Synthesis and Characterization of Novel Succinyl Chitosan-Dexamethasone Conjugates for Potential Intravitreal Dexamethasone Delivery
Abstract
1. Introduction
2. Results and Discussion
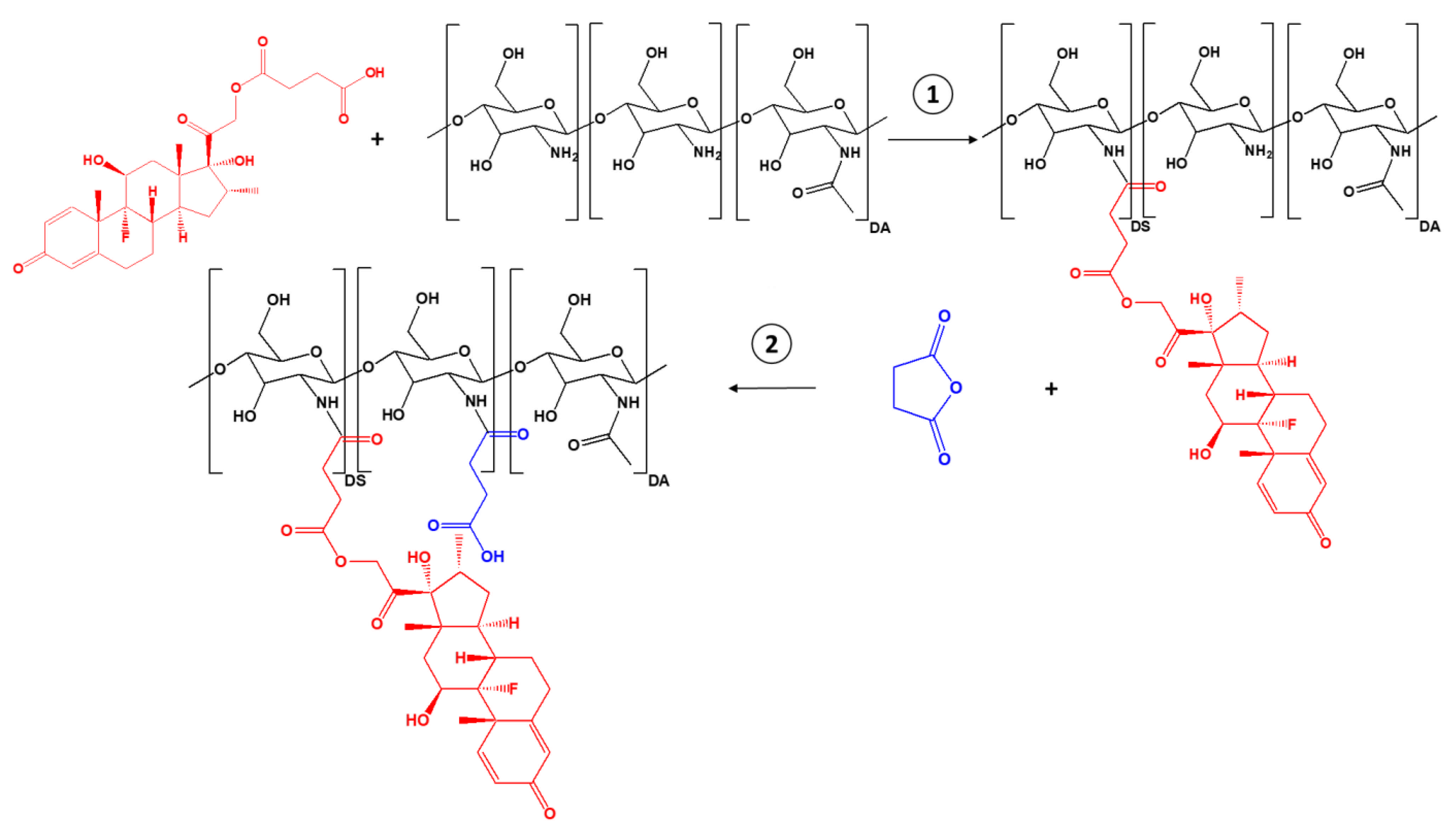
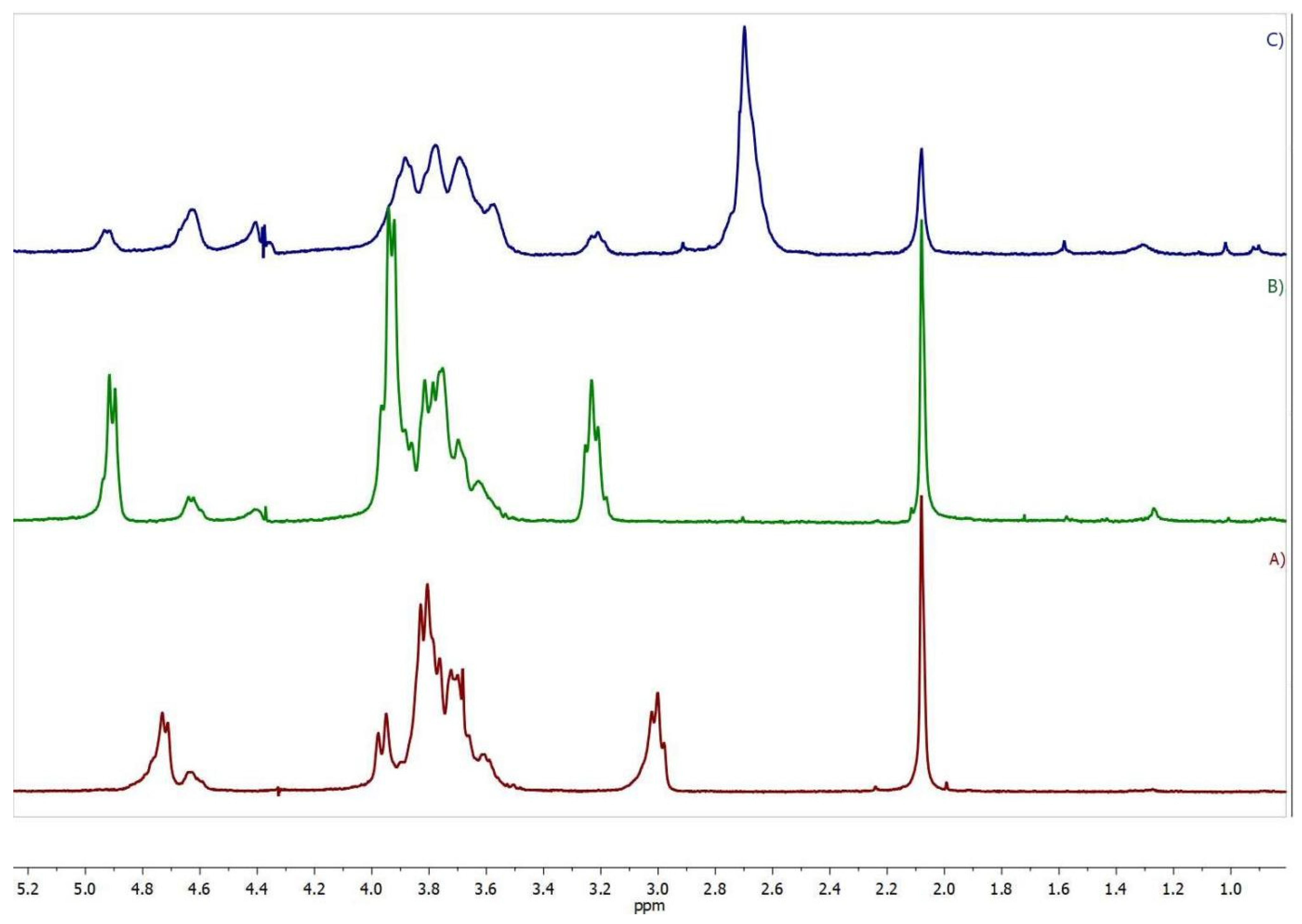
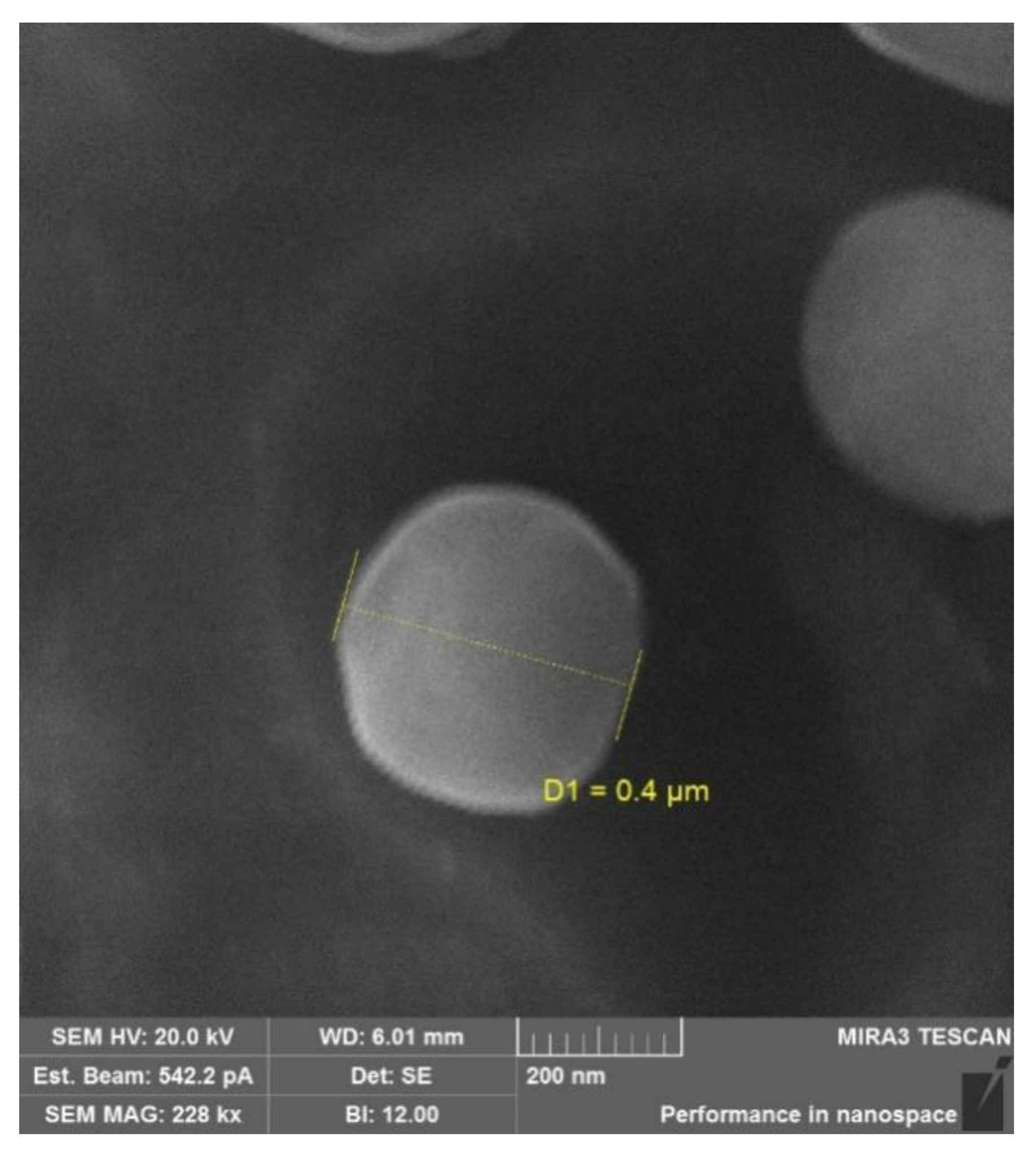
2.1. Synthesis and Characterization of the Succinyl Chitosan-Dexamethasone Conjugates (SucCS-DEX)
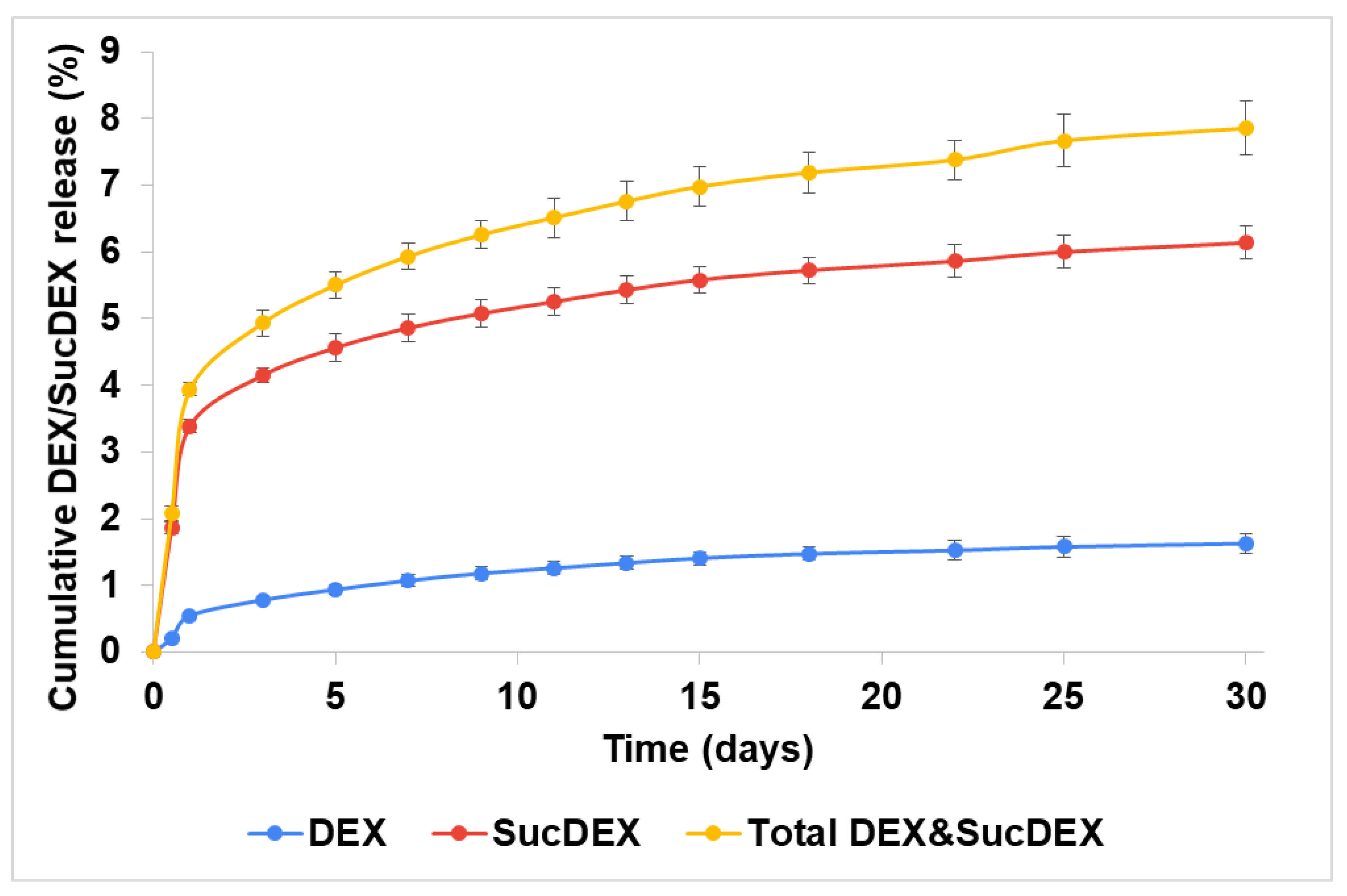
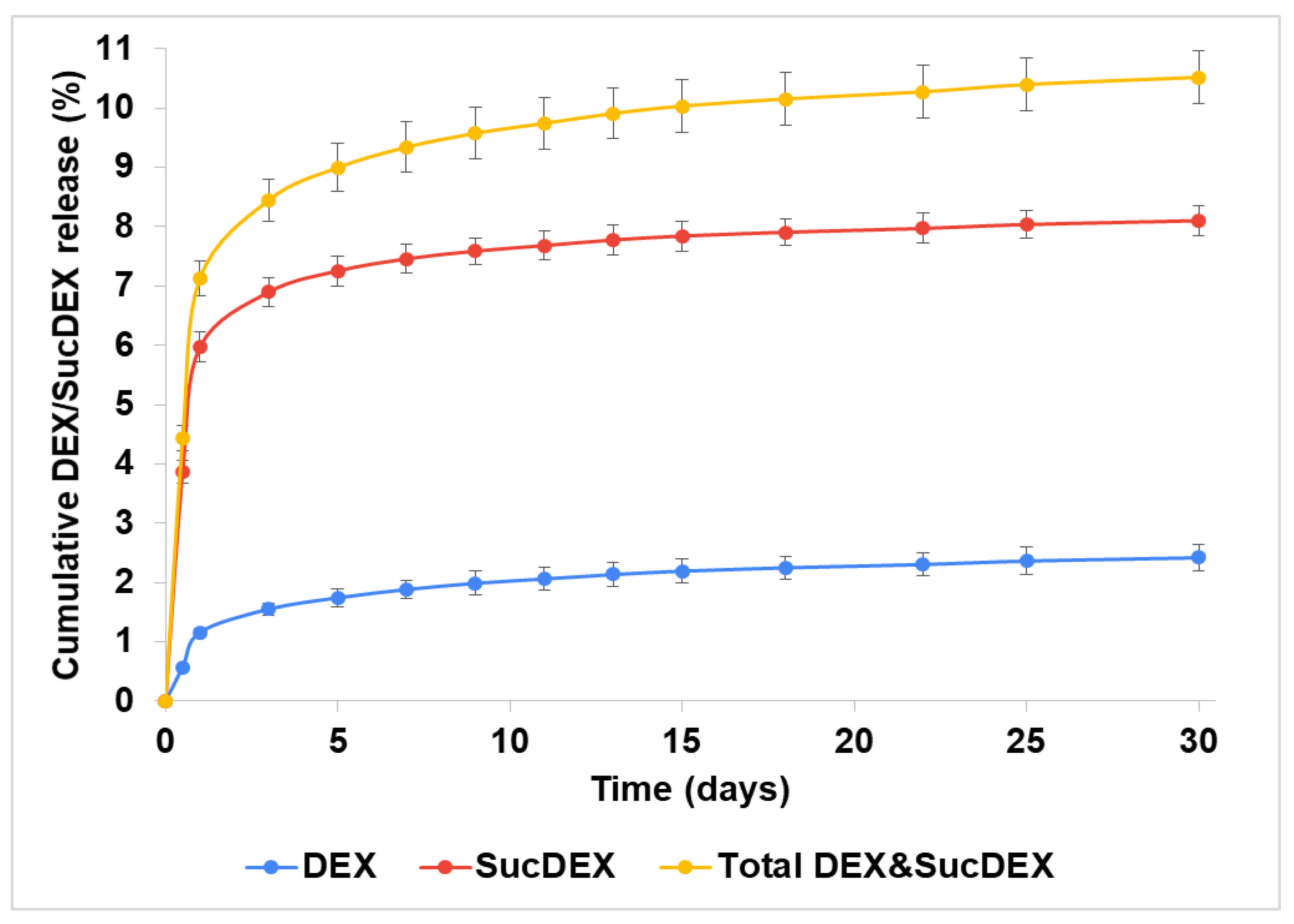
2.2. In Vitro DEX Release from the SucCS-DEX Conjugates
2.3. Anti-Inflammatory Activity of the SucCS-DEX Conjugates
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of SucDEX
3.3. Synthesis of the SucCS-DEX Conjugates
3.4. Characterization of Conjugates
3.5. Determination of DEX Content in the SucCS-DEX Conjugates
3.6. In Vitro DEX Release from the SucCS-DEX Conjugates
3.7. Anti-Inflammatory Activity of the SucCS-DEX Conjugates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharya, M.; Sadeghi, A.; Sarkhel, S.; Hagström, M.; Bahrpeyma, S.; Toropainen, E.; Auriola, S.; Urtti, A. Release of functional dexamethasone by intracellular enzymes: A modular peptide-based strategy for ocular drug delivery. J. Control. Release 2020, 327, 584–594. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Li, G.; Xu, F.; Li, S.; Teng, L.; Li, Y.; Sun, F. Anti-angiogenic activity of bevacizumab-bearing dexamethasone-loaded plga nanoparticles for potential intravitreal applications. Int. J. Nanomed. 2019, 14, 8819–8834. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Bozdağ Pehlivan, S.; Sümer Bolu, B.; Nomak Sanyal, R.; Vural, İ.; Ünlü, N. Dexamethasone–pamam dendrimer conjugates for retinal delivery: Preparation, characterization and in vivo evaluation. J. Pharm. Pharmacol. 2016, 68, 1010–1020. [Google Scholar] [CrossRef]
- Raghava, S.; Hammond, M.; Kompella, U.B. Periocular routes for retinal drug delivery. Expert Opin. Drug Deliv. 2004, 1, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Urtti, A.; Salminen, L.; Miinalainen, O. Systemic absorption of ocular pilocarpine is modified by polymer matrices. Int. J. Pharm. 1985, 23, 147–161. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M.; Serpe, L.; Foglietta, F.; Muntoni, E.; del Pozo Rodriguez, A.; Aspiazu, M.A.S. Ocular delivery of solid lipid nanoparticles. In Lipid Nanocarriers for Drug Targeting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 269–312. [Google Scholar]
- Urtti, A.; Pipkin, J.D.; Rork, G.; Repta, A. Controlled drug delivery devices for experimental ocular studies with timolol 1. In vitro release studies. Int. J. Pharm. 1990, 61, 235–240. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Poshina, D.N.; Raik, S.V.; Urtti, A.; Skorik, Y.A. Polysaccharides in ocular drug delivery. Pharmaceutics 2020, 12, 22. [Google Scholar] [CrossRef]
- Cox, J.T.; Eliott, D.; Sobrin, L. Inflammatory complications of intravitreal anti-vegf injections. J. Clin. Med. 2021, 10, 981. [Google Scholar] [CrossRef]
- Melo, G.B.; da Cruz, N.F.S.; Emerson, G.G.; Rezende, F.A.; Meyer, C.H.; Uchiyama, S.; Carpenter, J.; Shiroma, H.F.; Farah, M.E.; Maia, M.; et al. Critical analysis of techniques and materials used in devices, syringes, and needles used for intravitreal injections. Prog. Retin. Eye Res. 2021, 80, 100862. [Google Scholar] [CrossRef]
- Li, Q.; Weng, J.; Wong, S.N.; Thomas Lee, W.Y.; Chow, S.F. Nanoparticulate drug delivery to the retina. Mol. Pharm. 2020, 18, 506–521. [Google Scholar] [CrossRef]
- Kim, H.M.; Woo, S.J. Ocular drug delivery to the retina: Current innovations and future perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef]
- Mehta, H.; Gillies, M.; Fraser-Bell, S. Perspective on the role of ozurdex (dexamethasone intravitreal implant) in the management of diabetic macular oedema. Ther. Adv. Chronic Dis. 2015, 6, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Ryu, M.; Nakazawa, T.; Akagi, T.; Tanaka, T.; Watanabe, R.; Yasuda, M.; Himori, N.; Maruyama, K.; Yamashita, T.; Abe, T.; et al. Suppression of phagocytic cells in retinal disorders using amphiphilic poly (γ-glutamic acid) nanoparticles containing dexamethasone. J. Control. Release 2011, 151, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Manickavasagam, D.; Oyewumi, M.O. Critical assessment of implantable drug delivery devices in glaucoma management. J. Drug Deliv. 2013, 2013, 895013. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Golovkin, A.S.; Kudryavtsev, I.V.; Prikhodko, S.S.; Trulioff, A.S.; Bokatyi, A.N.; Poshina, D.N.; Raik, S.V.; Skorik, Y.A. Mucoadhesive cholesterol-chitosan self-assembled particles for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2020, 158, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Vellonen, K.S.; Kidron, H.; Urtti, A. Intravitreal clearance and volume of distribution of compounds in rabbits: In silico prediction and pharmacokinetic simulations for drug development. Eur. J. Pharm. Biopharm. 2015, 95, 215–226. [Google Scholar] [CrossRef]
- Shatz, W.; Hass, P.E.; Mathieu, M.; Kim, H.S.; Leach, K.; Zhou, M.; Crawford, Y.; Shen, A.; Wang, K.; Chang, D.P.; et al. Contribution of antibody hydrodynamic size to vitreal clearance revealed through rabbit studies using a species-matched fab. Mol. Pharm. 2016, 13, 2996–3003. [Google Scholar] [CrossRef]
- Peynshaert, K.; Devoldere, J.; De Smedt, S.C.; Remaut, K. In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv. Drug Deliv. Rev. 2018, 126, 44–57. [Google Scholar] [CrossRef]
- Pitkanen, L.; Pelkonen, J.; Ruponen, M.; Ronkko, S.; Urtti, A. Neural retina limits the nonviral gene transfer to retinal pigment epithelium in an in vitro bovine eye model. AAPS J. 2004, 6, 72–80. [Google Scholar] [CrossRef]
- Mains, J.; Wilson, C.G. The vitreous humor as a barrier to nanoparticle distribution. J. Ocul. Pharmacol. Ther. 2013, 29, 143–150. [Google Scholar] [CrossRef]
- Bishop, P.N. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog. Retin. Eye Res. 2000, 19, 323–344. [Google Scholar] [CrossRef]
- Thakur, S.S.; Barnett, N.L.; Donaldson, M.J.; Parekh, H.S. Intravitreal drug delivery in retinal disease: Are we out of our depth? Expert Opin. Drug Deliv. 2014, 11, 1575–1590. [Google Scholar] [CrossRef]
- Pitkanen, L.; Ruponen, M.; Nieminen, J.; Urtti, A. Vitreous is a barrier in nonviral gene transfer by cationic lipids and polymers. Pharm. Res. 2003, 20, 576–583. [Google Scholar] [CrossRef]
- Martens, T.F.; Vercauteren, D.; Forier, K.; Deschout, H.; Remaut, K.; Paesen, R.; Ameloot, M.; Engbersen, J.F.; Demeester, J.; De Smedt, S.C.; et al. Measuring the intravitreal mobility of nanomedicines with single-particle tracking microscopy. Nanomedicine 2013, 8, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Shatz, W.; Aaronson, J.; Yohe, S.; Kelley, R.F.; Kalia, Y.N. Strategies for modifying drug residence time and ocular bioavailability to decrease treatment frequency for back of the eye diseases. Expert Opin. Drug Deliv. 2019, 16, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1603–1611. [Google Scholar] [CrossRef]
- Altiok, E.I.; Santiago-Ortiz, J.L.; Svedlund, F.L.; Zbinden, A.; Jha, A.K.; Bhatnagar, D.; Loskill, P.; Jackson, W.M.; Schaffer, D.V.; Healy, K.E. Multivalent hyaluronic acid bioconjugates improve sflt-1 activity in vitro. Biomaterials 2016, 93, 95–105. [Google Scholar] [CrossRef]
- Altiok, E.I.; Browne, S.; Khuc, E.; Moran, E.P.; Qiu, F.; Zhou, K.; Santiago-Ortiz, J.L.; Ma, J.-x.; Chan, M.F.; Healy, K.E. Sflt multivalent conjugates inhibit angiogenesis and improve half-life in vivo. PLoS ONE 2016, 11, e0155990. [Google Scholar] [CrossRef]
- Famili, A.; Crowell, S.R.; Loyet, K.M.; Mandikian, D.; Boswell, C.A.; Cain, D.; Chan, J.; Comps-Agrar, L.; Kamath, A.; Rajagopal, K. Hyaluronic acid–antibody fragment bioconjugates for extended ocular pharmacokinetics. Bioconjugate Chem. 2019, 30, 2782–2789. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Skorik, Y.A. Dexamethasone conjugates: Synthetic approaches and medical prospects. Biomedicines 2021, 9, 341. [Google Scholar] [CrossRef]
- Skorik, Y.A.; Kritchenkov, A.S.; Moskalenko, Y.E.; Golyshev, A.A.; Raik, S.V.; Whaley, A.K.; Vasina, L.V.; Sonin, D.L. Synthesis of n-succinyl-and n-glutaryl-chitosan derivatives and their antioxidant, antiplatelet, and anticoagulant activity. Carbohydr. Polym. 2017, 166, 166–172. [Google Scholar] [CrossRef]
- Pandit, J.; Sultana, Y.; Aqil, M. Chitosan coated nanoparticles for efficient delivery of bevacizumab in the posterior ocular tissues via subconjunctival administration. Carbohydr. Polym. 2021, 267, 118217. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, N.; Jackson, T.L.; Elsaid, Z.; Alqathania, A.; Somavarapu, S. Plga microparticles entrapping chitosan-based nanoparticles for the ocular delivery of ranibizumab. Mol. Pharm. 2016, 13, 2923–2940. [Google Scholar] [CrossRef] [PubMed]
- Chaharband, F.; Daftarian, N.; Kanavi, M.R.; Varshochian, R.; Hajiramezanali, M.; Norouzi, P.; Arefian, E.; Atyabi, F.; Dinarvand, R. Trimethyl chitosan-hyaluronic acid nano-polyplexes for intravitreal vegfr-2 sirna delivery: Formulation and in vivo efficacy evaluation. Nanomed. Nanotechnol. Biol. Med. 2020, 26, 102181. [Google Scholar] [CrossRef]
- Ugurlu, N.; Asik, M.D.; Cakmak, H.B.; Tuncer, S.; Turk, M.; Cag il, N.; Denkbas, E.B. Transscleral delivery of bevacizumab-loaded chitosan nanoparticles. J. Biomed. Nanotechnol. 2019, 15, 830–838. [Google Scholar] [CrossRef]
- Villanueva, J.R.; Villanueva, L.R.; Navarro, M.G. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int. J. Pharm. 2017, 516, 342–351. [Google Scholar] [CrossRef]
- Wong, C.W.; Wong, T.T. Posterior segment drug delivery for the treatment of exudative age-related macular degeneration and diabetic macular oedema. Br. J. Ophthalmol. 2019, 103, 1356–1360. [Google Scholar] [CrossRef]
- Behar-Cohen, F. Recent advances in slow and sustained drug release for retina drug delivery. Expert Opin. Drug Deliv. 2019, 16, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Balasso, A.; Subrizi, A.; Salmaso, S.; Mastrotto, F.; Garofalo, M.; Tang, M.; Chen, M.; Xu, H.; Urtti, A.; Caliceti, P. Screening of chemical linkers for development of pullulan bioconjugates for intravitreal ocular applications. Eur. J. Pharm. Sci. 2021, 161, 105785. [Google Scholar] [CrossRef]
- Suzuki, T.; Sato, E.; Tada, H.; TOJIMA, Y. Examination of local anti-inflammatory activities of new steroids, hemisuccinyl methyl glycolates. Biol. Pharm. Bull. 1999, 22, 816–821. [Google Scholar] [CrossRef][Green Version]
- Koo, H.; Moon, H.; Han, H.; Na, J.H.; Huh, M.S.; Park, J.H.; Woo, S.J.; Park, K.H.; Kwon, I.C.; Kim, K.; et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials 2012, 33, 3485–3493. [Google Scholar] [CrossRef]
- Chen, S.W.; Xin, Q.; Kong, W.X.; Min, L.; Li, J.F. Anxiolytic-like effect of succinic acid in mice. Life Sci. 2003, 73, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Onishi, H.; Machida, Y. Biological characteristics of lactosaminated n-succinyl-chitosan as a liver-specific drug carrier in mice. J. Control. Release 2001, 70, 295–307. [Google Scholar] [CrossRef]
- Kato, Y.; Onishi, H.; Machida, Y. Evaluation of n-succinyl-chitosan as a systemic long-circulating polymer. Biomaterials 2000, 21, 1579–1585. [Google Scholar] [CrossRef]
- Fattal-German, M.; Le Roy Ladurie, F.; Lecerf, F.; Berrih-Aknin, S. Expression of ICAM-1 and TNFα in human alveolar macrophages from lung-transplant recipients. Ann. N. Y. Acad. Sci. 1996, 796, 138–148. [Google Scholar] [CrossRef]
- Yang, L.; Froio, R.M.; Sciuto, T.E.; Dvorak, A.M.; Alon, R.; Luscinskas, F.W. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood 2005, 106, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Raik, S.V.; Poshina, D.N.; Lyalina, T.A.; Polyakov, D.S.; Vasilyev, V.B.; Kritchenkov, A.S.; Skorik, Y.A. N-[4-(n,n,n-trimethylammonium)benzyl]chitosan chloride: Synthesis, interaction with DNA and evaluation of transfection efficiency. Carbohydr. Polym. 2018, 181, 693–700. [Google Scholar] [CrossRef]
- Pogodina, N.; Pavlov, G.; Bushin, S.; Mel’nikov, A.; Lysenko, Y.B.; Nud’ga, L.; Marsheva, V.; Marchenko, G.; Tsvetkov, V. Conformational characteristics of chitosan molecules as demonstrated by diffusion-sedimentation analysis and viscometry. Polym. Sci. USSR 1986, 28, 251–259. [Google Scholar] [CrossRef]
| Sample | Molar Ratio of Reagents | ω (%) | DSDEX ** (%) by EA | DSDEX (%) by NMR | ||||
|---|---|---|---|---|---|---|---|---|
| CS | EDC * | NHS * | SucDEX | C | N | |||
| CS-DEX-5 | 1 | 0.05 | 0.05 | 0.05 | 40.07 | 6.749 | 1.7 | 1.8 |
| CS-DEX-10 | 1 | 0.1 | 0.1 | 0.1 | 40.09 | 6.424 | 3.1 | 2.9 |
| CS-DEX-20 | 1 | 0.2 | 0.2 | 0.2 | 39.27 | 6.145 | 3.8 | 3.9 |
| CS | - | - | - | - | 41.55 | 7.449 | - | - |
| Sample | ω (%) | DSSuc * (%) by EA | DSSuc (%) by NMR | DSDEX (%) by NMR | DEX Content (μg/mg) | |
|---|---|---|---|---|---|---|
| C | N | |||||
| SucCS-DEX-5 | 31.58 | 3.868 | 65 | 64 | 1.8 | 50 |
| SucCS-DEX-10 | 30.40 | 3.575 | 66 | 68 | 3.0 | 85 |
| Sample | 2Rh (nm) | ζ-Potential (mV) |
|---|---|---|
| CS-DEX-5 | 816 ± 268 | 22.5 ± 0.5 |
| SucCS-DEX-5 | 916 ± 326 | −32.1 ± 0.5 |
| CS-DEX-10 | 700 ± 252 | 14.9 ± 0.8 |
| SucCS-DEX-10 | 950 ± 330 | −30.9 ± 0.7 |
| Samples | Concentration (μg/mL) | w/o LPS or TNF | LPS (1 μg/mL) | TNFα (2 ng/mL) |
|---|---|---|---|---|
| Negative control (n = 11) | - | 95.8 ± 0.3 | 92.40 ± 0.7 | 87.8 ± 1.6 |
| DEX (n = 6) | 1 | 94.0 ± 0.8 * | 91.49 ± 1.2 | 87.1 ± 0.9 |
| 10 | 92.6 ± 0.8 * | 89.54 ± 1.5 | 85.9 ± 1.4 | |
| SucDEX (n = 6) | 1 | 94.7 ± 0.5 | 91.94 ± 1.1 | 88.6 ± 0.8 |
| 10 | 94.0 ± 0.6 * | 88.8 ± 1.8 | 84.6 ± 1.1 | |
| SucCS (n = 6) | 1 | 94.2 ± 0.5 * | 92.9 ± 0.7 | 88.8 ± 1.1 |
| 10 | 94.4 ± 0.6 * | 92.1 ± 0.9 | 89.5 ± 0.9 | |
| SucCS-DEX-10 (n = 6) | 1 | 94.4 ± 0.5 * | 91.2 ± 0.8 | 87.33 ± 1.3 |
| 10 | 91.8 ± 0.9 * | 91.4 ± 0.7 | 85.23 ± 1.2 |
| Sample | Concentration (μg/mL) | w/o TNF | TNFα (2 ng/mL) | TNF vs. w/o TNF, p |
|---|---|---|---|---|
| Negative control (n = 11) | - | 0.51 ± 0.02 | 3.3 ± 0.5 | <0.001 |
| DEX (n = 6) | 1 | 0.47 ± 0.03 | 1.9 ± 0.2 | <0.001 |
| 10 | 0.55 ± 0.05 | 2.01 ± 0.10 | <0.001 | |
| SucDEX (n = 6) | 1 | 0.46 ± 0.01 | 2.0 ± 0.2 | <0.001 |
| 10 | 0.46 ± 0.02 | 2.1 ± 0.2 | <0.001 | |
| SucCS (n = 6) | 1 | 0.43 ± 0.06 | 2.2 ± 0.4 | <0.001 |
| 10 | 0.43 ± 0.06 | 2.4 ± 0.4 | <0.001 | |
| SucCS-DEX-10 (n = 6) | 1 | 0.48 ± 0.02 | 1.2 ± 0.3 * | 0.030 |
| 10 | 0.49 ± 0.02 | 1.04 ± 0.19 ** | 0.018 |
| Sample | Concentration (μg/mL) | w/o LPS | LPS (1 μg/mL) | LPS vs. w/o LPS, p |
|---|---|---|---|---|
| Negative control (n = 11) | - | 0.51 ± 0.02 | 2.6 ± 0.9 | <0.001 |
| DEX (n = 6) | 1 | 0.47 ± 0.03 | 2.1 ± 0.9 | 0.012 |
| 10 | 0.55 ± 0.05 | 3.0 ± 1.2 | <0.001 | |
| SucDEX (n = 6) | 1 | 0.46 ± 0.01 | 1.4 ± 0.5 | <0.001 |
| 10 | 0.46 ± 0.02 | 1.8 ± 0.8 | <0.001 | |
| SucCS (n = 6) | 1 | 0.43 ± 0.06 | 1.1 ± 0.2 | 0.016 |
| 10 | 0.43 ± 0.06 | 1.0 ± 0.3 * | 0.039 | |
| SucCS-DEX-10, (n = 6) | 1 | 0.48 ± 0.02 | 0.69 ± 0.16 ** | 0.231 |
| 10 | 0.49 ± 0.02 | 0.54 ± 0.12 ** | 0.693 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubashynskaya, N.V.; Bokatyi, A.N.; Golovkin, A.S.; Kudryavtsev, I.V.; Serebryakova, M.K.; Trulioff, A.S.; Dubrovskii, Y.A.; Skorik, Y.A. Synthesis and Characterization of Novel Succinyl Chitosan-Dexamethasone Conjugates for Potential Intravitreal Dexamethasone Delivery. Int. J. Mol. Sci. 2021, 22, 10960. https://doi.org/10.3390/ijms222010960
Dubashynskaya NV, Bokatyi AN, Golovkin AS, Kudryavtsev IV, Serebryakova MK, Trulioff AS, Dubrovskii YA, Skorik YA. Synthesis and Characterization of Novel Succinyl Chitosan-Dexamethasone Conjugates for Potential Intravitreal Dexamethasone Delivery. International Journal of Molecular Sciences. 2021; 22(20):10960. https://doi.org/10.3390/ijms222010960
Chicago/Turabian StyleDubashynskaya, Natallia V., Anton N. Bokatyi, Alexey S. Golovkin, Igor V. Kudryavtsev, Maria K. Serebryakova, Andrey S. Trulioff, Yaroslav A. Dubrovskii, and Yury A. Skorik. 2021. "Synthesis and Characterization of Novel Succinyl Chitosan-Dexamethasone Conjugates for Potential Intravitreal Dexamethasone Delivery" International Journal of Molecular Sciences 22, no. 20: 10960. https://doi.org/10.3390/ijms222010960
APA StyleDubashynskaya, N. V., Bokatyi, A. N., Golovkin, A. S., Kudryavtsev, I. V., Serebryakova, M. K., Trulioff, A. S., Dubrovskii, Y. A., & Skorik, Y. A. (2021). Synthesis and Characterization of Novel Succinyl Chitosan-Dexamethasone Conjugates for Potential Intravitreal Dexamethasone Delivery. International Journal of Molecular Sciences, 22(20), 10960. https://doi.org/10.3390/ijms222010960










