Ectopic Endometrium: The Pathologist’s Perspective
Abstract
:1. Introduction
2. Historical Aspects
3. Pathological Features
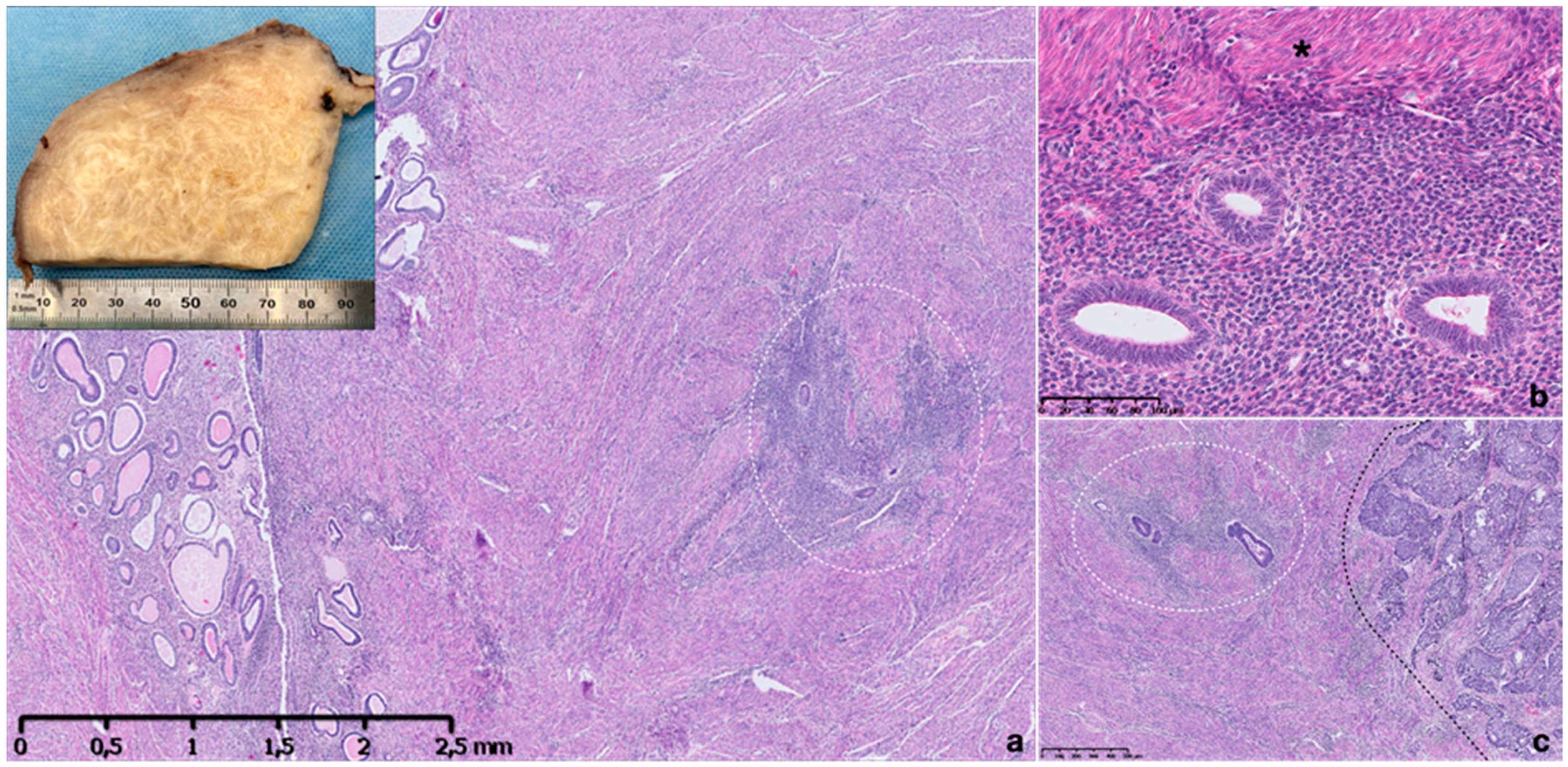
3.1. Pathological Features of Adenomyosis
Pathological Classification
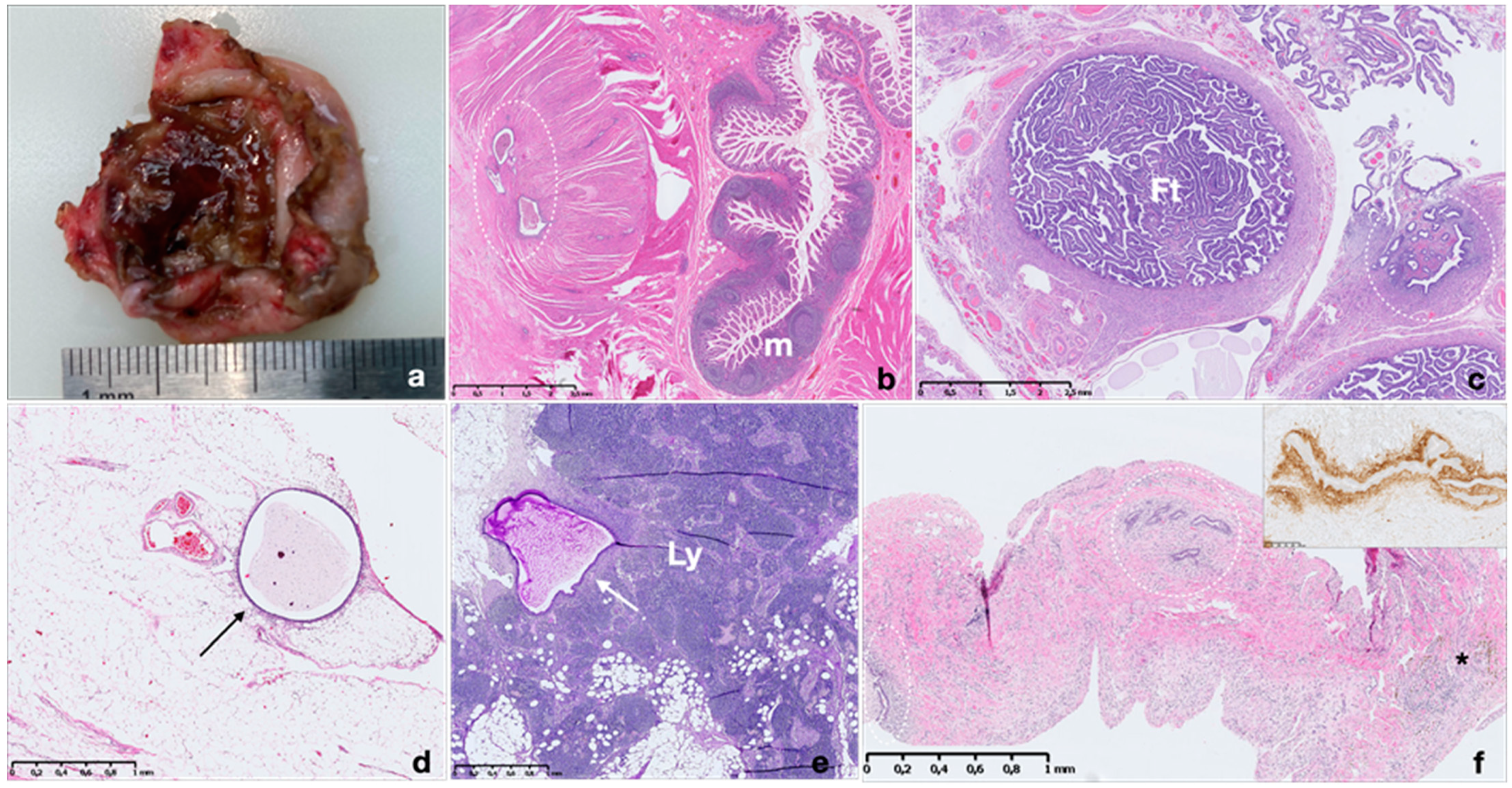
3.2. Pathological Features of Endometriosis
Pathological Classification
4. Association with Others Gynecological Condition and Malignant Transformation
5. Association between Adenomyosis and Endometriosis
6. Pathogenesis
6.1. Endometriosis
6.2. Adenomyosis
6.3. Molecular Aspects in the Pathogenesis of Endometriosis and Adenomyosis
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Tempest, N.; Jansen, M.; Baker, A.M.; Hill, C.J.; Hale, M.; Magee, D.; Treanor, D.; Wright, N.A.; Hapangama, D.K. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J. Pathol. 2020, 251, 440–451. [Google Scholar] [CrossRef]
- Devlieger, R.; D’Hooghe, T.; Timmerman, D. Uterine adenomyosis in the infertility clinic. Hum. Reprod. Update 2003, 9, 139–147. [Google Scholar] [CrossRef] [Green Version]
- García-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.M. Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef]
- Rokitansky, K. Uber Uterusdrüsen-Neubildung. Z Ges. Aerzte 1860, 16, 577–581. [Google Scholar]
- Mayer, R. Uber eine adenomatose Wucherung der Serosa in einer Bauchnarde. Z Geburtshilfe Gynäkol 1903, 49, 32–41. [Google Scholar]
- Cullen, T.S. Adenomyoma of the Uterus; W.B. Saunders: Philadelphia, PA, USA, 1908. [Google Scholar]
- Cullen, T.S. The distribution of adenomyomata containing uterine mucosa. Arch. Surg. 1920, 1, 215–283. [Google Scholar] [CrossRef] [Green Version]
- Benagiano, G.; Brosens, I. Who identified endometriosis? Fertil. Steril. 2011, 95, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Antero, M.F.; Ayhan, A.; Segars, J.; Shih, I.M. Pathology and Pathogenesis of Adenomyosis. Semin. Reprod. Med. 2020, 38, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Bengiano, G.; Brosens, I. History of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Frankl, O. Adenomyosis uteri. Am. J. Obstet. Gynecol. 1925, 10, 680–684. [Google Scholar] [CrossRef]
- Benagiano, G.; Brosens, I.; Lippi, D. The history of endometriosis. Gynecol. Obstet. Investig. 2014, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef]
- Mathur, B.B.; Shah, B.S.; Bhende, Y.M. Adenomyosis uteri. A pathologic study of 290 cases. Am J Obstet. Gynecol. 1962, 84, 1820–1829. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Yachida, N.; Ueda, H.; Sugino, K.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. Iscience 2021, 24, 102258. [Google Scholar] [CrossRef]
- Hirschowitz, L.; Mayall, F.G.; Ganesan, R.; McCluggage, W.G. Intravascular adenomyomatosis: Expanding the morphologic spectrum of intravascular leiomyomatosis. Am. J. Surg. Pathol. 2013, 37, 1395–1400. [Google Scholar] [CrossRef]
- Soslow, R.A.; Longcare, T.A. Uterine Pathology (Cambridge Illustrated Surgical Pathology); Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Goldblum, J.R.; Clement, P.B.; Hart, W.R. Adenomyosis with sparse glands. A potential mimic of low-grade endometrial stromal sarcoma. Am. J. Clin. Pathol. 1995, 103, 218–223. [Google Scholar] [CrossRef]
- Munro, M.G. Classification and Reporting Systems for Adenomyosis. J. Minim. Invasive Gynecol. 2020, 27, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Sampson, J.A. Perforating hemorrhagic (chocolate) cysts of the ovary. Arch. Surg. 1921, 3, 245–323. [Google Scholar] [CrossRef] [Green Version]
- Benson, R.C.; Sneeden, V.D. Adenomyosis: A reappraisal of symptomatology. Am. J. Obstet. Gynecol. 1958, 76, 1044–1057. [Google Scholar] [CrossRef]
- Sandberg, E.C.; Cohn, F. Adenomyosis in the gravid uterus at term. Am. J. Obstet. Gynecol. 1962, 84, 1457–1465. [Google Scholar] [CrossRef]
- Owolabi, T.O.; Strickler, R.C. Adenomyosis: A neglected diagnosis. Obstet. Gynecol. 1977, 50, 424–427. [Google Scholar]
- Novak, E.R.; Woodruff, J.D. Adenomyosis uteri. In Gynecologic and Obstetric Pathology, 7th ed.; Novak, E.R., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1974; p. 261. [Google Scholar]
- Hendrickson, M.R.; Kempson, R.L. Non-neoplastic conditions of the myometrium and uterine serosa. In Surgical Pathology of the Uterine Corpus; W.B. Saunders: Philadelphia, PA, USA, 1980; pp. 452–467. [Google Scholar]
- Gompel, C.; Silverberg, S.G. Pathology in Gynecology and Obstetrics; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1985. [Google Scholar]
- Nishida, M. Relationship between the onset of dysmenorrhea and histologic findings in adenomyosis. Am. J. Obstet. Gynecol. 1991, 165, 229–231. [Google Scholar] [CrossRef]
- McCausland, A.M. Hysteroscopic myometrial biopsy: Its use in diagnosing adenomyosis and its clinical application. Am. J. Obstet. Gynecol. 1992, 166, 1619–1626. [Google Scholar] [CrossRef]
- Vercellini, P.; Ragni, G.; Trespidi, L.; Oldani, S.; Panazza, S.; Crosignani, P.G. Adenomyosis: A déjà vu? Obstet. Gynecol. Surv. 1993, 48, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Siegler, A.M.; Camilien, L. Adenomyosis. J. Reprod. Med. 1994, 39, 841–853. [Google Scholar] [PubMed]
- Vercellini, P.; Parazzini, F.; Oldani, S.; Panazza, S.; Bramante, T.; Crosignani, P.G. Adenomyosis at hysterectomy: A study on frequency distribution and patient characteristics. Hum. Reprod. 1995, 10, 1160–1162. [Google Scholar] [CrossRef]
- Parazzini, F.; Vercellini, P.; Panazza, S.; Chatenoud, L.; Oldani, S.; Crosignani, P.G. Risk factors for adenomyosis. Hum. Reprod. 1997, 12, 1275–1279. [Google Scholar] [CrossRef] [Green Version]
- Ferenczy, A. Pathophysiology of adenomyosis. Hum. Reprod. Update 1998, 4, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Levgur, M.; Abadi, M.A.; Tucker, A. Adenomyosis: Symptoms, histology, and pregnancy terminations. Obstet. Gynecol. 2000, 95, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Zaloudek, C.; Hendrickson, M.R. Mesenchymal tumors of the uterus. In Blaustein’s Pathology of the Female Genital Tract; Kurman, R.J., Ed.; Springer: New York, NY, USA; Berlin, Germany, 2002; pp. 561–616. [Google Scholar]
- Bergholt, T.; Eriksen, L.; Berendt, N.; Jacobsen, M.; Hertz, J.B. Prevalence and risk factors of adenomyosis at hysterectomy. Hum. Reprod. 2001, 16, 2418–2421. [Google Scholar] [CrossRef] [Green Version]
- Bazot, M.; Cortez, A.; Darai, E.; Rouger, J.; Chopier, J.; Antoine, J.M.; Uzan, S. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: Correlation with histopathology. Hum. Reprod. 2001, 16, 2427–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulka, C.A.; Hall, D.A.; McCarthy, K.; Simeone, J. Sonographic findings in patients with adenomyosis: Can sonography assist in predicting extent of disease? AJR Am. J. Roentgenol. 2002, 179, 379–383. [Google Scholar] [CrossRef]
- Sammour, A.; Pirwany, I.; Usubutun, A.; Arseneau, J.; Tulandi, T. Correlations between extent and spread of adenomyosis and clinical symptoms. Gynecol. Obstet. Investig. 2002, 54, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Daguati, R.; Abbiati, A.; Fedele, L. Adenomyosis: Epidemiological factors. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 465–477. [Google Scholar] [CrossRef]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012, 207, 114–117. [Google Scholar] [CrossRef]
- Pistofidis, G.; Makrakis, E.; Koukoura, O.; Bardis, N.; Balinakos, P.; Anaf, V. Distinct types of uterine adenomyosis based on laparoscopic and histopathologic criteria. Clin. Exp. Obstet. Gynecol. 2014, 41, 113–118. [Google Scholar]
- Grimbizis, G.F.; Mikos, T.; Tarlatzis, B. Uterus-sparing operative treatment for adenomyosis. Fertil. Steril. 2014, 101, 472–487. [Google Scholar] [CrossRef]
- Habiba, M.; Gordts, S.; Bazot, M.; Brosens, I.; Benagiano, G. Exploring the challenges for a new classification of adenomyosis. Reprod. Biomed. Online 2020, 40, 569–581. [Google Scholar] [CrossRef]
- Uduwela, A.; Perera, M.; Aiqing, L. Endometrial-myometrial interface: Relationship to adenomyosis and changes in pregnancy. Obstet. Gynaecol. Surv. 2000, 55, 390–400. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Agarwal, N.; Subramanian, A. Endometriosis—Morphology, clinical presentations and molecular pathology. J. Lab. Physicians 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Woodward, P.J.; Sohaey, R.; Mezzetti, T.P., Jr. Endometriosis: Radiologic-pathologic correlation. Radiographics 2001, 21, 193–216. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Puttemans, P.; Deprest, J. Appearances of endometriosis. Baillière’s Clin. Obstet. Gynaecol. 1993, 7, 741–757. [Google Scholar] [CrossRef]
- Parker, R.L.; Dadmanesh, F.; Young, R.H.; Clement, P.B. Polypoid endometriosis: A clinicopathologic analysis of 24 cases and a review of the literature. Am. J. Surg. Pathol. 2004, 28, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.A. Endometriosis—A disease because it is characterized by bleeding. Am. J. Obstet. Gynecol. 1997, 176, 263–267. [Google Scholar] [CrossRef]
- Keckstein, J.; Becker, C.M.; Canis, M. Recommendations for the surgical treatment of endometriosis. Part 2: Deep endometriosis. Hum. Reprod. Open 2020, 1, hoaa002. [Google Scholar] [CrossRef] [Green Version]
- Clement, P.B. The pathology of endometriosis: A survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv. Anat. Pathol. 2007, 14, 241–260. [Google Scholar] [CrossRef]
- McCluggage, W.G. Endometriosis-related pathology: A discussion of selected uncommon benign, premalignant and malignant lesions. Histopathology 2020, 76, 76–92. [Google Scholar] [CrossRef]
- Sumathi, V.P.; McCluggage, W.G. CD10 is useful in demonstrating endometrial stroma at ectopic sites and in confirming a diagnosis of endometriosis. J. Clin. Pathol. 2002, 55, 391–392. [Google Scholar] [CrossRef] [Green Version]
- Terada, S.; Miyata, Y.; Nakazawa, H.; Higashimori, T.; Arai, T.; Kikuchi, Y.; Nozaki, M. Immunohistochemical analysis of an ectopic endometriosis in the uterine round ligament. Diagn. Pathol. 2006, 9, 1–5. [Google Scholar]
- Parra-Herran, C.E.; Yuan, L.; Nucci, M.R.; Quade, B.J. Targeted development of specific biomarkers of endometrial stromal cell differentiation using bioinformatics: The IFITM1 model. Mod. Pathol. 2014, 27, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Busca, A.; Djordjevic, B.; Giassi, A.; Parra-Herran, C. IFITM1 Is Superior to CD10 as a Marker of Endometrial Stroma in the Evaluation of Myometrial Invasion by Endometrioid Adenocarcinoma. Am. J. Clin. Pathol. 2016, 145, 486–496. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Fukuda, S.; Hirata, T.; Arakawa, T.; Ma, S.; Neriishi, K.; Wang, Y.; Takeuchi, A.; Saeki, A.; Harada, M.; et al. IFITM1 is a Novel, Highly Sensitive Marker for Endometriotic Stromal Cells in Ovarian and Extragenital Endometriosis. Reprod. Sci. 2019, 21, 1933719119831782. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Fukuda, S.; Hirata, T.; Neriishi, K.; Wang, Y.; Takeuchi, A.; Saeki, A.; Harada, M.; Hirota, Y.; Matsumoto, T.; et al. PAX8: A Highly Sensitive Marker for the Glands in Extragenital Endometriosis. Reprod. Sci. 2019, 14, 1933719119828095. [Google Scholar] [CrossRef]
- Tanase, Y.; Furukawa, N.; Kobayashi, H.; Matsumoto, T. Malignant Transformation from Endometriosis to Atypical Endometriosis and Finally to Endometrioid Adenocarcinoma within 10 Years. Case Rep. Oncol. 2013, 6, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Wicks, M.J.; Larson, C.R. Histologic criteria for evaluating endometriosis. Northwest Med. 1949, 48, 611–613. [Google Scholar] [CrossRef]
- Acosta, A.A.; Buttram, V.C., Jr.; Besch, P.K.; Malinak, L.R.; Franklin, R.R.; Vanderheyden, J.D. A proposed classification of pelvic endometriosis. Obstet. Gynecol. 1973, 42, 19–25. [Google Scholar] [PubMed]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noë, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [Green Version]
- Chapron, C.; Fauconnier, A.; Vieira, M.; Barakat, H.; Dousset, B.; Pansini, V.; Vacher-Lavenu, M.; Dubuisson, J. Anatomical distribution of deeply infiltrating endometriosis: Surgical implications and proposition for a classification. Hum. Reprod. 2003, 18, 157–161. [Google Scholar] [CrossRef]
- Abrao, M.S.; Neme, R.M.; Carvalho, F.M.; Aldrighi, J.M.; Pinotti, J.A. Histological classification of endometriosis as a predictor of response to treatment. Int. J. Gynaecol. Obstet. 2003, 82, 31–40. [Google Scholar] [CrossRef]
- Andres, M.P.; Borrelli, G.M.; Abrão, M.S. Endometriosis classification according to pain symptoms: Can the ASRMclassification be improved? Best Pract. Res. Clin Obstet. Gynaecol. 2018, 51, 111–118. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar]
- Takai, N.; Akizuki, S.; Nasu, K.; Etoh, Y.; Miyakawa, I. Endometrioid adenocarcinoma arising from adenomyosis. Gynecol. Obstet. Investig. 1999, 48, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Gün, I.; Oner, O.; Bodur, S.; Ozdamar, O.; Atay, V. Is adenomyosis associated with the risk of endometrial cancer? Med. Glas. 2012, 9, 268–272. [Google Scholar]
- Giammalvo, J.T.; Kaplan, K. The incidence of endometriosis interna in 120 cases of carcinoma of the endometrium. Am. J. Obstet. Gynecol 1958, 75, 161–166. [Google Scholar] [CrossRef]
- Johnatty, S.E.; Stewart, C.J.R.; Smith, D.; Nguyen, A.; Dwyer, J.O.; O’Mara, T.A.; Webb, P.M.; Spurdle, A.B. Co-existence of leiomyomas, adenomyosis and endometriosis in women with endometrial cancer. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Kairi-Vassilatou, E.; Kontogianni, K.; Salamale- kis, M.; Sykiotis, K.; Kondi-Pafitis, A. A clinico-pathological study of the relationship be- tween adenomyosis and other hormone-de- pendent uterine lesions. Eur. J. Gynaecol. Oncol. 2004, 25, 222–224. [Google Scholar]
- Kucera, E.; Hejda, V.; Dankovcik, R.; Valha, P.; Dudas, M.; Feyereisl, J. Malignant changes in adenomyosis in patients with endometrioid adenocarcinoma. Eur. J. Gynaecol. Oncol. 2011, 32, 182–184. [Google Scholar]
- Zouzoulas, O.D.; Tsolakidis, D.; Efstratiou, I.; Pervana, S.; Pazarli, E.; Grimbizis, G. Correlation between adenomyosis and endometrial cancer: 6-year experience of a single center. Facts Views Vis. Obgyn. 2018, 10, 147–152. [Google Scholar]
- Habiba, M.; Pluchino, N.; Petignat, P.; Bianchi, P.; Brosens, I.A.; Benagiano, G. Adenomyosis and Endometrial Cancer: Literature Review. Gynecol. Obstet. Investig. 2018, 83, 313–328. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S. WHO Classification of Tumors of the Female Reproductive Organs; WHO Press: Lyon, France, 2014. [Google Scholar]
- Sampson, J.A. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch. Surg. 1925, 10, 1–72. [Google Scholar] [CrossRef]
- Siufi Neto, J.; Kho, R.M.; Siufi, D.F.; Baracat, E.C.; Anderson, K.S.; Abrão, M.S. Cellular, histologic, and molecular changes associated with endometriosis and ovarian cancer. J. Minim. Invasive Gynecol. 2014, 21, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.B. Malignant changes in endometriosis. Obstet. Gynecol. 1953, 2, 283–289. [Google Scholar] [PubMed]
- Nezhat, F.; Datta, M.S.; Hanson, V.; Pejovic, T.; Nezhat, C.; Nezhat, C. The relationship of endometriosis and ovarian malignancy: A review. Fertil. Steril. 2008, 90, 1559–1570. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Chen, C.C.; Tsai, E.M.; Er, T.K. Endometriosis-associated ovarian cancer: What have we learned so far? Clin. Chim. Acta 2019, 493, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Erzen, M.; Rakar, S.; Klancnik, B.; Syrjänen, K. Endometriosis-associated ovarian carcinoma (EAOC): An entity distinct from other ovarian carcinomas as suggested by a nested case-control study. Gynecol. Oncol. 2001, 83, 100–108. [Google Scholar] [CrossRef]
- Yu, H.C.; Lin, C.Y.; Chang, W.C.; Shen, B.J.; Chang, W.P.; Chuang, C.M. Task Force on Carcinogenesis of Endometrial Cancer. Increased association between endometriosis and endometrial cancer: A nationwide population-based retrospective cohort study. Int. J. Gynecol. Cancer 2015, 25, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Chapron, C.; Tosti, C.; Marcellin, L.; Bourdon, M.; Lafay-Pillet, M.C.; Millischer, A.E.; Streuli, I.; Borghese, B.; Petraglia, F.; Santulli, P. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum. Reprod. 2017, 32, 1393–1401. [Google Scholar] [CrossRef] [Green Version]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P. Mall, G.; Böttcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932. [Google Scholar] [CrossRef] [Green Version]
- Novak, E.R.; de Lima, O.A. A correlative study of adenomyosis and pelvic endo- metriosis, with special reference to the hormonal reaction of ectopic endo- metrium. Am. J. Obstet. Gynecol. 1948, 56, 634–644. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Fellah, L. What if deep endometriotic nodules and uterine adenomyosis were actually two forms of the same disease? Fertil. Steril. 2019, 111, 454–456. [Google Scholar] [CrossRef] [Green Version]
- Kunz, G.; Beil, D.; Huppert, P.; Noe, M.; Kissler, S.; Leyendecker, G. Adenomyosis in endometriosis—Prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum. Reprod. 2005, 20, 2309–2316. [Google Scholar] [CrossRef] [Green Version]
- Benagiano, G.; Brosens, I.; Habiba, M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum. Reprod. Update 2014, 20, 386–402. [Google Scholar] [CrossRef]
- Giudice, L.C.; Khao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and Pathophysiology of Endometriosis. Fertil. Steril. 2012, 98, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, S.; Kim, Y.M.; Ji, Y.I. Uterine adenomyosis which developed from hypoplastic uterus in postmenopausal woman with Mayer- Rokitansky-Kuster-Hauser syndrome: A case report. J. Menopausal Med. 2013, 19, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratopoulou, C.A.; Donnez, J.; Dolmans, M.M. Origin and Pathogenic Mechanisms of Uterine Adenomyosis: What Is Known So Far. Reprod. Sci. 2021, 28, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Batt, R.E.; Yeh, J. Müllerianosis: Four developmental (embryonic) Müllerian diseases. Reprod. Sci. 2013, 20, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Zhao, L.; Wang, L.; Wu, Z.; Mei, Q.; Nie, J.; Li, X.; Li, Y.; Fu, X.; et al. Whole-exome sequencing of endometriosis identifies fre- quent alterations in genes involved in cell adhesion and chromatin-remodeling complexes. Hum. Mol. Genet. 2014, 23, 6008–6021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, S.; Hirota, Y.; Ueno, T.; Fukui, Y.; Yoshida, E.; Hayashi, T.; Kojima, S.; Takeyama, R.; Hashimoto, T.; Kiyono, T.; et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat. Commun. 2019, 10, 5785. [Google Scholar] [CrossRef]
- Moore, L.; Leongamornlert, D.; Coorens, T.H.H.; Sanders, M.A.; Ellis, P.; Dentro, S.C.; Dawson, K.J.; Butler, T.; Rahbari, R.; Mitchell, T.J.; et al. The mutational landscape of normal human endometrial epithelium. Nature 2020, 580, 640–646. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Z.; Liu, H.; Niu, W.; Wang, X.; Liang, N.; Wang, X.; Wang, Y.; Shi, Y.; Xu, L.; et al. Single-cell transcriptomic analysis of eutopic endometrium and ectopic lesions of adenomyosis. Cell Biosci. 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yildiz, S.; Adli, M.; Wei, J.J. Adenomyosis pathogenesis: Insights from next-generation sequencing. Hum. Reprod. Update 2021. [Google Scholar] [CrossRef]

| Reference | Diagnostic Cut-Off Point | Classification |
|---|---|---|
| Sampson, 1921 [22] | N/A | Group 1: Invasion from within Group 2: Invasion from without Group 3: Adenomyoma (intramyometrial) |
| Bensen and Sneedens, 1958 [23] | >2 LPP + muscle changes | Degree of uterine involvement: Slight Moderate Marked |
| Sandberg and Cohn, 1962 [24] | >2 LPF (8 mm) | N/A |
| Bird et al., 1972 [15] | ≥1 LPF (2 mm) below the endometrium basal layer | Depth of invasion: Grade I: sub-basal lesions within one LPF Grade II: up to mid-myometrium Grade III: beyond mid-myometrium. |
| Degree of involvement: Slight: 1–3 glands/LPF Moderate: 4–9 glands/LPF Marked: ≥10 glands/LPF | ||
| Owolabi and Strickler, 1977 [25] | >1 LPF | N/A |
| Novak and Woodruff, 1974 [26] | >1 HPF | N/A |
| Hendrickson and Kempson, 1980 [27] | >1/4 of total uterine wall thickness | N/A |
| Gompel and Silverberg, 1985 [28] | >1 MPF (×100) | N/A |
| Nishida et al., 1991 [29] | N/A | Type 1: Continuous from the endometrium Type 2: Continuous from the serosa |
| McCausland et al., 1992 [30] | ≥1 mm depth | Minimal Deep |
| Vercellini et al., 1993 [31] | >1 LPF (4 mm) | |
| Siegler and Camilien, 1994 [32] | N/A | Depth of penetration from the basal layer of endometrium: grades 1–3 Degree of involvement: Mild: 1–3 islands/LPF Moderate: 4–9 islands/LPF Severe: >10 islands/LPF |
| Configuration: diffuse, discrete (nodular/focal) | ||
| Vercellini et al., 1995 [33] | >0.5 LPF (2.5 mm) | N/A |
| Parazzini et al., 1997 [34] | >0.5 LPF (2.5 mm) | N/A |
| Ferenczy et al., 1998 [35] | Distance between the endomyometrial junction to the nearest adenomyotic focus should be ~25% of the myometrial thickness | |
| Levgur et al., 2000 [36] | ≥2 mm below endomyometrial junction myometrial hyperplasia | Superficial: <40% uterine wall thickness Intermediate: 40–80% wall thickness Deep: >80% wall thickness |
| Zaloudek and Hendrickson, 2002 [37] | >0.5 LPF (2.5 mm) | N/A |
| Bergholt et al., 2001 [38] | Prevalence varied when ≥1, ≥2, or ≥3 mm from the endometrial–myometrial junction was used as a cut-off point. | |
| Bazot et al., 2001 [39] | >2.5 mm beyond the endometrial-myometrial junction | Depth of myometrial involvement: Grade 1: 1/3 (superficial adenomyosis) Grade 2: 2/3 Grade 3: entire myometrium (deep adenomyosis) Grading according to the number of endometrial islets: Mild: 1–3 Moderate: 4–9 Severe: ≥10 |
| Hulka et al., 2002 [40] | >0.5 LPF (2−3 mm) | Category 1 (mild): microscopic foci or only affecting the inner 1/3 of myometrium Category 2 (focal lesions) Category 3 (severe): affecting the outer 2/3 of the myometrium |
| Sammour et al., 2002 [41] | ≥2 mm below endomyometrial junction myometrial hyperplasia | Group A: up to 25% Group B: 26–50% Group C: 51–75% Group D: >75% of myometrial thickness |
| Vercellini et al., 2006 [42] | >2.5 mm from endometrial junction | Depth of myometrial involvement: Mild, 1/3 Moderate, 1/3–2/3 Severe >2/3 of uterine wall |
| Grades based on degree of spread: Grade 1: 1–3 islets/LPF Grade 2: 4–10 islets/LPF Grade 3: >10 islets/LPF | ||
| Configuration: diffuse, focal or nodular. | ||
| Kishi et al., 2012 [43] | N/A | Subtypes based on magnetic resonance imaging, surgical, and histologic findings: I: intrinsic: Inner uterine layer. II: extrinsic: outer uterine layer (normal junctional zone). III: solitary adenomyosis no connection to the junctional zone or to the serosa. IV: indeterminate |
| Pistofidis et al., 2014 [44] | N/A | Types based on laparoscopic and histopathologic criteria: Sclerotic Nodular Cystic |
| Grimbizis et al., 2014 [45] | N/A | Diffuse: disease scattered throughout the musculature. Focal: affecting a restricted area (includes adenomyoma and cystic variety) Polypoid (typical and atypical) Special (rare forms) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camboni, A.; Marbaix, E. Ectopic Endometrium: The Pathologist’s Perspective. Int. J. Mol. Sci. 2021, 22, 10974. https://doi.org/10.3390/ijms222010974
Camboni A, Marbaix E. Ectopic Endometrium: The Pathologist’s Perspective. International Journal of Molecular Sciences. 2021; 22(20):10974. https://doi.org/10.3390/ijms222010974
Chicago/Turabian StyleCamboni, Alessandra, and Etienne Marbaix. 2021. "Ectopic Endometrium: The Pathologist’s Perspective" International Journal of Molecular Sciences 22, no. 20: 10974. https://doi.org/10.3390/ijms222010974
APA StyleCamboni, A., & Marbaix, E. (2021). Ectopic Endometrium: The Pathologist’s Perspective. International Journal of Molecular Sciences, 22(20), 10974. https://doi.org/10.3390/ijms222010974





