Diets with Higher ω-6/ω-3 Ratios Show Differences in Ceramides and Fatty Acid Levels Accompanied by Increased Amyloid-Beta in the Brains of Male APP/PS1 Transgenic Mice
Abstract
:1. Introduction
2. Results
2.1. Diet Composition
2.2. Effect of Diet on Body Weight
2.3. Effect of Diet on the Brain Lipidome
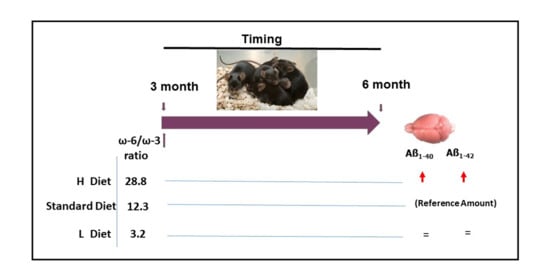
2.4. Effect of Diet on Burden of Amyloid Deposition
2.5. Effect of Diet on Brain Protein Markers
3. Discussion
4. Materials and Methods
4.1. Animals and Husbandry
4.2. Genotyping
4.3. Diet
4.4. Tissue Processing
4.5. Lipid Analysis
4.6. Quantitative Determination of Aβ1-40 and Aβ1-42
4.7. Western Blot
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiatr. Psych. Gerichtl. Med. 1907, 64, 146–148. [Google Scholar]
- Selkoe, D.J. Alzheimer’s Disease Is a Synaptic Failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.E.; Huang, H.M. Oxidative stress in Alzheimer’s disease. Neurobiol Aging 2005, 26, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Mullan, M.; Crawford, F.; Axelman, K.; Houlden, H.; Lilius, L.; Winblad, B.; Lannfelt, L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992, 1, 345–347. [Google Scholar] [CrossRef]
- Levy-Lahad, E.; Wasco, W.; Poorkaj, P.; Romano, D.M.; Oshima, J.; Pettingell, W.H.; E Yu, C.; Jondro, P.D.; Schmidt, S.D.; Wang, K.; et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 1995, 269, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, R.; Froelich, S.; Sorbi, S.; Campion, D.; Chi, H.; Rogaeva, E.A.; Levesque, G.; Rogaev, E.I.; Lin, C.; Liang, Y.; et al. Alzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum. Mol. Genet. 1996, 5, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Ettcheto, M.; Petrov, D.; Pedrós, I.; de Lemos, L.; Pallàs, M.; Alegret, M.; Laguna, J.C.; Folch, J.; Camins, A. Hypercholesterolemia and neurodegeneration. Comparison of hippocampal phenotypes in LDLr knockout and APPswe/PS1dE9 mice. Exp. Gerontol. 2015, 65, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Dar, T.; Sheikh, I.; Ganie, S.; Ali, R.; Singh, L.; Gan, S.H.; Kamal, M.A.; Zargar, M. Molecular Linkages Between Diabetes and Alzheimer’s Disease: Current Scenario and Future Prospects. CNS Neurol. Disord.-Drug Targets 2014, 13, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.H.; Choi, K.H.; Jang, Y.J.; Bae, S.S.; Choi, B.T.; Shin, H.K. Hypercholesterolemia accelerates amyloid beta-induced cognitive deficits. Int. J. Mol. Med. 2013, 31, 577–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciarelli, R.; Canepa, E.; Marengo, B.; Marinari, U.M.; Poli, G.; Pronzato, M.A.; Domenicotti, C. Cholesterol and Alzheimer’s disease: A still poorly understood correlation. IUBMB Life 2012, 64, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of Fish and n-3 Fatty Acids and Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.-X.; Mayeux, R.; Luchsinger, J. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, Y.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, S.M.; Giovagnoni, C.; Virsseren, L.; Scheithauer, A.L.; de Wit, N.; den Hoedt, S.; Losen, M.; Mulder, M.T.; Walter, J.; de Vries, H.E.; et al. Sphingolipids in Alzheimer’s disease, how can we target them? Adv. Drug Deliv. Rev. 2020, 159, 214–231. [Google Scholar] [CrossRef]
- Bourre, J.M. Nature, origin and role of fatty acids of the nervous system: An essential fatty acid, an alpha-linolenic acid, changing the structure and the cerebral function. Bull. l’Academie Natl. Med. 1989, 173, 1137. [Google Scholar] [PubMed]
- Cervera, M.A.R.; Valenzuela, R.; Hernandez-Rodas, M.C.; Barrera, C.; Espinosa, A.; Marambio, M.; Valenzuela, A. Vegetable oils rich in alpha linolenic acid increment hepatic n-3 LCPUFA, modulating the fatty acid metabolism and antioxidant response in rats. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Joint, F.A.O. Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr Pap. 2010, 91, 1–166. [Google Scholar]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [Green Version]
- Crawford, M.A.; Broadhurst, C.L. The role of docosahexaenoic and the marine food web as determinants of evolution and hominid brain development: The challenge for human sustainability. Nutr. Health 2012, 21, 17–39. [Google Scholar] [CrossRef]
- Mazza, M.; Pomponi, M.; Janiri, L.; Bria, P.; Mazza, S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2007, 31, 12–26. [Google Scholar] [CrossRef]
- Catalan, J.; Moriguchi, T.; Slotnick, B.; Murthy, M.; Greiner, R.S.; Salem, N. Cognitive deficits in docosahexaenoic acid-deficient rats. Behav. Neurosci. 2002, 116, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, A.; Ohishi, M.; Sato, Y.; Hata, N.; Misawa, Y.; Fujii, Y.; Okuyama, H. Reversibility of n-3 fatty acid deficiency-induced alterations of learning behavior in the rat: Level of n-6 fatty acids as another critical factor. J. Lipid Res. 2001, 42, 1655–1663. [Google Scholar] [CrossRef]
- Wiesmann, M.; Zerbi, V.; Jansen, D.; Haast, R.; Lütjohann, D.; Broersen, L.M.; Heerschap, A.; Kiliaan, A.J. A Dietary Treatment Improves Cerebral Blood Flow and Brain Connectivity in Aging apoE4 Mice. Neural Plast. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Jr, N.S.; Frautschy, S.A.; Cole, G.M. A Diet Enriched with the Omega-3 Fatty Acid Docosahexaenoic Acid Reduces Amyloid Burden in an Aged Alzheimer Mouse Model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, S.E.; He, B.; Muhammad, N.; Oh, K.J.; Fahnestock, M.; Ikonomovic, M.D.; Mufson, E.J. Cholinotrophic basal forebrain system alterations in 3xTg-AD transgenic mice. Neurobiol. Dis. 2011, 41, 338–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsenault, D.; Julien, C.; Tremblay, C.; Calon, F. DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3xTg-AD mice. PLoS ONE 2011, 6, e17397. [Google Scholar] [CrossRef] [Green Version]
- Herrera, J.L.; Ordoñez-Gutierrez, L.; Fabrias, G.; Casas, J.; Morales, A.; Hernández, G.; Acosta, N.G.; Rodriguez, C.; Prieto-Valiente, L.; Garcia-Segura, L.M.; et al. Ovarian Hormone-Dependent Effects of Dietary Lipids on APP/PS1 Mouse Brain. Front. Aging Neurosci. 2019, 11, 346. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Sholar, P.W.; Bilbo, S. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2011, 120, 948–963. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Crespo-Castrillo, A.; de Ceballos, M.L.; Chowen, J.; Azcoitia, I.; Arevalo, M.-A.; Garcia-Segura, L.M. Sex differences in the phagocytic and migratory activity of microglia and their impairment by palmitic acid. Glia 2017, 66, 522–537. [Google Scholar] [CrossRef]
- Morselli, E.; Criollo, A.; Rodriguez-Navas, C.; Clegg, D.J. Chronic High Fat Diet Consumption Impairs Metabolic Health of Male Mice. Inflamm. Cell Signal. 2015, 1, e561. [Google Scholar] [CrossRef]
- Argente-Arizón, P.; Díaz, F.; Ros, P.; Barrios, V.; Tena-Sempere, M.; García-Segura, L.M.; Argente, J.; A Chowen, J. The Hypothalamic Inflammatory/Gliosis Response to Neonatal Overnutrition Is Sex and Age Dependent. Endocrinology 2017, 159, 368–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, J.L.; Ordoñez-Gutierrez, L.; Fabrias, G.; Casas, J.; Morales, A.; Hernández, G.; Acosta, N.G.; Rodriguez, C.; Prieto-Valiente, L.; Garcia-Segura, L.M.; et al. Ovarian Function Modulates the Effects of Long-Chain Polyunsaturated Fatty Acids on the Mouse Cerebral Cortex. Front. Cell. Neurosci. 2018, 12, 103. [Google Scholar] [CrossRef]
- Koivisto, H.; Grimm, M.; Rothhaar, T.L.; Berkecz, R.; Lütjohann, D.; Giniatullina, R.; Takalo, M.; Miettinen, P.O.; Lahtinen, H.-M.; Giniatullin, R.; et al. Special lipid-based diets alleviate cognitive deficits in the APPswe/PS1dE9 transgenic mouse model of Alzheimer’s disease independent of brain amyloid deposition. J. Nutr. Biochem. 2013, 25, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Interplay Between n-3 and n-6 Long-Chain Polyunsaturated Fatty Acids and the Endocannabinoid System in Brain Protection and Repair. Lipids 2017, 52, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A. Beneficial Effects of Fish. In Oil on Human Brain; Springer: New York, NY, USA, 2009; 396p. [Google Scholar]
- Green, K.N.; Martinez-Coria, H.; Khashwji, H.; Hall, E.B.; Yurko-Mauro, K.A.; Ellis, L.; LaFerla, F.M. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J. Neurosci. 2007, 27, 4385–4395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, S.E.; Berg, B.M.; Moore, K.A.; He, B.; Counts, S.E.; Fritz, J.J.; Hu, Y.; Lazarov, O.; Lah, J.J.; Mufson, E.J. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: Possible gender effects. J. Neurosci. Res. 2010, 88, 1026–1040. [Google Scholar] [PubMed] [Green Version]
- Samieri, C.; Lorrain, S.; Buaud, B.; Vaysse, C.; Berr, C.; Peuchant, E.; Cunnane, S.C.; Barberger-Gateau, P. Relationship between diet and plasma long-chain n-3 PUFAs in older people: Impact of apolipoprotein E genotype. J. Lipid Res. 2013, 54, 2559–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivera-Perez, H.M.; Lam, L.; Dang, J.; Jiang, W.; Rodriguez, F.; Rigali, E.; Weitzman, S.; Porter, V.; Rubbi, L.; Morselli, M.; et al. Omega-3 fatty acids increase the unfolded protein response and improve amyloid-beta phagocytosis by macrophages of patients with mild cognitive impairment. FASEB J. 2017, 31, 4359–4369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjorth, E.; Zhu, M.; Cortés-Toro, V.; Vedin, I.; Palmblad, J.; Cederhom, T.; Freund-Levi, Y.; Wahlund, L.; Basun, H.; Eriksdotter, M.; et al. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-beta42 by human microglia and decrease inflammatory markers. J. Alzheimers Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Tanila, H.; Puoliväli, J.; Kadish, I.; van Groen, T. Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol. Dis. 2003, 14, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.M.; Rosario, E.R.; Elteriefi, R.; Pike, C.J. Sex-specific effects of high fat diet on indices of metabolic syndrome in 3xTg-AD mice: Implications for Alzheimer’s disease. PLoS ONE 2013, 8, e78554. [Google Scholar] [CrossRef]
- Arcones, A.; Cruces-Sande, M.; Ramos, P.; Mayor, F.; Murga, C. Sex Differences in High Fat Diet-Induced Metabolic Alterations Correlate with Changes in the Modulation of GRK2 Levels. Cells 2019, 8, 1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, A.; Barrington, W.T.; Dearth, S.; May, A.; Threadgill, D.W.; Campagna, S.R.; Voy, B.H. Tissue Level Diet. and Sex.-by-Diet. Interactions Reveal Unique Metabolite and Clustering Profiles Using Untargeted Liquid Chromatography-Mass Spectrometry on Adipose, Skeletal Muscle, and Liver Tissue in C57BL6/J. Mice. J. Proteome Res. 2018, 17, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rutters, F.; Dederen, P.; Gambarota, G.; Veltien, A.; van Groen, T.; Broersen, L.; Lütjohann, D.; Heerschap, A.; Tanila, H.; et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD). Neurobiol. Dis. 2007, 28, 16–29. [Google Scholar] [CrossRef]
- Barberger-Gateau, P.; Letenneur, L.; Deschamps, V.; Peres, K.; Dartigues, J.-F.; Renaud, S. Fish, meat, and risk of dementia: Cohort study. BMJ 2002, 325, 932–933. [Google Scholar] [CrossRef] [Green Version]
- Calon, F.; Lim, G.P.; Yang, F.; Morihara, T.; Teter, B.; Ubeda, O.; Rostaing, P.; Triller, A.; Salem, N.; Ashe, K.H.; et al. Docosahexaenoic Acid Protects from Dendritic Pathology in an Alzheimer’s Disease Mouse Model. Neuron 2004, 43, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Palmblad, J. Are omega-3 fatty acids options for prevention and treatment of cognitive decline and dementia? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.N.; Feng, Q.; Azizkhanian, I.; Rawat, V.; Castor, K.; Fonteh, A.N.; Harrington, M.; Zheng, L.; Reed, B.R.; DeCarli, C.; et al. Association of Serum Docosahexaenoic Acid With Cerebral Amyloidosis. JAMA Neurol. 2016, 73, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Panchal, M.; Gaudin, M.; Lazar, A.N.; Salvati, E.; Rivals, I.; Ayciriex, S.; Dauphinot, L.; Dargère, D.; Auzeil, N.; Masserini, M.; et al. Ceramides and sphingomyelinases in senile plaques. Neurobiol. Dis. 2014, 65, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, L.; Ellis, B.C.; Saunders, A.J.; Kovacs, D.M. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J. Biol. Chem. 2003, 278, 19777–19783. [Google Scholar] [PubMed] [Green Version]
- Patil, S.; Melrose, J.; Chan, C. Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur. J. Neurosci. 2007, 26, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Huang, Y.; Li, B.; Gong, C.-X.; Schuchman, E.H. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol. Aging 2010, 31, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippov, V.; Song, M.A.; Zhang, K.; Vinters, H.V.; Tung, S.; Kirsch, W.M.; Yang, J.; Duerksen-Hughes, P.J. Increased Ceramide in Brains with Alzheimer’s and Other Neurodegenerative Diseases. J. Alzheimer’s Dis. 2012, 29, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Ellis, B.; Hye, A.; Snowden, S.G. Metabolic Modifications in Human Biofluids Suggest the Involvement of Sphingolipid, Antioxidant, and Glutamate Metabolism in Alzheimer’s Disease Pathogenesis. J. Alzheimers Dis. 2015, 46, 313–327. [Google Scholar]
- Bandaru, V.V.R.; Troncoso, J.; Wheeler, D.; Pletnikova, O.; Wang, J.; Conant, K.; Haughey, N.J. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer’s but not normal brain. Neurobiol. Aging 2009, 30, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Cutler, R.G.; Kelly, J.; Storie, K.; Pedersen, W.A.; Tammara, A.; Hatanpaa, K.; Troncoso, J.C.; Mattson, M.P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2070–2075. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, M.; Shimizu, Y.; Yoshioka, T.; Wakabayashi, M.; Tanaka, Y.; Higashino, K.; Numata, Y.; Sakai, S.; Kihara, A.; Igarashi, Y.; et al. Histological analyses by matrix-assisted laser desorption/ionization-imaging mass spectrometry reveal differential localization of sphingomyelin molecular species regulated by particular ceramide synthase in mouse brains. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 1554–1565. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Spassieva, S.D.; Jucius, T.J.; Shultz, L.D.; Shick, H.E.; Macklin, W.B.; Hannun, Y.A.; Obeid, L.; Ackerman, S.L. A Deficiency of Ceramide Biosynthesis Causes Cerebellar Purkinje Cell Neurodegeneration and Lipofuscin Accumulation. PLoS Genet. 2011, 7, e1002063. [Google Scholar] [CrossRef] [Green Version]
- Laviad, E.L.; Albee, L.; Pankova-Kholmyansky, I.; Epstein, S.; Park, H.; Merrill, A.H.; Futerman, A.H. Characterization of ceramide synthase 2: Tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 2008, 283, 5677–5684. [Google Scholar] [CrossRef] [Green Version]
- Babenko, N.A.; Semenova, Y.A. Effects of long-term fish oil-enriched diet on the sphingolipid metabolism in brain of old rats. Exp. Gerontol. 2010, 45, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cong, P.; Lu, L.; Wang, Y.; Tang, Q.; Zhang, H.; Xu, J.; Xue, C. Effects of dietary glucocerebrosides from sea cucumber on the brain sphingolipid profiles of mouse models of Alzheimer’s disease. Food Funct. 2017, 8, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of Dietary Sphingomyelin on Central Nervous System Myelination in Developing Rats. Pediatr. Res. 2003, 53, 589–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allende, M.L.; Proia, R.L. Simplifying complexity: Genetically resculpting glycosphingolipid synthesis pathways in mice to reveal function. Glycoconj. J. 2014, 31, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrier, L.; Ingrand, S.; Damjanac, M.; Bilan, A.R.; Hugon, J.; Page, G. Genotype-related changes of ganglioside composition in brain regions of transgenic mouse models of Alzheimer’s disease. Neurobiol. Aging 2007, 28, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Fabelo, N.; Martín, M.V.; Marín, R.; Santpere, G.; Aso, E.; Ferrer, I.; Díaz, M. Evidence for Premature Lipid Raft Aging in APP/PS1 Double-Transgenic Mice, a Model of Familial Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2012, 71, 868–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiley, L.; Sen, A.; Heaton, J.; Proitsi, P.; García-Gómez, D.; Leung, R.; Smith, N.; Thambisetty, M.; Kloszewska, I.; Mecocci, P.; et al. Evidence of altered phosphatidylcholine metabolism in Alzheimer’s disease. Neurobiol. Aging 2013, 35, 271–278. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Moffat, T.C.; Gable, K.; Ganesan, S.; Niewola-Staszkowska, K.; Johnston, A.; Nislow, C.; Giaever, G.; Harris, L.J.; Loewith, R.; et al. A Signaling Lipid Associated with Alzheimer’s Disease Promotes Mitochondrial Dysfunction. Sci. Rep. 2016, 6, srep19332. [Google Scholar] [CrossRef] [Green Version]
- Brenna, J.T.; Salem, N.; Sinclair, A.J.; Cunnane, S.C. Alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Xu, N.; Li, A.-D.; Ji, L.-L.; Ye, Y.; Wang, Z.-Y.; Tong, L. miR-132 regulates the expression of synaptic proteins in APP/PS1 transgenic mice through C1q. Eur. J. Histochem. 2019, 63. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Mei, Y.; Qu, Z.-L.; Zhang, S.-J.; Zhao, W.; Fang, J.-S.; Wu, J.; Yang, C.; Liu, S.-J.; Fang, Y.-Q.; et al. Ligustilide Ameliorates Memory Deficiency in APP/PS1 Transgenic Mice via Restoring Mitochondrial Dysfunction. BioMed Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, L.-J.; Shi, S.; Xu, S.-F.; Wang, X.-L.; Peng, Y. L-3-n-butylphthalide Rescues Hippocampal Synaptic Failure and Attenuates Neuropathology in Aged APP/PS1 Mouse Model of Alzheimer’s Disease. CNS Neurosci. Ther. 2016, 22, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Pedrós, I.; Petrov, D.; Allgaier, M.; Sureda, F.X.; Barroso, E.; Beas-Zarate, C.; Auladell, C.; Pallàs, M.; Vázquez-Carrera, M.; Casadesus, G.; et al. Early alterations in energy metabolism in the hippocampus of APPswe/PS1dE9 mouse model of Alzheimer’s disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1556–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Kou, J.; Li, F.; Huo, D.; Xu, J.; Zhou, X.; Meng, D.; Ghulam, M.; Artyom, B.; Gao, X.; et al. Lemon essential oil ameliorates age-associated cognitive dysfunction via modulating hippocampal synaptic density and inhibiting acetylcholinesterase. Aging 2020, 12, 8622–8639. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Luo, Y.; Xue, Q.; Li, G.; Tan, Y.; Xiao, J.; Yu, B. Docosahexaenoic Acid Rescues Synaptogenesis Impairment and Long-Term Memory Deficits Caused by Postnatal Multiple Sevoflurane Exposures. BioMed Res. Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophocleous, A.; Idris, A. Ovariectomy/Orchiectomy in Rodents. In Bone Research Protocols; Humana Press: New York, NY, USA, 2019; pp. 261–267. [Google Scholar]
- Jankowsky, J.L.; Slunt, H.H.; Ratovitski, T.; Jenkins, N.A.; Copeland, N.G.; Borchelt, D.R. Co-expression of multiple transgenes in mouse CNS: A comparison of strategies. Biomol. Eng. 2001, 17, 157–165. [Google Scholar] [CrossRef]
- Ordoñez-Gutierrez, L.; Antón, M.; Wandosell, F. Peripheral amyloid levels present gender differences associated with aging in AbPP/PS1 mice. J. Alz. Dis. 2015, 44, 1063–1068. [Google Scholar]
- Cingolani, F.; Casasampere, M.; Sanllehí, P.; Casas, J.; Fàbrias, G. Inhibition of dihydroceramide desaturase activity by the sphingosine kinase inhibitor SKII. J. Lipid. Res. 2014, 55, 1711–1720. [Google Scholar] [CrossRef] [Green Version]
- Garanto, A.; Mandal, N.A.; Egido-Gabás, M.; Marfany, G.; Fabrias, G.; Anderson, R.E.; Casas, J.; Gonzàlez-Duarte, R. Specific sphingolipid content decrease in Cerkl knockdown mouse retinas. Exp. Eye Res. 2013, 110, 96–106. [Google Scholar] [CrossRef] [Green Version]









| L | SF | H | |||||
|---|---|---|---|---|---|---|---|
| Total fat contain | 6.9 g/100g | 4.6 g/100g | 7.3 g/100g | ||||
| relative % | mg/100g | relative % | mg/100g | relative % | mg/100g | ||
| Saturated fatty acids | |||||||
| Total saturated fatty acids | SFA | 12.7 | 871 | 15.1 | 654 | 8.3 | 579 |
| Palmitic acid | C16:0 | 8 | 552 | 11.4 | 495 | 5.1 | 356 |
| Stearic acid | C18:0 | 2.4 | 162 | 2.4 | 104 | 2.2 | 156 |
| Unsaturated fatty acids | |||||||
| Total unsaturated fatty acids | UFA | 87.3 | 5858 | 84.9 | 3605 | 91.7 | 6287 |
| Oleic acid | C18:1 | 40.5 | 2733 | 47 | 2002 | 55 | 3784 |
| Monounsaturated fatty acids | MUFA | 47.5 | 3201 | 47.8 | 2037 | 55.6 | 3825 |
| Polyunsaturated fatty acids | PUFA | 39.9 | 2657 | 37.1 | 1568 | 36.1 | 2463 |
| Omega 3 (ω3) | |||||||
| Omega 3 | ω-3 | 9.4 | 615 | 2.8 | 119 | 1.4 | 94 |
| Alpha-linolenic acid | ALA | 2.9 | 193 | 2.8 | 116 | 1.2 | 80 |
| Eicosapentaenoic acid | EPA | 1.7 | 108 | nd | nd | nd | nd |
| Docosohexaenoic acid | DHA | 2.8 | 177 | nd | nd | 0.2 | 14 |
| Docosapentaenoic acid | DPA | 0.8 | 52 | nd | nd | nd | nd |
| Omega 6 (ω6) | |||||||
| Omega 6 | ω-6 | 30.2 | 2025 | 34.3 | 1449 | 34.5 | 2356 |
| Linoleic acid | LA | 29.1 | 1952 | 34.2 | 1446 | 34.5 | 2356 |
| Ratio | |||||||
| LA/ALA | 10 | 12.2 | 28.8 | ||||
| ω -6/ω -3 | 3.2 | 12.3 | 24.6 | ||||
| C18:1/C16:0 | 5.1 | 4.1 | 10.8 | ||||
| SFA/ω-3 | 1.4 | 5.4 | 5.9 | ||||
| C16:0/SFA | 0.6 | 0.8 | 0.6 | ||||
| C16:0/ALA | 2.8 | 4.1 | 4.3 | ||||
| PUFA/ALA | 13.8 | 13.3 | 30.1 | ||||
| SFA/ALA | 4.4 | 5.4 | 6.9 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordóñez-Gutiérrez, L.; Fábrias, G.; Casas, J.; Wandosell, F. Diets with Higher ω-6/ω-3 Ratios Show Differences in Ceramides and Fatty Acid Levels Accompanied by Increased Amyloid-Beta in the Brains of Male APP/PS1 Transgenic Mice. Int. J. Mol. Sci. 2021, 22, 10907. https://doi.org/10.3390/ijms222010907
Ordóñez-Gutiérrez L, Fábrias G, Casas J, Wandosell F. Diets with Higher ω-6/ω-3 Ratios Show Differences in Ceramides and Fatty Acid Levels Accompanied by Increased Amyloid-Beta in the Brains of Male APP/PS1 Transgenic Mice. International Journal of Molecular Sciences. 2021; 22(20):10907. https://doi.org/10.3390/ijms222010907
Chicago/Turabian StyleOrdóñez-Gutiérrez, Lara, Gemma Fábrias, Josefina Casas, and Francisco Wandosell. 2021. "Diets with Higher ω-6/ω-3 Ratios Show Differences in Ceramides and Fatty Acid Levels Accompanied by Increased Amyloid-Beta in the Brains of Male APP/PS1 Transgenic Mice" International Journal of Molecular Sciences 22, no. 20: 10907. https://doi.org/10.3390/ijms222010907
APA StyleOrdóñez-Gutiérrez, L., Fábrias, G., Casas, J., & Wandosell, F. (2021). Diets with Higher ω-6/ω-3 Ratios Show Differences in Ceramides and Fatty Acid Levels Accompanied by Increased Amyloid-Beta in the Brains of Male APP/PS1 Transgenic Mice. International Journal of Molecular Sciences, 22(20), 10907. https://doi.org/10.3390/ijms222010907