Type 1 Diabetes Mellitus and the First Trimester Placenta: Hyperglycemia-Induced Effects on Trophoblast Proliferation, Cell Cycle Regulators, and Invasion
Abstract
1. Introduction
2. Results
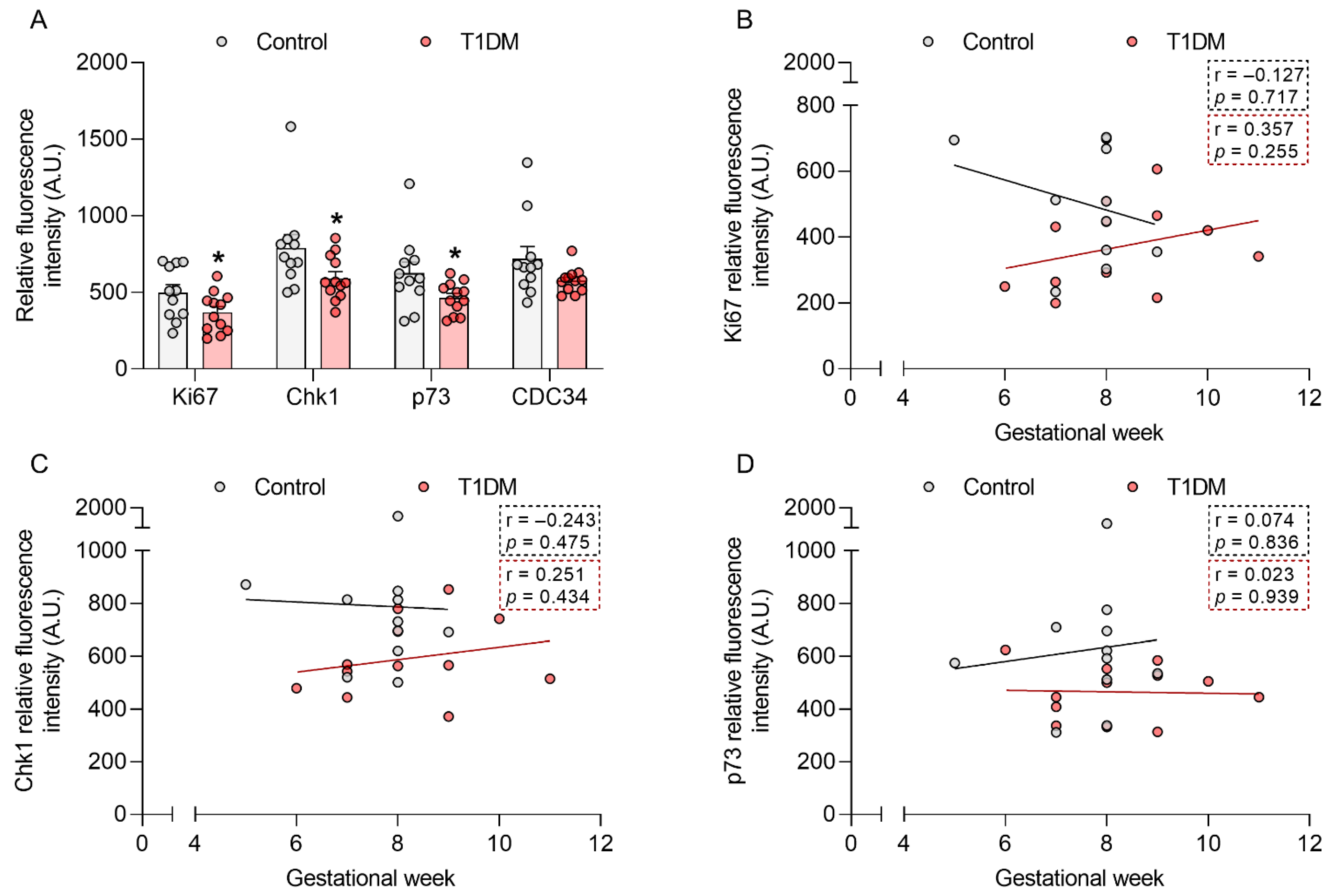
2.1. T1DM Affects Placental Cell Cycle Modulators in the First Trimester of Pregnancy
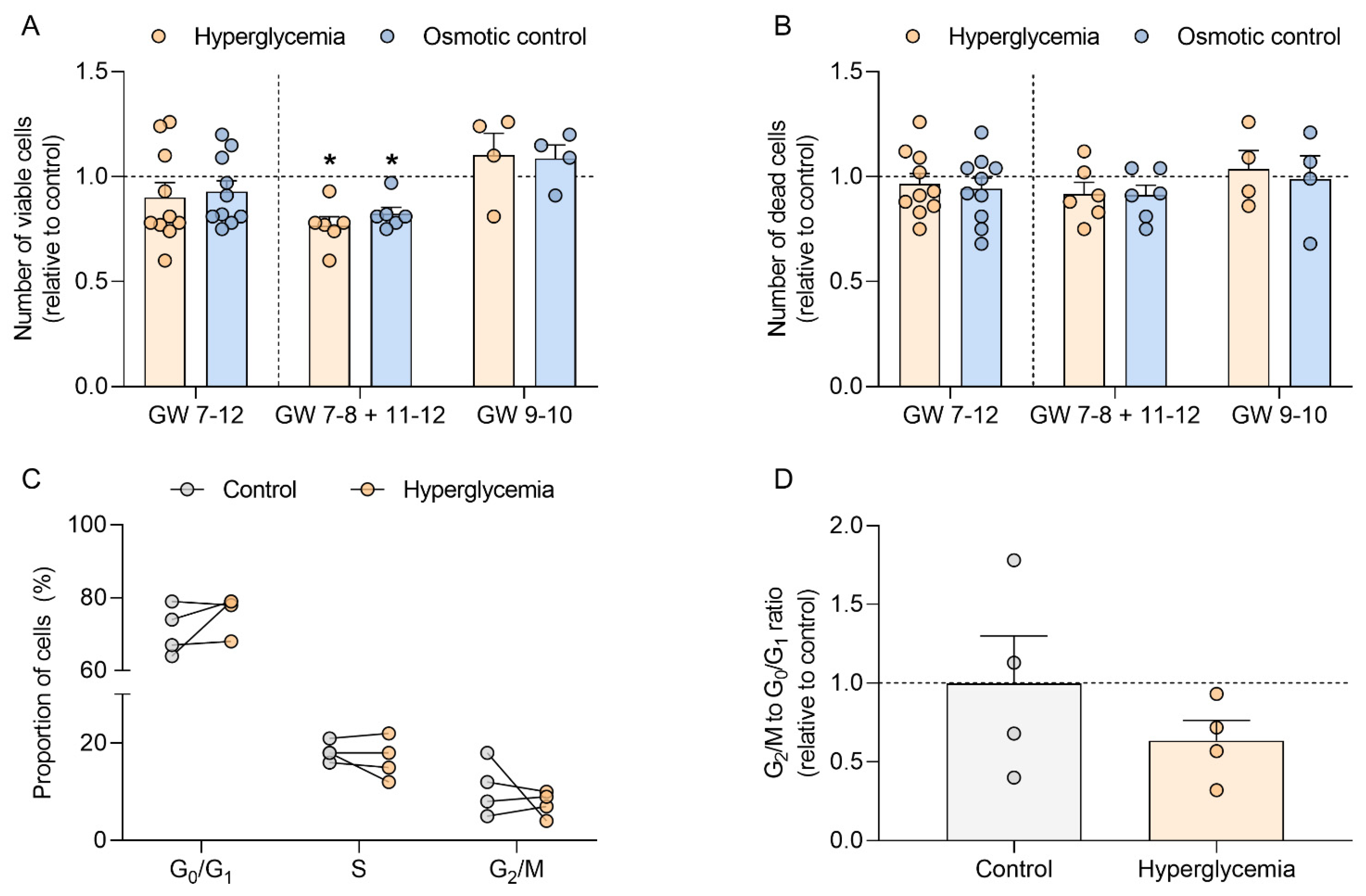
2.2. Hyperglycemia Decreases Trophoblast Cell Number in Early Pregnancy
2.3. Hyperglycemia Increases Trophoblast Invasion in Early Pregnancy
3. Discussion
4. Materials and Methods
4.1. First Trimester Placental Tissue Collection
4.2. First Trimester Trophoblast Isolation
4.3. Protein Array
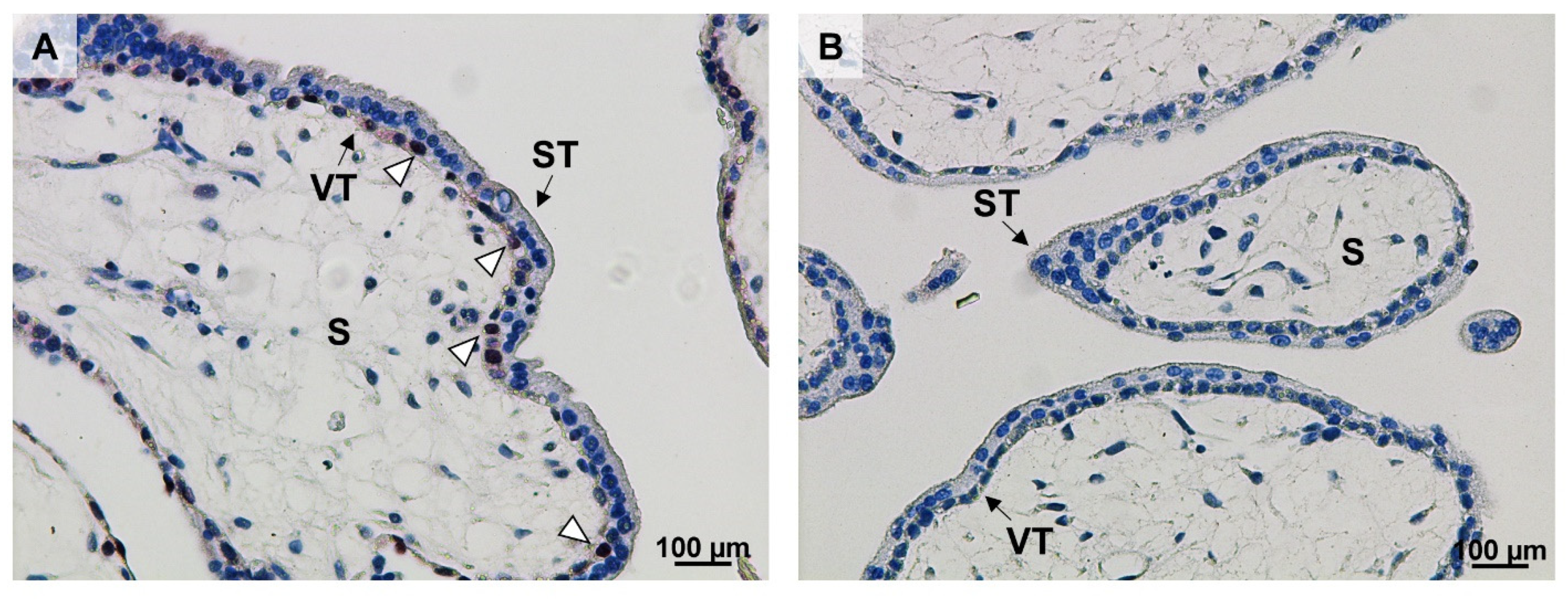
4.4. Ki67 Immunolocalization
4.5. Trophoblast Cell Number Determination and FACS Analysis
4.6. Invasion Assay
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef]
- Chang, C.W.; Wakeland, A.K.; Parast, M.M. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J. Endocrinol. 2018, 236, R43–R56. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E.; Charnock-Jones, D.S. The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 2010, 54, 303–312. [Google Scholar] [CrossRef]
- Siwetz, M.; Blaschitz, A.; El-Heliebi, A.; Hiden, U.; Desoye, G.; Huppertz, B.; Gauster, M. TNF-α alters the inflammatory secretion profile of human first trimester placenta. Lab. Investig. J. Tech. Methods Pathol. 2016, 96, 428–438. [Google Scholar] [CrossRef]
- Hoch, D.; Bachbauer, M.; Pöchlauer, C.; Algaba-Chueca, F.; Tandl, V.; Novakovic, B.; Megia, A.; Gauster, M.; Saffery, R.; Glasner, A.; et al. Maternal Obesity Alters Placental Cell Cycle Regulators in the First Trimester of Human Pregnancy: New Insights for BRCA1. Int. J. Mol. Sci. 2020, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Cervar-Zivkovic, M. Diabetes Mellitus, Obesity, and the Placenta. Obstet. Gynecol. Clin. North Am. 2020, 47, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- McCance, D.R.; Casey, C. Type 1 Diabetes in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Pitchika, A.; Jolink, M.; Winkler, C.; Hummel, S.; Hummel, N.; Krumsiek, J.; Kastenmüller, G.; Raab, J.; Kordonouri, O.; Ziegler, A.G.; et al. Associations of maternal type 1 diabetes with childhood adiposity and metabolic health in the offspring: A prospective cohort study. Diabetologia 2018, 61, 2319–2332. [Google Scholar] [CrossRef]
- Lin, S.F.; Kuo, C.F.; Chiou, M.J.; Chang, S.H. Maternal and fetal outcomes of pregnant women with type 1 diabetes, a national population study. Oncotarget 2017, 8, 80679–80687. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.A.; Mills, J.L.; Metzger, B.E.; Knopp, R.H.; Simpson, J.L.; Jovanovic-Peterson, L.; Scheer, K.; Van Allen, M.I.; Aarons, J.H.; Reed, G.F. Early sonographic evaluation for fetal growth delay and congenital malformations in pregnancies complicated by insulin-requiring diabetes. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care 1992, 15, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Mulder, E.J.; Visser, G.H. Growth and motor development in fetuses of women with type-1 diabetes. I. Early growth patterns. Early Hum. Dev. 1991, 25, 91–106. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Mølsted-Pedersen, L.; Lebech, P.E. Is the early growth delay in the diabetic pregnancy accompanied by a delay in placental development? Acta Obstet. Gynecol. Scand. 1986, 65, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.F.; Sørensen, S.; Mølsted-Pedersen, L. Serum levels of human placental lactogen, pregnancy-associated plasma protein A and endometrial secretory protein PP14 in first trimester of diabetic pregnancy. Acta Obstet. Gynecol. Scand. 1998, 77, 155–158. [Google Scholar] [PubMed]
- Gauster, M.; Majali-Martinez, A.; Maninger, S.; Gutschi, E.; Greimel, P.H.; Ivanisevic, M.; Djelmis, J.; Desoye, G.; Hiden, U. Maternal Type 1 diabetes activates stress response in early placenta. Placenta 2017, 50, 110–116. [Google Scholar] [CrossRef]
- Hiden, U.; Glitzner, E.; Ivanisevic, M.; Djelmis, J.; Wadsack, C.; Lang, U.; Desoye, G. MT1-MMP expression in first-trimester placental tissue is upregulated in type 1 diabetes as a result of elevated insulin and tumor necrosis factor-alpha levels. Diabetes 2008, 57, 150–157. [Google Scholar] [CrossRef][Green Version]
- Majali-Martinez, A.; Hoch, D.; Tam-Amersdorfer, C.; Pollheimer, J.; Glasner, A.; Ghaffari-Tabrizi-Wizsy, N.; Beristain, A.G.; Hiden, U.; Dieber-Rotheneder, M.; Desoye, G. Matrix metalloproteinase 15 plays a pivotal role in human first trimester cytotrophoblast invasion and is not altered by maternal obesity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 10720–10730. [Google Scholar]
- Bandres-Meriz, J.; Dieberger, A.M.; Hoch, D.; Pöchlauer, C.; Bachbauer, M.; Glasner, A.; Niedrist, T.; van Poppel, M.N.M.; Desoye, G. Maternal Obesity Affects the Glucose-Insulin Axis During the First Trimester of Human Pregnancy. Front. Endocrinol. 2020, 11, 566673. [Google Scholar] [CrossRef]
- Hoch, D.; Gauster, M.; Hauguel-de Mouzon, S.; Desoye, G. Diabesity-associated oxidative and inflammatory stress signalling in the early human placenta. Mol. Asp. Med. 2019, 66, 21–30. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Korgun, E.T.; Celik-Ozenci, C.; Acar, N.; Cayli, S.; Desoye, G.; Demir, R. Location of cell cycle regulators cyclin B1, cyclin A, PCNA, Ki67 and cell cycle inhibitors p21, p27 and p57 in human first trimester placenta and deciduas. Histochem. Cell Biol. 2006, 125, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Zorn, T.M.; Zúñiga, M.; Madrid, E.; Tostes, R.; Fortes, Z.; Giachini, F.; San Martín, S. Maternal diabetes affects cell proliferation in developing rat placenta. Histol. Histopathol. 2011, 26, 1049–1056. [Google Scholar] [PubMed]
- Unek, G.; Ozmen, A.; Mendilcioglu, I.; Simsek, M.; Korgun, E.T. Immunohistochemical distribution of cell cycle proteins p27, p57, cyclin D3, PCNA and Ki67 in normal and diabetic human placentas. J. Mol. Histol. 2014, 45, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J.; Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 2003, 3, 421–429. [Google Scholar] [CrossRef]
- Zhong, A.; Chang, M.; Yu, T.; Gau, R.; Riley, D.J.; Chen, Y.; Chen, P.L. Aberrant DNA damage response and DNA repair pathway in high glucose conditions. J. Cancer Res. Updates 2018, 7, 64–74. [Google Scholar] [CrossRef]
- Lefkimmiatis, K.; Caratozzolo, M.F.; Merlo, P.; D’Erchia, A.M.; Navarro, B.; Levrero, M.; Sbisa, E.; Tullo, A. p73 and p63 sustain cellular growth by transcriptional activation of cell cycle progression genes. Cancer Res. 2009, 69, 8563–8571. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrand, J.L.; Bowen, M.E.; Vogel, H.; Barna, M.; Attardi, L.D. The p53 family members have distinct roles during mammalian embryonic development. Cell Death Differ. 2017, 24, 575–579. [Google Scholar] [CrossRef]
- Robinson, J.; Canavan, J.P.; el Haj, A.J.; Goldspink, D.F. Maternal diabetes in rats. I. Effects on placental growth and protein turnover. Diabetes 1988, 37, 1665–1670. [Google Scholar] [CrossRef]
- Siddiqi, T.A.; Miodovnik, M.; Mimouni, F.; Clark, E.A.; Khoury, J.C.; Tsang, R.C. Biphasic intrauterine growth in insulin-dependent diabetic pregnancies. J. Am. Coll. Nutr. 1989, 8, 225–234. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Kestenbaum, B.; Rue, T.C.; Steffes, M.W.; Cleary, P.A.; Molitch, M.E.; Lachin, J.M.; Weiss, N.S.; Brunzell, J.D. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch. Intern. Med. 2008, 168, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Blaschitz, A.; Weiss, U.; Dohr, G.; Desoye, G. Antibody reaction patterns in first trimester placenta: Implications for trophoblast isolation and purity screening. Placenta 2000, 21, 733–741. [Google Scholar] [CrossRef]
- Weiss, U.; Cervar, M.; Puerstner, P.; Schmut, O.; Haas, J.; Mauschitz, R.; Arikan, G.; Desoye, G. Hyperglycaemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast. Diabetologia 2001, 44, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Fröhlich, J.D.; Huppertz, B.; Abuja, P.M.; König, J.; Desoye, G. Oxygen modulates the response of first-trimester trophoblasts to hyperglycemia. Am. J. Pathol. 2012, 180, 153–164. [Google Scholar] [CrossRef]
- Lyall, F.; Bulmer, J.N.; Duffie, E.; Cousins, F.; Theriault, A.; Robson, S.C. Human trophoblast invasion and spiral artery transformation: The role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am. J. Pathol. 2001, 158, 1713–1721. [Google Scholar] [CrossRef]
- Illsley, N.P.; DaSilva-Arnold, S.C.; Zamudio, S.; Alvarez, M.; Al-Khan, A. Trophoblast invasion: Lessons from abnormally invasive placenta (placenta accreta). Placenta 2020, 102, 61–66. [Google Scholar] [CrossRef]
- Tao, J.; Xia, L.Z.; Chen, J.J.; Zeng, J.F.; Meng, J.; Wu, S.; Wang, Z. High glucose condition inhibits trophoblast proliferation, migration and invasion by downregulating placental growth factor expression. J. Obstet. Gynaecol. Res. 2020, 46, 1690–1701. [Google Scholar] [CrossRef]
- Belkacemi, L.; Lash, G.E.; Macdonald-Goodfellow, S.K.; Caldwell, J.D.; Graham, C.H. Inhibition of human trophoblast invasiveness by high glucose concentrations. J. Clin. Endocrinol. Metab. 2005, 90, 4846–4851. [Google Scholar] [CrossRef]
- Majali-Martinez, A.; Velicky, P.; Pollheimer, J.; Knöfler, M.; Yung, H.W.; Burton, G.J.; Tabrizi-Wizsy, N.G.; Lang, U.; Hiden, U.; Desoye, G.; et al. Endothelin-1 down-regulates matrix metalloproteinase 14 and 15 expression in human first trimester trophoblasts via endothelin receptor type B. Hum. Reprod. 2017, 32, 46–54. [Google Scholar] [CrossRef]
- Leopold, B.; Strutz, J.; Weiß, E.; Gindlhuber, J.; Birner-Gruenberger, R.; Hackl, H.; Appel, H.M.; Cvitic, S.; Hiden, U. Outgrowth, proliferation, viability, angiogenesis and phenotype of primary human endothelial cells in different purchasable endothelial culture media: Feed wisely. Histochem. Cell Biol. 2019, 152, 377–390. [Google Scholar] [CrossRef]

| Control (n = 11) | T1DM (n = 12) | p Value | |
|---|---|---|---|
| Gestational age (weeks) | 7.6 ± 1.0 | 8.3 ± 1.4 | n.s. |
| Maternal age (years) | 30.3 ± 6.7 | 30.5 ± 5.2 | n.s. |
| HbA1c (%) | n.d. | 7.7 ± 1.6 | n.d. |
| Maternal BMI (kg/m2) | 24.2 ± 2.5 | 22.9 ± 1.1 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majali-Martinez, A.; Weiss-Fuchs, U.; Miedl, H.; Forstner, D.; Bandres-Meriz, J.; Hoch, D.; Djelmis, J.; Ivanisevic, M.; Hiden, U.; Gauster, M.; et al. Type 1 Diabetes Mellitus and the First Trimester Placenta: Hyperglycemia-Induced Effects on Trophoblast Proliferation, Cell Cycle Regulators, and Invasion. Int. J. Mol. Sci. 2021, 22, 10989. https://doi.org/10.3390/ijms222010989
Majali-Martinez A, Weiss-Fuchs U, Miedl H, Forstner D, Bandres-Meriz J, Hoch D, Djelmis J, Ivanisevic M, Hiden U, Gauster M, et al. Type 1 Diabetes Mellitus and the First Trimester Placenta: Hyperglycemia-Induced Effects on Trophoblast Proliferation, Cell Cycle Regulators, and Invasion. International Journal of Molecular Sciences. 2021; 22(20):10989. https://doi.org/10.3390/ijms222010989
Chicago/Turabian StyleMajali-Martinez, Alejandro, Ursula Weiss-Fuchs, Heidi Miedl, Desiree Forstner, Julia Bandres-Meriz, Denise Hoch, Josip Djelmis, Marina Ivanisevic, Ursula Hiden, Martin Gauster, and et al. 2021. "Type 1 Diabetes Mellitus and the First Trimester Placenta: Hyperglycemia-Induced Effects on Trophoblast Proliferation, Cell Cycle Regulators, and Invasion" International Journal of Molecular Sciences 22, no. 20: 10989. https://doi.org/10.3390/ijms222010989
APA StyleMajali-Martinez, A., Weiss-Fuchs, U., Miedl, H., Forstner, D., Bandres-Meriz, J., Hoch, D., Djelmis, J., Ivanisevic, M., Hiden, U., Gauster, M., & Desoye, G. (2021). Type 1 Diabetes Mellitus and the First Trimester Placenta: Hyperglycemia-Induced Effects on Trophoblast Proliferation, Cell Cycle Regulators, and Invasion. International Journal of Molecular Sciences, 22(20), 10989. https://doi.org/10.3390/ijms222010989








