Protection of Teleost Fish against Infectious Diseases through Oral Administration of Vaccines: Update 2021
Abstract
:1. Introduction
2. Bacterial Diseases
2.1. Vibriosis
2.2. Motile Aeromonad Septicemia (MAS)
2.3. Edwardsiellosis
2.4. Yersiniosis
2.5. Lactococcosis/Streptococcosis
2.6. Furunculosis
2.7. Francisellosis
2.8. Piscirickettsiosis/Salmonid Rickettsial Septicaemia
3. Viral Diseases
3.1. Infectious Pancreas Necrosis (IPN)
3.2. Viral Nervous Necrosis
3.3. Grass Carp Reovirus (GCRV)
3.4. Spring Viremia of Carp (SVC)
3.5. Viral Haemorrhagic Septicaemia
3.6. Infectious Hematopoietic Necrosis (IHN)
3.7. Infectious Salmon Anaemia
3.8. Iridoviral Disease (IVD)
4. Parasitic Diseases
ClonorChiasis
5. Model Antigens
| Disease | Pathogen | Species | Vaccine | Reference |
|---|---|---|---|---|
| Clonorchiasis | Clonorchis sinensis | Grass carp | Recombinant | [61] |
| Ichthyophthiriosis | Ichthyophthirius multifiliis | Goldfish | [80] | |
| Rainbow trout | Nanoparticle gamma-ray irradiated vaccine | [81] | ||
| Model antigens | ||||
| Gilthead sea bream | Plasmid DNA β-galactosidase (GLB1) | [82] | ||
| [78] | ||||
| Red crucian carp | OVA model antigen | [83] | ||
| Zebrafish | GFP in Chlamydomonas reinhardtii | [77] |
6. Intestinal Immune Defense
6.1. Immune Molecules in Mucus and Bile
6.2. Anatomical Distribution of Immune Cells in the Gut
6.3. Lamina Propria Eosinophilic Granular Cells
6.4. Modulation of Intestinal Responses
6.5. Oral Tolerance
7. Conclusions and Future Direction
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mutoloki, S.; Munangandu, H.M.; Evensen, O. Oral vaccination of fish—Antigen preparations, uptake, and immune induction. Front. Immunol. 2015, 6, 519. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Atienza, E.; Diaz-Rosales, P.; Tafalla, C. Systemic and mucosal B and T cell responses upon mucosal vaccination of teleost fish. Front. Immunol. 2021, 11, 622377. [Google Scholar] [CrossRef]
- Salinas, I.; Fernandez-Montero, A.; Ding, Y.; Sunyer, J.O. Mucosal immunoglobulins of teleost fish: A decade of advances. Dev. Comp. Immunol. 2021, 121, 104079. [Google Scholar] [CrossRef]
- Xu, Z.; Takizawa, F.; Parra, D.; Gomez, D.; Jorgensen, L.V.; LaPatra, S.E.; Sunyer, J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016, 7, 10728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hordvik, I. Immunoglobulin Isotypes in Atlantic Salmon, Salmo Salar. Biomolecules 2015, 5, 166–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, N.J.; Jorgensen, P.E.V. Quantification of serum immunoglobulin in rainbow-trout salmo-gairdneri under various environmental-conditions. Dis. Aquat. Organ. 1986, 1, 183–189. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Kong, W.G.; Yin, Y.X.; Dong, F.; Huang, Z.Y.; Yin, G.M.; Dong, S.; Salinas, I.; Zhang, Y.A.; Xu, Z. Mucosal immunoglobulins protect the olfactory organ of teleost fish against parasitic infection. PLoS Pathog. 2018, 14, e1007251. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.J.; He, C.J.; Qiu, Y.Y.; Chen, J.G. Expression of Vibrio harveyi ompK in the yeast Pichia pastoris: The first step in developing an oral vaccine against vibriosis? Aquaculture 2011, 318, 268–272. [Google Scholar] [CrossRef]
- Galindo-Villegas, J.; Mulero, I.; Garcia-Alcazar, A.; Munoz, I.; Penalver-Mellado, M.; Streitenberger, S.; Scapigliati, G.; Meseguer, J.; Mulero, V. Recombinant TNF alpha as oral vaccine adjuvant protects European sea bass against vibriosis: Insights into the role of the CCL25/CCR9 axis. Fish Shellfish Immunol. 2013, 35, 1260–1271. [Google Scholar] [CrossRef]
- Sarropoulou, E.; Galindo-Villegas, J.; Garcia-Alcazar, A.; Kasapidis, P.; Mulero, V. Characterization of European sea bass transcripts by RNA SEQ after oral vaccine against v-anguillarum. Mar. Biotechnol. 2012, 14, 634–642. [Google Scholar] [CrossRef]
- Li, L.; Lin, S.L.; Deng, L.; Liu, Z.G. Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in black seabream Acanthopagrus schlegelii Bleeker to protect from Vibrio parahaemolyticus. J. Fish Dis. 2013, 36, 987–995. [Google Scholar] [CrossRef]
- Li, J.; Ma, S.Y.; Woo, N.Y.S. Vaccination of silver sea bream (Sparus sarba) against vibrio alginolyticus: Protective evaluation of different vaccinating modalities. Int. J. Mol. Sci. 2016, 17, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, G.D.; da Silva, B.C.; Vieira, F.D.; Seiffert, W.Q.; Ushizima, T.T.; Mourino, J.L.P.; Martins, M.L. Vaccination strategies with oral booster for surubim hybrid (Pseudoplatystoma corruscans × P. reticulatum) against haemorrhagic septicaemia. Aquac. Res. 2015, 46, 1831–1841. [Google Scholar] [CrossRef]
- Dubey, S.; Avadhani, K.; Mutalik, S.; Sivadasan, S.M.; Maiti, B.; Paul, J.; Girisha, S.K.; Venugopal, M.N.; Mutoloki, S.; Evensen, O.; et al. Aeromonas hydrophila OmpW PLGA Nanoparticle oral vaccine shows a dose-dependent protective immunity in rohu (Labeo rohita). Vaccines 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Chatakondi, N.; Peterson, B.C.; Greenway, T.E.; Byars, T.S.; Wise, D.J. Efficacy of a live-attenuated edwardsiella ictaluri oral vaccine in channel and hybrid catfish. J. World Aquac. Soc. 2018, 49, 686–691. [Google Scholar] [CrossRef]
- Dubey, S.; Avadhani, K.; Mutalik, S.; Sivadasan, S.M.; Maiti, B.; Girisha, S.K.; Venugopal, M.N.; Mutoloki, S.; Evensen, O.; Karunasagar, I.; et al. Edwardsiella tarda OmpA Encapsulated in chitosan nanoparticles shows superior protection over inactivated whole cell vaccine in orally vaccinated fringed-lipped peninsula carp (Labeo fimbriatus). Vaccines 2016, 4, 40. [Google Scholar] [CrossRef]
- Kole, S.; Kumari, R.; Anand, D.; Kumar, S.; Sharma, R.; Tripathi, G.; Makesh, M.; Rajendran, K.V.; Bedekar, M.K. Nanoconjugation of bicistronic DNA vaccine against Edwardsiella tarda using chitosan nanoparticles: Evaluation of its protective efficacy and immune modulatory effects in Labeo rohita vaccinated by different delivery routes. Vaccine 2018, 36, 2155–2165. [Google Scholar] [CrossRef]
- Ghosh, B.; Nguyen, T.D.; Crosbie, P.B.B.; Nowak, B.F.; Bridle, A.R. Oral vaccination of first-feeding Atlantic salmon, Salmo salar L., confers greater protection against yersiniosis than immersion vaccination. Vaccine 2016, 34, 599–608. [Google Scholar] [CrossRef]
- Romalde, J.L.; Luzardo-Alvarez, A.; Ravelo, C.; Toranzo, A.E.; Blanco-Wendez, J. Oral immunization using alginate microparticles as a useful strategy for booster vaccination against fish lactoccocosis. Aquaculture 2004, 236, 119–129. [Google Scholar] [CrossRef]
- Halimi, M.; Alishahi, M.; Abbaspour, M.R.; Ghorbanpoor, M.; Tabandeh, M.R. Efficacy of a Eudragit L30D-55 encapsulated oral vaccine containing inactivated bacteria (Lactococcus garvieae/Streptococcus iniae) in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 81, 430–437. [Google Scholar] [CrossRef]
- Nur-Nazifah, M.; Sabri, M.Y.; Siti-Zahrah, A. Development and efficacy of feed-based recombinant vaccine encoding the cell wall surface anchor family protein of Streptococcus agalactiae against streptococcosis in Oreochromis sp. Fish Shellfish Immunol. 2014, 37, 193–200. [Google Scholar] [CrossRef]
- Firdaus-Nawi, M.; Yusoff, S.M.; Yusof, H.; Abdullah, S.Z.; Zamri-Saad, M. Efficacy of feed-based adjuvant vaccine against Streptococcus agalactiae in Oreochromis spp. in Malaysia. Aquac. Res. 2013, 45, 87–96. [Google Scholar] [CrossRef]
- Hayat, M.; Yusoff, M.S.M.; Samad, M.J.; Razak, I.S.A.; Yasin, I.S.M.; Thompson, K.D.; Hasni, K. Efficacy of feed-based formalin-killed vaccine of streptococcus iniae stimulates the gut-associated lymphoid tissues and immune response of red hybrid tilapia. Vaccines 2021, 9, 51. [Google Scholar] [CrossRef]
- Monir, M.S.; Yusoff, S.B.; Zulperi, Z.B.M.; Abu Hassim, H.B.; Mohamad, A.; Ngoo, M.S.B.H.; Ina-Salwany, M.Y. Haemato-immunological responses and effectiveness of feed-based bivalent vaccine against Streptococcus iniaeand Aeromonas hydrophilainfections in hybrid red tilapia (Oreochromis mossambicus × O. niloticus). BMC Vet. Res. 2020, 16, 226. [Google Scholar] [CrossRef] [PubMed]
- Maurice, S.; Nussinovitch, A.; Jaffe, N.; Shoseyov, O.; Gertler, A. Oral immunization of Carassius auratus with modified recombinant A-layer proteins entrapped in alginate beads. Vaccine 2004, 23, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Santos, Y.; Garcia-Marquez, S.; Pereira, P.G.; Pazos, F.; Riaza, A.; Silva, R.; El Morabit, A.; Ubeira, F.M. Efficacy of furunculosis vaccines in turbot, Scophthalmus maximus (L.): Evaluation of immersion, oral and injection delivery. J. Fish Dis. 2005, 28, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Attaya, A.; Secombes, C.J.; Wang, T.H. Effective isolation of GALT cells: Insights into the intestine immune response of rainbow trout (Oncorhynchus mykiss) to different bacterin vaccine preparations. Fish Shellfish Immunol. 2020, 105, 378–392. [Google Scholar] [CrossRef]
- Hoare, R.; Leigh, W.; Limakom, T.; Wongwaradechkul, R.; Metselaar, M.; Shinn, A.P.; Ngo, T.P.H.; Thompson, K.D.; Adams, A. Oral vaccination of Nile tilapia (Oreochromis niloticus) against francisellosis elevates specific antibody titres in serum and mucus. Fish Shellfish Immunol. 2021, 113, 86–88. [Google Scholar] [CrossRef]
- Sotomayor-Gerding, D.; Troncoso, J.M.; Pino, A.; Almendras, F.; Diaz, M.R. Assessing the immune response of atlantic salmon (Salmo salar) after the oral intake of alginate-encapsulated piscirickettsia salmonis antigens. Vaccines 2020, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Tobar, I.; Arancibia, S.; Torres, C.; Vera, V.; Soto, P.; Carrasco, C.; Alvarado, M.; Neira, E.; Arcos, S.; Tobar, J.A. Successive oral immunizations against Piscirickettsia salmonis and infectious salmon anemia virus are required to maintain a long-term protection in farmed salmonids. Front. Immunol. 2015, 6, 244. [Google Scholar] [CrossRef] [Green Version]
- Akhlaghi, M. Passive immunisation of fish against vibriosis, comparison of intraperitoneal, oral and immersion routes. Aquaculture 1999, 180, 191–205. [Google Scholar] [CrossRef]
- Gao, P.; Xia, G.X.; Bao, Z.X.; Feng, C.; Cheng, X.J.; Kong, M.; Liu, Y.; Chen, X.G. Chitosan based nanoparticles as protein carriers for efficient oral antigen delivery. Int. J. Biol. Macromol. 2016, 91, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Asha, A.; Nayak, D.K.; Shankar, K.M.; Mohan, C.V. Antigen expression in biofilm cells of Aeromonas hydrophila employed in oral vaccination of fish. Fish Shellfish Immunol. 2004, 16, 429–436. [Google Scholar] [CrossRef]
- Tu, F.P.; Chu, W.H.; Zhuang, X.Y.; Lu, C.P. Effect of oral immunization with Aeromonas hydrophila ghosts on protection against experimental fish infection. Lett. Appl. Microbiol. 2010, 50, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, M.S.; Kim, K.H. Protection of olive flounder (Paralichthys olivaceus) against Edwardsiella tarda infection by oral administration of auxotrophic mutant E. tarda (Delta alr Delta asd E. tarda). Aquaculture 2011, 317, 48–52. [Google Scholar] [CrossRef]
- Villumsen, K.R.; Neumann, L.; Ohtani, M.; Strom, H.K.; Raida, M.K. Oral and anal vaccination confers full protection against enteric redmouth disease (ERM) in rainbow trout. PLoS ONE 2014, 9, e93845. [Google Scholar] [CrossRef] [PubMed]
- Munangandu, H.M.; Paul, J.; Evensen, O. An overview of vaccination strategies and antigen delivery systems for streptococcus agalactiae vaccines in Nile Tilapia (Oreochromis niloticus). Vaccines 2016, 4, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, E.L.; Wang, X.L.; Wang, K.Y.; He, J.; Zhu, L.; He, Y.; Chen, D.F.; Ouyang, P.; Geng, Y.; Huang, X.L.; et al. Preparation, characterization and evaluation of the immune effect of alginate/chitosan composite microspheres encapsulating recombinant protein of Streptococcus iniae designed for fish oral vaccination. Fish Shellfish Immunol. 2018, 73, 262–271. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Z.Z.; Hu, C.H.; Bellis, S.L.; Yang, W.D.; Su, Y.T.; Zhang, X.Y.; Wu, Y.K. Controlled and targeted release of antigens by intelligent shell for improving applicability of oral vaccines. Biomaterials 2016, 77, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Tobar, J.A.; Jerez, S.; Caruffo, M.; Bravo, C.; Contreras, F.; Bucarey, S.A.; Harel, M. Oral vaccination of Atlantic salmon (Salmo salar) against salmonid rickettsial septicaemia. Vaccine 2011, 29, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Kang, Y.H.; Chen, L.; Siddiqui, S.A.; Wang, C.F.; Qian, A.D.; Shan, X.F. Oral immunization with recombinant Lactobacillus casei expressing OmpAI confers protection against Aeromonas veronii challenge in common carp, Cyprinus carpio. Fish Shellfish Immunol. 2018, 72, 552–563. [Google Scholar] [CrossRef]
- Wise, D.J.; Greenway, T.E.; Byars, T.S.; Griffin, M.J.; Khoo, L.H. Oral Vaccination of channel catfish against enteric septicemia of catfish using a live attenuated edwardsiella ictaluri isolate. J. Aquat. Anim. Health 2015, 27, 135–143. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Soltani, M.; Behdani, M.; Evensen, O.; Alirahimi, E.; Hassanzadeh, R.; Soltani, E. Oral DNA vaccines based on CS-TPP nanoparticles and alginate microparticles confer high protection against infectious pancreatic necrosis virus (IPNV) infection in trout. Dev. Comp. Immunol. 2017, 74, 178–189. [Google Scholar] [CrossRef]
- De las Heras, A.I.; Saint-Jean, S.R.; Perez-Prieto, S.I. Immunogenic and protective effects of an oral DNA vaccine against infectious pancreatic necrosis virus in fish. Fish Shellfish Immunol. 2010, 28, 562–570. [Google Scholar] [CrossRef]
- Ballesteros, N.A.; Saint-Jean, S.S.R.; Perez-Prieto, S.I.; Coll, J.M. Trout oral VP2 DNA vaccination mimics transcriptional responses occurring after infection with infectious pancreatic necrosis virus (IPNV). Fish Shellfish Immunol. 2012, 33, 1249–1257. [Google Scholar] [CrossRef]
- Ballesteros, N.A.; Castro, R.; Abos, B.; Saint-Jean, S.S.R.; Perez-Prieto, S.I.; Tafalla, C. The pyloric caeca area is a major site for IgM(+) and IgT(+) B cell recruitment in response to oral vaccination in rainbow trout. PLoS ONE 2013, 8, e66118. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.H.; Klaric, G.; Wadsworth, S.; Jayasinghe, S.; Kuo, T.Y.; Evensen, O.; Mutoloki, S. Augmentation of the antibody response of atlantic salmon by oral administration of alginate-encapsulated IPNV Antigens. PLoS ONE 2014, 9, e109337. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Ramirez, C.; Ñancucheo, I.; Villegas, R.; Schaffeld, G.; Kriman, L.; Gonzalez, J.; Oyarzun, P. A novel “in-feed” delivery platform applied for oral DNA vaccination against IPNV enables high protection in Atlantic salmon (Salmon salar). Vaccine 2017, 35, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, N.A.; St-Jean, S.R.; Perez-Prieto, S.I. Food pellets as an effective delivery method for a DNA vaccine against infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss, Walbaum). Fish Shellfish Immunol. 2014, 37, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Shih, C.H.; Liu, H.C.; Wu, C.L.; Lin, C.C.; Wang, H.C.; Chen, T.Y.; Yang, H.L.; Lin, J.H.Y. An oral nervous necrosis virus vaccine using Vibrio anguillarum as an expression host provides early protection. Aquaculture 2011, 321, 26–33. [Google Scholar] [CrossRef]
- Gonzalez-Silvera, D.; Guardiola, F.A.; Espinosa, C.; Chaves-Pozo, E.; Esteban, M.A.; Cuesta, A. Recombinant nodavirus vaccine produced in bacteria and administered without purification elicits humoral immunity and protects European sea bass against infection. Fish Shellfish Immunol. 2019, 88, 458–463. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, J.H.Y.; Chen, M.S.; Yang, H.L. An oral nervous necrosis virus vaccine that induces protective immunity in larvae of grouper (Epinephelus coioides). Aquaculture 2007, 268, 265–273. [Google Scholar] [CrossRef]
- Kai, Y.H.; Wu, Y.C.; Chi, S.C. Immune gene expressions in grouper larvae (Epinephelus coioides) induced by bath and oral vaccinations with inactivated betanodavirus. Fish Shellfish Immunol. 2014, 40, 563–569. [Google Scholar] [CrossRef]
- Wi, G.R.; Hwang, J.Y.; Kwon, M.G.; Kim, H.J.; Kang, H.A.; Kim, H.J. Protective immunity against nervous necrosis virus in convict grouper Epinephelus septemfasciatus following vaccination with virus-like particles produced in yeast Saccharomyces cerevisiae. Vet. Microbiol. 2015, 177, 214–218. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, H.J.; Lan, N.T.; Han, H.J.; Lee, D.C.; Hwang, J.Y.; Kwon, M.G.; Kang, B.K.; Han, S.Y.; Moon, H.; et al. Oral vaccination through voluntary consumption of the convict grouper Epinephelus septemfasciatus with yeast producing the capsid protein of red-spotted grouper nervous necrosis virus. Vet. Microbiol. 2017, 204, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.H.; Wu, S.Y.; Lin, C.H. Oral immunization with cell-free self-assembly virus-like particles against orange-spotted grouper nervous necrosis virus in grouper larvae, Epinephelus coioides. Vet. Immunol. Immunopathol. 2018, 197, 69–75. [Google Scholar] [CrossRef]
- Vimal, S.; Majeed, S.A.; Nambi, K.S.N.; Madan, N.; Farook, M.A.; Venkatesan, C.; Taju, G.; Venu, S.; Subburaj, R.; Thirunavukkarasu, A.R.; et al. Delivery of DNA vaccine using chitosan–tripolyphosphate (CS/TPP) nanoparticles in Asian sea bass, Lates calcarifer (Bloch, 1790) for protection against nodavirus infection. Aquaculture 2014, 420–421, 240–246. [Google Scholar] [CrossRef]
- Cho, H.S.; Seo, J.Y.; Park, S.I.; Kim, T.G.; Kim, T.J. Oral immunization with recombinant protein antigen expressed in tobacco against fish nervous necrosis virus. J. Vet. Med. Sci. 2018, 80, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, A.Y.; Yamashita, H.; Istiqomah, I.; Kawato, Y.; Ninomiya, K.; Younes, A.; Nakai, T. An oral vaccination method with the aid of capsaicin against viral nervous necrosis (VNN). Fish Pathol. 2018, 53, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Valero, Y.; Awad, E.; Buonocore, F.; Arizcun, M.; Esteban, M.A.; Meseguer, J.; Chaves-Pozo, E.; Cuesta, A. An oral chitosan DNA vaccine against nodavirus improves transcription of cell-mediated cytotoxicity and interferon genes in the European sea bass juveniles gut and survival upon infection. Dev. Comp. Immunol. 2016, 65, 64–72. [Google Scholar] [CrossRef]
- Sun, H.C.; Shang, M.; Tang, Z.L.; Jiang, H.Y.; Dong, H.M.; Zhou, X.Y.; Lin, Z.P.; Shi, C.B.; Ren, P.L.; Zhao, L.; et al. Oral delivery of Bacillus subtilis spores expressing Clonorchis sinensis paramyosin protects grass carp from cercaria infection. Appl. Microbiol. Biotechnol. 2020, 104, 1633–1646. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, M.; Chen, H.; Wei, Y.; Ning, D.G. Germination-arrest bacillus subtilis spores as an oral delivery vehicle of grass carp reovirus (GCRV) Vp7 antigen augment protective immunity in grass carp (Ctenopharyngodon idella). Genes 2020, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xu, H.; He, Y.; Li, J. Protection of grass carp, Ctenopharyngon idellus (Valenciennes), through oral administration of a subunit vaccine against reovirus. J. Fish Dis. 2011, 34, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.C.; Guan, X.T.; Liu, Z.M.; Tian, C.Y.; Xu, Y.G. Recombinant lactobacillus expressing G protein of spring viremia of carp virus (SVCV) combined with ORF81 protein of koi herpesvirus (KHV): A promising way to induce protective immunity against SVCV and KHV infection in cyprinid fish via oral vaccination. Vaccine 2015, 33, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Zhou, K.; Pan, R.H.; Wei, J.; Liu, Z.M.; Xu, Y.G. Oral immunization of carps with chitosan-alginate microcapsule containing probiotic expressing spring viremia of carp virus (SVCV) G protein provides effective protection against SVCV infection. Fish Shellfish Immunol. 2020, 105, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Kang, B.J.; Park, S.I.; Kim, T.J. Development of an oral vaccine using recombinant viral haemorrhagic septicaemia virus glycoproteins produced in tobacco. Vet. Med. 2019, 64, 456–461. [Google Scholar] [CrossRef]
- Ballesteros, N.A.; Alonso, M.; Saint-Jean, S.R.; Perez-Prieto, S.I. An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dose-dependent immune responses and significant protection in rainbow trout (Oncorrhynchus mykiss). Fish Shellfish Immunol. 2015, 45, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Aravena, A.; Fuentes, Y.; Cartagena, J.; Brito, T.; Poggio, V.; La Torre, J.; Mendoza, H.; Gonzalez-Nilo, F.; Sandino, A.M.; Spencer, E. Development of a nanoparticle-based oral vaccine for Atlantic salmon against ISAV using an alphavirus replicon as adjuvant. Fish Shellfish Immunol. 2015, 45, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Chung, H.J.; Kim, T.J. Codon-optimized expression of fish iridovirus capsid protein in yeast and its application as an oral vaccine candidate. J. Fish Dis. 2013, 36, 763–768. [Google Scholar] [CrossRef]
- Shin, Y.J.; Kwon, T.H.; Seo, J.Y.; Kim, T.J. Oral immunization of fish against iridovirus infection using recombinant antigen produced from rice callus. Vaccine 2013, 31, 5210–5215. [Google Scholar] [CrossRef]
- Chen, L.H.; Evensen, O.; Mutoloki, S. IPNV Antigen uptake and distribution in atlantic salmon following oral administration. Viruses 2015, 7, 2507–2517. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.D.; Yao, Y.Y.; Cui, Z.W.; Zhang, X.Y.; Guo, X.; Zhou, Y.Y.; Zhang, Y.A. Comparative study of the immunoprotective effect of two grass carp-sourced Bacillus subtilis spore-based vaccines against grass carp reovirus. Aquaculture 2019, 504, 88–95. [Google Scholar] [CrossRef]
- Enzmann, P.J.; Fichtner, D.; Schutze, H.; Walliser, G. Development of vaccines against VHS and IHN: Oral application, molecular marker and discrimination of vaccinated fish from infected populations. J. Appl. Ichthyol. 1998, 14, 179–183. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Xu, L.M.; Liu, M.; Cao, Y.S.; LaPatra, S.E.; Yin, J.S.; Liu, H.B.; Lu, T.Y. Preliminary study of an oral vaccine against infectious hematopoietic necrosis virus using improved yeast surface display technology. Mol. Immunol. 2017, 85, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Speare, D.J.; Beaman, H.J.; Jones, S.R.M.; Markham, R.J.F.; Arsenault, G.J. Induced resistance in rainbow trout, Oncorhynchus mykiss (Walbaum), to gill disease associated with the microsporidian gill parasite Loma salmonae. J. Fish Dis. 1998, 21, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Chen, T.J.; Sun, H.C.; Tang, Z.L.; Yu, J.Y.; Lin, Z.P.; Ren, P.L.; Zhou, X.Y.; Huang, Y.; Li, X.R.; et al. Immune response induced by oral delivery of Bacillus subtilis spores expressing enolase of Clonorchis sinensis in grass carps (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2017, 60, 318–325. [Google Scholar] [CrossRef]
- Kwon, K.C.; Lamb, A.; Fox, D.; Jegathese, S.J.P. An evaluation of microalgae as a recombinant protein oral delivery platform for fish using green fluorescent protein (GFP). Fish Shellfish Immunol. 2019, 87, 414–420. [Google Scholar] [CrossRef]
- Saez, M.I.; Vizcaino, A.J.; Alarcon, F.J.; Martinez, T.F. Feed pellets containing chitosan nanoparticles as plasmid DNA oral delivery system for fish: In vivo assessment in gilthead sea bream (Sparus aurata) juveniles. Fish Shellfish Immunol. 2018, 80, 458–466. [Google Scholar] [CrossRef]
- Sato, A.; Okamoto, N. Characterization of the cell-mediated cytotoxic responses of isogeneic ginbuna crucian carp induced by oral immunisation with hapten-modified cellular antigens. Fish Shellfish Immunol. 2008, 24, 684–692. [Google Scholar] [CrossRef]
- Yao, J.Y.; Yuan, X.M.; Xu, Y.; Yin, W.L.; Lin, L.Y.; Pan, X.Y.; Yang, G.L.; Wang, C.F.; Shen, J.Y. Live recombinant Lactococcus lactis vaccine expressing immobilization antigen (i-Ag) for protection against Ichthyophthirius multifiliis in goldfish. Fish Shellfish Immunol. 2016, 58, 302–308. [Google Scholar] [CrossRef]
- Heidarieh, M.; Moodi, S.; Katuli, K.K.; Unger, H. Biochemical Effects of encapsulated radiovaccine via alginate nanoparticles as useful strategy for booster in immunized rainbow trout against ichthyophytirius multifiliis. Acta Sci. Vet. 2015, 43, 1330. [Google Scholar]
- Saez, M.I.; Vizcaino, A.J.; Alarcon, F.J.; Martinez, T.F. Comparison of lacZ reporter gene expression in, gilthead sea bream (Sparus aurata) following oral or intramuscular administration of plasmid DNA in chitosan nanoparticles. Aquaculture 2017, 474, 1–10. [Google Scholar] [CrossRef]
- Yan, N.N.; Xu, K.; Li, X.Y.; Liu, Y.W.; Bai, Y.C.; Zhang, X.H.; Han, B.Q.; Chen, Z.L.; Zhang, Z.Y. Recombinant Saccharomyces cerevisiae serves as novel carrier for oral DNA vaccines in Carassius auratus. Fish Shellfish Immunol. 2015, 47, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Song, Y.L.; Wang, B.; Zhang, X.Y.; Zhang, X.J.; Wang, Y.L.; Cheng, Y.Y.; Chen, D.D.; Xia, X.Q.; Lu, Y.S.; et al. Fish gut-liver immunity during homeostasis or inflammation revealed by integrative transcriptome and proteome studies. Sci. Rep. 2016, 6, 36048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2020, 13, 642–663. [Google Scholar] [CrossRef]
- Nadal, A.L.; Ikeda-Ohtsubo, W.; Sipkema, D.; Peggs, D.; McGurk, C.; Forlenza, M.; Wiegertjes, G.F.; Brugman, S. Feed, microbiota, and gut immunity: Using the zebrafish model to understand fish health. Front. Immunol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Sitja-Bobadilla, A.; Estensoro, I.; Perez-Sanchez, J. Immunity to gastrointestinal microparasites of fish. Dev. Comp. Immunol. 2016, 64, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol 2016, 64, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dezfuli, B.S.; Bosi, G.; DePasquale, J.A.; Manera, M.; Giari, L. Fish innate immunity against intestinal helminths. Fish Shellfish Immunol. 2016, 50, 274–287. [Google Scholar] [CrossRef]
- Scapigliati, G.; Fausto, A.M.; Picchietti, S. Fish Lymphocytes: An evolutionary equivalent of mammalian innate-like lymphocytes? Front. Immunol. 2018, 9, 971. [Google Scholar] [CrossRef] [Green Version]
- Brinchmann, M.F.; Patel, D.M.; Pinto, N.; Iversen, M.H. Functional aspects of fish mucosal lectinsinteraction with non-self. Molecules 2018, 23, 1119. [Google Scholar] [CrossRef] [Green Version]
- Lokka, G.; Austbo, L.; Falk, K.; Bjerkas, I.; Koppang, E.O. Intestinal morphology of the wild Atlantic salmon (Salmo salar). J. Morphol. 2013, 274, 859–876. [Google Scholar] [CrossRef]
- Verdile, N.; Pasquariello, R.; Scolari, M.; Scire, G.; Brevini, T.A.L.; Gandolfi, F. A detailed study of rainbow trout (Onchorhynchus mykiss) intestine revealed that digestive and absorptive functions are not linearly distributed along its length. Animals 2020, 10, 745. [Google Scholar] [CrossRef]
- Tafalla, C.; Leal, E.; Yamaguchi, T.; Fischer, U. T cell immunity in the teleost digestive tract. Dev. Comp. Immunol. 2016, 64, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Loken, O.M.; Bjorgen, H.; Hordvik, I.; Koppang, E.O. A teleost structural analogue to the avian bursa of Fabricius. J. Anat. 2020, 236, 798–808. [Google Scholar] [CrossRef]
- Inami, M.; Taverne-Thiele, A.J.; Schroder, M.B.; Kiron, V.; Rombout, J.H.W.M. Immunological differences in intestine and rectum of Atlantic cod (Gadus morhua L.). Fish Shellfish Immunol. 2009, 26, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Willms, R.J.; Hocking, J.C.; Foley, E. A Cell Atlas of Microbe-Responsive Processes in the Zebrafish Intestine; Cell Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Casteleyn, C.; Van den Broeck, W.; Gebert, A.; Tambuyzer, B.R.; Van Cruchten, S.; Van Ginneken, C. M cell specific markers in man and domestic animals: Valuable tools in vaccine development. Comp. Immunol. Microb. 2013, 36, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Wang, Q.C.; Huang, Z.Y.; Ding, L.G.; Xu, Z. Immunoglobulins, mucosal immunity and vaccination in teleost fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Sfacteria, A.; Brines, M.; Blank, U. The mast cell plays a central role in the immune system of teleost fish. Mol. Immunol. 2015, 67, 668. [Google Scholar] [CrossRef]
- Reite, O.B.; Evensen, O. Inflammatory cells of teleostean fish: A review focusing on mast cells/eosinophilic granule cells and rodlet cells. Fish Shellfish Immunol. 2006, 20, 192–208. [Google Scholar] [CrossRef]
- Manera, M.; Giammarino, A.; Borreca, C.; Giari, L.; Dezfuli, B.S. Degranulation of mast cells due to compound 48/80 induces concentration-dependent intestinal contraction in rainbow trout (Oncorhynchus mykiss Walbaum) ex vivo. J. Exp. Zool Part. A 2011, 315, 447–457. [Google Scholar] [CrossRef]
- Reite, O.B.; Evensen, O. Mast-cells in the swimbladder of atlantic salmon salmo-salar—Histochemistry and responses to compound 48/80 and formalin-inactivated aeromonas-salmonicida. Dis. Aquat. Organ. 1994, 20, 95–100. [Google Scholar] [CrossRef]
- Mulero, I.; Sepulcre, M.P.; Meseguer, J.; Garcia-Ayala, A.; Mulero, V. Histamine is stored in mast cells of most evolutionarily advanced fish and regulates the fish inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 19434–19439. [Google Scholar] [CrossRef] [Green Version]
- Booman, M.; Forster, I.; Vederas, J.C.; Groman, D.B.; Jones, S.R.M. Soybean meal-induced enteritis in Atlantic salmon (Salmo salar) and Chinook salmon (Oncorhynchus tshawytscha) but not in pink salmon (O-gorbuscha). Aquaculture 2018, 483, 238–243. [Google Scholar] [CrossRef]
- Abdelhamed, H.; Ibrahim, I.; Baumgartner, W.; Lawrence, M.L.; Karsi, A. Characterization of histopathological and ultrastructural changes in channel catfish experimentally infected with virulent aeromonas hydrophila. Front. Microbiol. 2017, 8, 1519. [Google Scholar] [CrossRef]
- Declercq, A.M.; Chiers, K.; Van den Broeck, W.; Dewulf, J.; Eeckhaut, V.; Cornelissen, M.; Bossier, P.; Haesebrouck, F.; Decostere, A. Interactions of highly and low virulent Flavobacterium columnare isolates with gill tissue in carp and rainbow trout. Vet. Res. 2015, 46, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silphaduang, U.; Colorni, A.; Noga, E.J. Evidence for widespread distribution of piscidin antimicrobial peptides in teleost fish. Dis. Aquat. Organ. 2006, 72, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Lui, A.; Giari, L.; Pironi, F.; Manera, M.; Lorenzoni, M.; Noga, E.J. Piscidins in the intestine of European perch, Perca fluviatilis, naturally infected with an enteric worm. Fish Shellfish Immunol. 2013, 35, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Da’as, S.; Teh, E.M.; Dobson, J.T.; Nasrallah, G.K.; McBride, E.R.; Wang, H.; Neuberg, D.S.; Marshall, J.S.; Lin, T.J.; Berman, J.N. Zebrafish mast cells possess an Fc epsilon RI-like receptor and participate in innate and adaptive immune responses. Dev. Comp. Immunol. 2011, 35, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Zhao, Y.T.; Cao, S.L.; Wang, H.; Zhang, J.J. Integrated transcriptomic and proteomic analyses of grass carp intestines after vaccination with a double-targeted DNA vaccine of Vibrio mimicus. Fish Shellfish Immunol. 2020, 98, 641–652. [Google Scholar] [CrossRef]
- Mohamad, A.; Zamri-Saad, M.; Amal, M.N.A.; Al-saari, N.; Monir, M.S.; Chin, Y.K.; Yasin, I.S.M. Vaccine efficacy of a newly developed feed-based whole-cell polyvalent vaccine against vibriosis, streptococcosis and motile aeromonad septicemia in asian seabass, lates calcarifer. Vaccines 2021, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Marana, M.H.; Chettri, J.K.; Salten, M.B.; Bach-Olesen, N.E.; Kania, P.W.; Dalsgaard, I.; Buchmann, K. Primary immunization using low antigen dosages and immunological tolerance in rainbow trout. Fish Shellfish Immunol. 2020, 105, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Joosten, P.H.M.; Engelsma, M.Y.; van der Zee, M.D.; Rombout, J.H.W.M. Induction of oral tolerance in carp (Cyprinus carpio L) after feeding protein antigens. Vet. Immunol. Immunopathol. 1997, 60, 187–196. [Google Scholar] [CrossRef]
- Joosten, P.H.M.; Avilestrigueros, M.; Sorgeloos, P.; Rombout, J.H.W.M. Oral vaccination of juvenile carp (Cyprinus-Carpio) and gilthead seabream (Sparus-Aurata) with bioencapsulated vibrio-anguillarum bacterin. Fish Shellfish Immunol. 1995, 5, 289–299. [Google Scholar] [CrossRef]
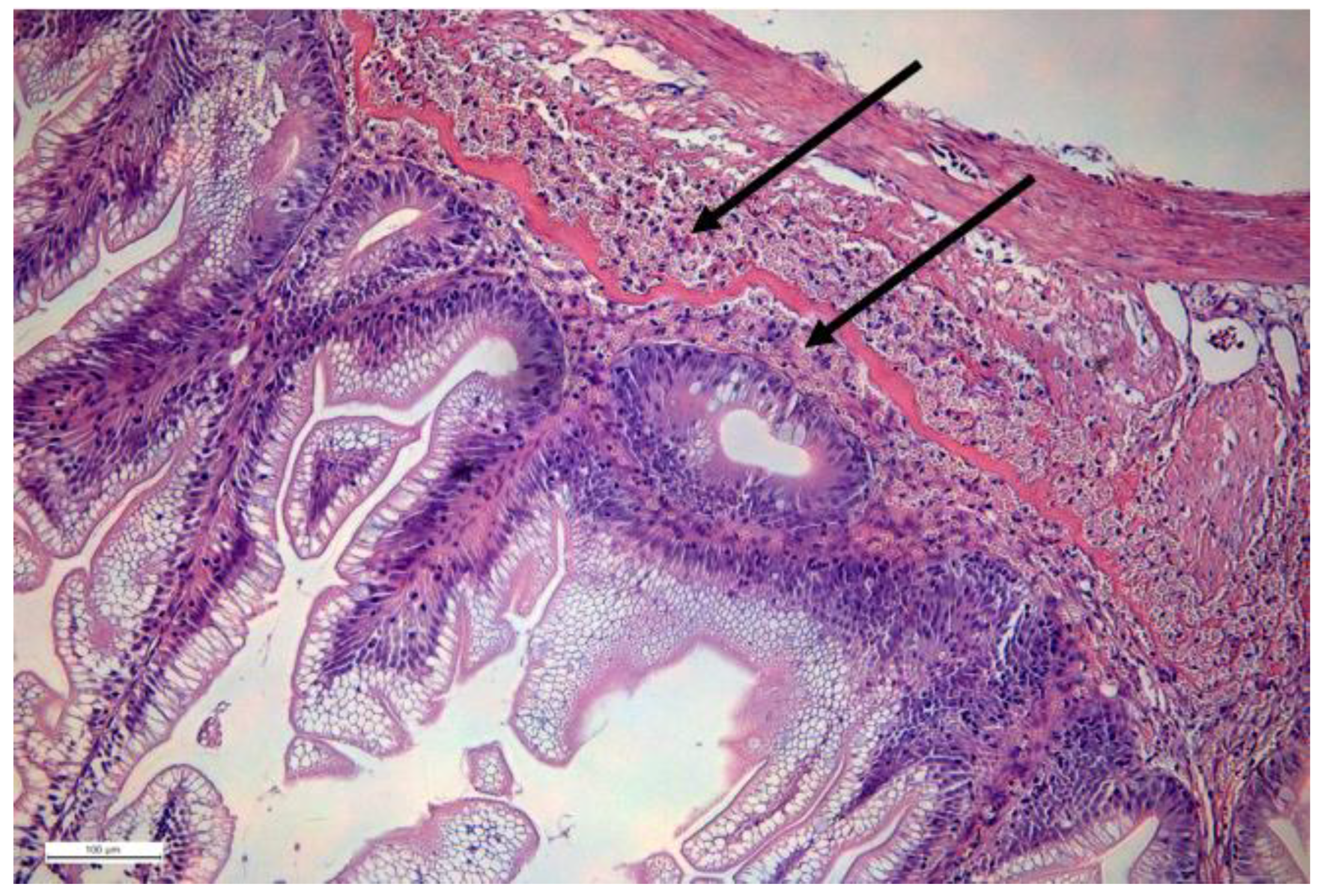
| Disease | Pathogen | Fish Species | Vaccine | Reference |
|---|---|---|---|---|
| Vibriosis | V. anguillarum | Rainbow trout | Anti-V. anguillarum antiserum | [31] |
| Turbot | Extracellular products | [32] | ||
| V. alginolyticus | Silver sea bream | Inactivated | [12] | |
| V. anguillarum | European sea bass | Aquavac Vibrio oral, ISPAH, commercial | [9] | |
| V. parahaemolyticus | Black seabream | DNA in chitosan particles | [11] | |
| V. anguillarum | European sea bass | Commercial Aquavac Vibrio oral | [10] | |
| V. harveyi | Sea bass | Recombinant | [8] | |
| Motile aeromonad septicaemia (MAS) | A. hydrophila | LPS+S-layer protein | [33] | |
| Surubim hybrid | Inactivated bacterin | [13] | ||
| Rohu | Recombinant | [14] | ||
| Haemorrhagic septicaemia | Carp | A. hydrophila ghosts | [34] | |
| Enteric septicemia of carp (ESC) | E. ictaluri | Channel & hybrid catfish | Live attenuated | [15] |
| Edwardsiellosis | E. tarda | Olive flounder | Mutated bacteria | [35] |
| Rohu | DNA in chitosan | [17] | ||
| Fringed-lipped peninsula carp | Recombinant outer membrane protein A in chitosan | [16] | ||
| Yersiniosis | Y. ruckeri | Atlantic salmon | Y. ruckeri lysate encapsulation in alginate | [18] |
| Rainbow trout | Bacterins | [27] | ||
| Enteric redmouth disease (ERM) | AquaVac ERM Oral vet (Merck) | [36] | ||
| Lactococcosis Streptococcosis | L. garviae S. iniae | Eudragit-coated bacteria | [20] | |
| Streptococcosis | S. agalactiae | Nile tilapia | Live attenuated/DNA vaccine | [37] |
| Red tilapia | Inactivated | [22] | ||
| S. iniae | Channel catfish | Recombinant protein in alginate/chitosan | [38] | |
| S. agalactiae | Tilapia | Surface immunogenic protein (SIP) from group B Streptococcus in PMMMA-PLGA | [39] | |
| S. iniae | Red tilapia | Inactivated | [23] | |
| Streptococcosis Motile aeromonad septicemia (MAS) | S. iniae A. hydrophila | Hybrid tilapia | [24] | |
| Lactococcosis | L. garviae | Rainbow trout | [19] | |
| Furunculosis | A. salmonicida | Bacterin | [27] | |
| Turbot | Commercial furunculosis vaccine, Aquavac Furovac | [26] | ||
| Salmon rickettsial septicaemia (SRS) | P. salmonis | Atlantic salmon | Providean Aquatech 1 Anasac in alginate | [29] |
| P. salmonis PS2C field strain in MicroMatrix | [40] | |||
| Salmonid rickettsial septicaemia Infectious salmon anaemia | P. salmonis ISA virus | Atlantic salmon, rainbow trout and coho salmon | Commercial vaccines | [30] |
| Epizootic ulcerative syndrome | A. veronii | Common carp | Recombinant OmpAI | [41] |
| Enteric septicaemia of catfish | E. ictaluri | Channel catfish | Live attenuated E. ictaluri | [42] |
| Disease | Pathogen | Fish Species | Vaccine | Reference |
|---|---|---|---|---|
| Infectious pancreas necrosis | IPNV | Rainbow trout | DNA | [43] |
| [45] | ||||
| [67] | ||||
| [49] | ||||
| [46] | ||||
| Atlantic salmon | Alginate-encapsulated IPNV antigens | [47] | ||
| Inactivated or live IPNV | [71] | |||
| VP2 DNA vaccine | [48] | |||
| Brown trout & Rainbow trout | DNA | [44] | ||
| Nervous necrosis | Red-spotted grouper NNV (RGNNV) | Red-spotted Grouper | Recombinant | [58] |
| Nervous necrosis | NNV | Orange-spotted Grouper | Subunit | [50] |
| Viral haemorrhagic disease | Grass carp rheovirus II (GCRVII) | Grass carp | Recombinant | [72] |
| Spring viremia of carp (SVCV) | SVCV | Common carp | [64] | |
| VHS, IHN | VHS virus, IHN virus | Rainbow trout | Attenuated | [73] |
| Viral haemorrhagic septicaemia | VHS virus | Olive flounder | Recombinant | [66] |
| Viral nervous necrosis (VNN) | NNV | Grouper | Inactivated betanodavirus | [53] |
| Viral nervous necrosis | VNNV | Recombinant | [52] | |
| Viral nervous necrosis (VNN) | VNNV/ Betanodavirus | Seven-band grouper | [58] | |
| Viral nervous necrosis | Piscine nodavirus/Betanodavirus | Inactivated | [59] | |
| Viral nervous necrosis (VNN) | Nodavirus | Orange-spotted grouper | Recombinant | [56] |
| Viral nervous necrosis (VNN) | Nodavirus | European sea bass | DNA in chitosan | [60] |
| Viral nervous necrosis (VNN) | Nodavirus | Asian sea bass | DNA in chitosan- tripolyphosphate | [57] |
| Infectious salmon anaemia | ISAV | Atlantic salmon | Inactivated | [68] |
| Rock bream iridovirus (RBIV) | Rock bream | Recombinant major capsid protein | [69] | |
| Rock bream iridovirus | [70] | |||
| Infectious hematopoietic necrosis | IHNV | Rainbow trout | Yeast vaccine EBY 100/pYD1-bi-G | [74] |
| Grass carp hemorrhagic disease | Grass carp reovirus | Grass carp | Recombinant | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bøgwald, J.; Dalmo, R.A. Protection of Teleost Fish against Infectious Diseases through Oral Administration of Vaccines: Update 2021. Int. J. Mol. Sci. 2021, 22, 10932. https://doi.org/10.3390/ijms222010932
Bøgwald J, Dalmo RA. Protection of Teleost Fish against Infectious Diseases through Oral Administration of Vaccines: Update 2021. International Journal of Molecular Sciences. 2021; 22(20):10932. https://doi.org/10.3390/ijms222010932
Chicago/Turabian StyleBøgwald, Jarl, and Roy A. Dalmo. 2021. "Protection of Teleost Fish against Infectious Diseases through Oral Administration of Vaccines: Update 2021" International Journal of Molecular Sciences 22, no. 20: 10932. https://doi.org/10.3390/ijms222010932
APA StyleBøgwald, J., & Dalmo, R. A. (2021). Protection of Teleost Fish against Infectious Diseases through Oral Administration of Vaccines: Update 2021. International Journal of Molecular Sciences, 22(20), 10932. https://doi.org/10.3390/ijms222010932







