activated receptor gamma have de-pot-specific effects on human preadipocyte differentiation. J. Clin. Invesig. 1997, 100, 3149–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchkonia, T.; Giorgadze, N.; Pirtskhalava, T.; Tchoukalova, Y.; Karagiannides, I.; Forse, R.A.; DePonte, M.; Stevenson, M.; Guo, W.; Han, J.; et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1286–R1296. [Google Scholar] [CrossRef] [Green Version]
- Qayyum, A.A.; Mathiasen, A.B.; Helqvist, S.; Jørgensen, E.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, A. Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results. J. Transl. Med. 2019, 17, 360. [Google Scholar] [CrossRef]
- Vieira, S.S.; Antonio, E.L.; Melo, B.L.; Portes, L.A.; Montemor, J.; Oliveira, H.A.; Martins, F.L.; Zogbi, C.; Girardi, A.C.; Silva, J.A., Jr. Exercise training potentiates the cardioprotective effects of stem cells post-infarction. Heart Lung Circ. 2019, 28, 263–271. [Google Scholar] [CrossRef]
- Kato, H.; Minamizato, H.; Ohno, H.; Ohira, Y.; Izawa, T. Exercise ameliorates high-fat diet-induced impairment of differentiation of adipose-derived stem cells into neuron-like cells in rats. J. Cell. Physiol. 2018, 234, 1452–1460. [Google Scholar] [CrossRef]
- Sakurai, T.; Endo, S.; Hatano, D.; Ogasawara, J.; Kizaki, T.; Oh-Ishi, S.; Izawa, T.; Ishida, H.; Ohno, H. Effects of exercise training on adipogenesis of stromal-vascular fraction cells in rat epididymal white adipose tissue. Acta Physiol. 2010, 200, 325–338. [Google Scholar] [CrossRef]
- Hatano, D.; Ogasawara, J.; Endoh, S.; Sakurai, T.; Nomura, S.; Kizaki, T.; Ohno, H.; Komabayashi, T.; Izawa, T. Effect of exercise training on the density of endothelial cells in the white adipose tissue of rats. Scand. J. Med. Sci. Sports 2010, 21, e115–e121. [Google Scholar] [CrossRef] [PubMed]
- Shyh-Chang, N.; Daley, G.Q.; Lewis, C.; Cantley, L.C. Stem cell metabolism in tissue development and aging. Development 2013, 140, 2535–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Marsboom, G.; Toth, P.T.; Rehman, J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS ONE 2013, 8, e77077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drehmer, D.L.; Aguiar, A.M.; Brandt, A.P.; Petiz, L.; Cadena, S.M.C.; Rebelatto, C.K.; Brofman, P.R.S.; Francisco, F.N.; Dallagiovanna, B.; Abud, A.P.R. Metabolic switches during the first steps of adipogenic stem cells differ-entiation. Stem Cell Res. 2016, 17, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, J.G.; Gardner, D.K.; Harvey, A.J. Pluripotent stem cell metabolism and mitochondria: Beyond ATP. Stem Cells Int. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackett, J.A.; Surani, M.A. DNA methylation dynamics during the mammalian life cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110328. [Google Scholar] [CrossRef] [Green Version]
- Seisenberger, S.; Peat, J.R.; Hore, T.A.; Santos, F.; Dean, W.; Reik, W. Reprogramming DNA methylation in the mammalian life cycle: Building and breaking epigenetic barriers. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110330. [Google Scholar] [CrossRef] [Green Version]
- Kraushaar, D.C.; Zhao, K. The epigenomics of embryonic stem cell differentiation. Int. J. Biol. Sci. 2013, 9, 1134–1144. [Google Scholar] [CrossRef] [Green Version]
- Kilberg, M.S.; Terada, N.; Shan, J. Influence of amino acid metabolism on embryonic stem cell function and differentiation. Adv. Nutr. 2016, 7, 780S–789S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Furuhashi, M.; Sugaya, T.; Oikawa, T.; Matsumoto, M.; Funahashi, Y.; Matsukawa, Y.; Gotoh, M.; Miura, T. Transcriptome and metabolome analyses in exogenous FABP4- and FABP5-Treated Adipose-Derived Stem Cells. PLoS ONE 2016, 11, e0167825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyh-Chang, N.; Locasale, J.W.; Lyssiotis, C.A.; Zheng, Y.; Teo, R.Y.; Ratanasirintrawoot, S.; Zhang, J.; Onder, T.; Unternaehrer, J.J.; Zhu, H.; et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 2013, 339, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Shiraki, N.; Shiraki, Y.; Tsuyama, T.; Obata, F.; Miura, M.; Nagae, G.; Aburatani, H.; Kume, K.; Endo, F.; Kume, S. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 2014, 19, 780–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, B.W.; Finley, L.W.S.; Cross, J.R.; Allis, C.D.; Thompson, C.B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nat. Cell Biol. 2015, 518, 413–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, N.J.; Galbo, H. Sympathetic nervous activity during exercise. Annu. Rev. Physiol. 1983, 45, 139–153. [Google Scholar] [CrossRef]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef]
- Mildmay-White, A.; Khan, W. Cell surface markers on adipose-derived stem cells: A systematic review. Curr. Stem Cell Res. Ther. 2016, 12, 484–492. [Google Scholar] [CrossRef]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [Green Version]
- Lehnig, A.C.; Dewal, R.S.; Baer, L.A.; Kitching, K.M.; Munoz, V.R.; Arts, P.J.; Sindeldecker, D.A.; May, F.J.; Lau-ritzen, H.P.M.M.; Goodyear, L.J.; et al. Exercise training induces depot-specific adaptations to white and brown adipose tissue. IScience 2019, 11, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-L.; Lin, S.-P.; Hsieh, P.C.; Hung, S.-C. Concomitant beige adipocyte differentiation upon induction of mesenchymal stem cells into brown adipocytes. Biochem. Biophys. Res. Commun. 2016, 478, 689–695. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Hallen, S.; Huang, L.; Svensson, P.-A.; Momo, R.A.; Wallin, S.; Carlsson, E.K.; Forslöw, A.; Seale, P.; Peng, X.-R. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol. Endocrinol. 2014, 29, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enevoldsen, L.H.; Stallknecht, B.; Fluckey, J.D.; Galbo, H. Effect of exercise training on in vivo lipolysis in intra-abdominal adipose tissue in rats. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E585–E592. [Google Scholar] [CrossRef] [PubMed]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M. The production and release of glycerol by adipose tissue incubated in vitro. J. Biol. Chem. 1962, 237, 3354–3358. [Google Scholar] [CrossRef]
- Vaughan, M.; Steinberg, D. Effect of hormones on lipolysis and esterification of free fatty acids during incubation of adipose tissue in vitro. J. Lipid Res. 1963, 4, 193–199. [Google Scholar] [CrossRef]
- Brooks, B.; Arch, J.R.; Newsholme, E.A. Effects of hormones on the rate of the triacylglycerol/fatty acid substrate cycle in adipocytes and epididymal fat pads. FEBS Lett. 1982, 146, 327–330. [Google Scholar] [CrossRef] [Green Version]
- Reilly, S.M.; Hung, C.-W.; Ahmadian, M.; Zhao, P.; Keinan, O.; Gomez, A.V.; DeLuca, J.H.; Dadpey, B.; Lu, D.; Zaid, J.; et al. Catecholamines suppress fatty acid re-esterification and increase oxidation in white adipocytes via STAT3. Nat. Metab. 2020, 2, 620–634. [Google Scholar] [CrossRef]
- Yehuda-Shnaidman, E.; Buehrer, B.; Pi, J.; Kumar, N.; Collins, S. Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes 2010, 59, 2474–2483. [Google Scholar] [CrossRef] [Green Version]
- Pistor, K.E.; Sepa-Kishi, D.M.; Hung, S.; Ceddia, R.B. Lipolysis, lipogenesis, and adiposity are reduced while fat-ty acid oxidation is increased in visceral and subcutaneous adipocytes of endurance-trained rats. Adipocyte 2014, 4, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Deveaud, C.; Beauvoit, B.; Salin, B.; Schaeffer, J.; Rigoulet, M. Regional differences in oxidative capacity of rat white adipose tissue are linked to the mitochondrial content of mature adipocytes. Mol. Cell. Biochem. 2004, 267, 157–166. [Google Scholar] [CrossRef]
- Chapados, N.; Collin, P.; Imbeault, P.; Corriveau, P.; Lavoie, J.-M. Exercise training decreases in vitro stimulated lipolysis in a visceral (mesenteric) but not in the retroperitoneal fat depot of high-fat-fed rats. Br. J. Nutr. 2008, 100, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Rodrigues, S.; Rodríguez, A.; Becerril, S.; Ramírez, B.; Gonçalves, O.I.; Beleza, J.; Frühbeck, G.; Ascensão, A.; Magalhães, J. Physical exercise remodels visceral adipose tissue and mitochondrial lipid metabolism in rats fed a high-fat diet. Clin. Exp. Pharmacol. Physiol. 2017, 44, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, G.; Kato, H.; Izawa, T. Endurance exercise training induces fat depot-specific differences in basal autophagic activity. Biochem. Biophys. Res. Commun. 2015, 466, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.J.; Lorin, S.; Blommaart, E.F.; Codogno, P. Regulation of autophagy by amino acids and MTOR-dependent signal transduction. Amino Acids 2015, 47, 2037–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannon, N.P.; Schnuck, J.K.; Vaughan, R.A. BCAA Metabolism and Insulin Sensitivity—Dysregulated by Meta-bolic Status? Mol. Nutr. Food Res. 2018, 62, e1700756. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, Y.; Tadaishi, M.; Nagaike, Y.; Morita, A.; Ogawa, Y.; Ezaki, O.; Takai-Igarashi, T.; Kitaura, Y.; Shimomura, Y.; Kamei, Y.; et al. PGC-1α-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS ONE 2014, 9, e91006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, R.; Murakami, T.; Obayashi, M.; Nakai, N.; Jaskiewicz, J.; Fujiwara, Y.; Shimomura, Y.; Harris, R.A. Clofibric acid stimulates branched-chain amino acid catabolism by three mechanisms. Arch. Biochem. Biophys. 2002, 407, 231–240. [Google Scholar] [CrossRef]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.H.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Metab. 2013, 304, E1175–E1187. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, J.; Sakurai, T.; Kizaki, T.; Ishibashi, Y.; Izawa, T.; Sumitani, Y.; Ishida, H.; Radak, Z.; Haga, S.; Ohno, H. Higher levels of ATGL are associated with exercise-induced enhancement of lipolysis in rat epididymal adipocytes. PLoS ONE 2012, 7, e40876. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Panadero, M.I.; Pérez, L.M.; Gálvez, B.G.; García, A.; Barbas, C.; Rupérez, F.J. New insight on obesity and adipose-derived stem cells using comprehensive metabolomics. Biochem. J. 2016, 473, 2187–2203. [Google Scholar] [CrossRef]
- Liu, S.Y.; He, Y.B.; Deng, S.Y.; Zhu, W.T.; Xu, S.Y.; Ni, G.X. Exercise affects biological characteristics of mesenchymal stromal cells derived from bone marrow and adipose tissue. Int. Orthop. 2017, 41, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.R.; Walvoord, R.R.; Chang, A.Y.; Kozlowski, M.C.; Gamper, H.; Hou, Y.M. Amino acid-dependent stability of the acyl linkage in aminoacyl-tRNA. RNA 2014, 20, 758–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francklyn, C.S.; Mullen, P. Progress and challenges in aminoacyl-tRNA synthetase-based therapeutics. J. Biol. Chem. 2019, 294, 5365–5385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, Y.; Hashimoto, O.; Shindo, D.; Sugiyama, M.; Tomonaga, S.; Murakami, M.; Matsui, T.; Funaba, M. Metabolic changes in adipose tissues in response to β3-adrenergic receptor activation in mice. J. Cell. Biochem. 2019, 120, 821–835. [Google Scholar] [CrossRef] [Green Version]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groussard, C.; Maillard, F.; Vazeille, E.; Barnich, N.; Sirvent, P.; Otero, Y.F.; Combaret, L.; Madeuf, E.; Sourdrille, A.; Delcros, G.; et al. Tissue-specific oxidative stress modulation by exercise: A comparison between mict and hiit in an obese rat model. Oxid. Med. Cell. Longev. 2019, 2019, 1965364–11. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T.; Izawa, T.; Kizaki, T.; Ogasawara, J.-E.; Shirato, K.; Imaizumi, K.; Takahashi, K.; Ishida, H.; Ohno, H. Exercise training decreases expression of inflammation-related adipokines through reduction of oxidative stress in rat white adipose tissue. Biochem. Biophys. Res. Commun. 2009, 379, 605–609. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and sex differences in adipose tissue. Curr. Diabetes Rep. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Ogasawara, J.; Izawa, T.; Sakurai, T.; Shirato, K.; Ishibashi, Y.; Ohira, Y.; Ishida, H.; Ohno, H.; Kizaki, T. Habitual exercise training acts as a physiological stimulator for constant activation of lipolytic enzymes in rat primary white adipocytes. Biochem. Biophys. Res. Commun. 2015, 464, 348–353. [Google Scholar] [CrossRef]
- Kato, H.; Ogasawara, J.; Takakura, H.; Shirato, K.; Sakurai, T.; Kizaki, T.; Izawa, T. Exercise training-enhanced lipolytic potency to catecholamine depends on the time of the day. Int. J. Mol. Sci. 2020, 21, 6920. [Google Scholar] [CrossRef]
- Gleeson, T.T.; Baldwin, K.M. Cardiovascular response to treadmill exercise in untrained rats. J. Appl. Physiol. 1981, 50, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Rodriguesa, B.; Figueroa, D.M.; Mostarda, C.T.; Heeren, M.V.; Irigoyen, M.C.; De Angelis, K. Maximal exercise test is a useful method for physical capacity and oxygen consumption determination in streptozotocin-diabetic rats. Cardiovasc. Diabetol. 2007, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisløff, U.; Helgerud, J.; Kemi, O.J.; Ellingsen, Ø. Intensity-controlled treadmill running in rats: Vo 2 max and cardiac hypertrophy. Am. J. Physiol. Circ. Physiol. 2001, 280, H1301–H1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh-Ishi, S.; Kizaki, T.; Nagasawa, J.; Izawa, T.; Komabayashi, T.; Nagata, N.; Suzuki, K.; Taniguchi, N.; Ohno, H. Effects of endurance training on superoxide dismutase activity, content and mRNA expression in rat muscle. Clin. Exp. Pharmacol. Physiol. 1997, 24, 326–332. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Deutsch, M.J.; Schriever, S.C.; Roscher, A.A.; Ensenauer, R. Digital image analysis approach for lipid droplet size quantitation of oil red O-stained cultured cells. Anal. Biochem. 2014, 445, 87–89. [Google Scholar] [CrossRef]






| Epididymal ADSC-Derived Adipocytes | Inguinal ADSC-Derived Adipocytes | |||||
|---|---|---|---|---|---|---|
| ND-TR vs. ND-SED | HFD-SED vs. ND-SED | HFD-TR vs. HFD-SED | ND-TR vs. ND-SED | HFD-SED vs. ND-SED | HFD-TR vs. HFD-SED | |
| GSH/GSSG | 1.2 | 0.8 | 1.1 | 0.6 * | 0.9 | 1.2 ** |
| NADP+/NADPH | 0.7 | 0.5 | 1.4 | 0.7 | 1.4 | 0.7 |
| NAD+/NADH | 0.8 * | 0.7 * | 0.9 | 0.7 * | 1.4 * | 0.7 * |
| Lactate/Pyruvate | 1.1 | 1.4 * | 1.0 | 0.8 | 0.8 | 1.0 |
| Malate/Asp | 1.1 | 1.3 | 1.0 | 0.6 *** | 0.7 ** | 1.1 |
| Citrulline/Ornithine | 1.1 | 1.0 | 1.0 | 1.1 | 0.8 | 1.1 |
| Glu/2-Oxoglutarate | 1.3 ** | 1.5 | 1.5 | 1.7 * | 1.1 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osawa, S.; Kato, H.; Maeda, Y.; Takakura, H.; Ogasawara, J.; Izawa, T. Metabolomic Profiles in Adipocytes Differentiated from Adipose-Derived Stem Cells Following Exercise Training or High-Fat Diet. Int. J. Mol. Sci. 2021, 22, 966. https://doi.org/10.3390/ijms22020966
Osawa S, Kato H, Maeda Y, Takakura H, Ogasawara J, Izawa T. Metabolomic Profiles in Adipocytes Differentiated from Adipose-Derived Stem Cells Following Exercise Training or High-Fat Diet. International Journal of Molecular Sciences. 2021; 22(2):966. https://doi.org/10.3390/ijms22020966
Chicago/Turabian StyleOsawa, Seita, Hisashi Kato, Yuki Maeda, Hisashi Takakura, Junetsu Ogasawara, and Tetsuya Izawa. 2021. "Metabolomic Profiles in Adipocytes Differentiated from Adipose-Derived Stem Cells Following Exercise Training or High-Fat Diet" International Journal of Molecular Sciences 22, no. 2: 966. https://doi.org/10.3390/ijms22020966
APA StyleOsawa, S., Kato, H., Maeda, Y., Takakura, H., Ogasawara, J., & Izawa, T. (2021). Metabolomic Profiles in Adipocytes Differentiated from Adipose-Derived Stem Cells Following Exercise Training or High-Fat Diet. International Journal of Molecular Sciences, 22(2), 966. https://doi.org/10.3390/ijms22020966






